Abstract
An in vitro motility assay approach was used to investigate the mechanisms of the functional differences between myosin isoforms, by studying the effect of MgATP and MgADP on actin sliding velocity (Vf) of pure slow and fast rat skeletal myosin at different temperatures. The value of Vf depended on [MgATP] according to Michaelis–Menten kinetics, with an apparent constant (Km) of 54.2, 64.4 and 200 μm for the fast isoform and 18.6, 36.5 and 45.5 μm for the slow isoform at 20, 25 and 35°C, respectively. The presence of 2 mm MgADP decreased Vf and yielded an inhibition constant (Ki) of 377, 463 and 533 μm for the fast isoform at 20, 25 and 35°C, respectively, and 120 and 355 μm for the slow isoform at 25 and 35°C, respectively. The analysis of Km and Ki suggested that slow and fast isoforms differ in the kinetics limiting Vf. Moreover, the higher sensitivity of the fast myosin isoform to a drop in [MgATP] is consistent with the higher fatigability of fast fibres than slow fibres. From the Michaelis–Menten relation in the absence of MgADP, we calculated the rate of actomyosin dissociation by MgATP (k+ATP) and the rate of MgADP release (k–ADP). We found values of k+ATP of 4.8 × 106, 6.5 × 106 and 6.6 × 106 M−1 s−1 for the fast isoform and 3.3 × 106, 2.9 × 106 and 6.7 × 106 M−1 s−1 for the slow isoform and values of k–ADP of 263, 420 and 1320 s−1 for the fast isoform and 62, 107 and 306 s−1 for the slow isoform at 20, 25 and 35°C, respectively. The results suggest that k–ADP could be the major determinant of functional differences between the fast and slow myosin isoforms at physiological temperatures.
It is generally believed that the mechanical properties of skeletal muscle fibres, including unloaded shortening velocity (V0), are to a large extent determined by the myosin isoforms expressed in the fibres (Schiaffino & Reggiani, 1996). In a series of recent studies (Weiss et al. 2001; Capitanio et al. 2006; Nyitrai et al. 2006; Iorga et al. 2007), the mechanochemical properties of pure myosin isoforms isolated from skeletal muscle fibres were studied in order to define the kinetic and molecular basis of myosin diversity, but the issue remains important and somewhat unresolved.
Muscle contraction results from a cyclical interaction of myosin cross-bridges with actin, coupled with the hydrolysis of MgATP to MgADP and inorganic phosphate (Pi). The events in the cycle are essentially the same for all muscle myosins; during the actomyosin interaction, myosin propels an actin filament for a distance δ (or step size) in a time ton, i.e. the time it remains attached to actin. The sliding velocity of the actin filament and the shortening velocity of the muscle fibre are therefore equal to δ/ton (i.e. distance travelled/time required). The difference between slow and fast myosin isoforms could be due to a different step size or to a different duration of the attached state, or to both. To address this question, actomyosin kinetics of single fibres (Cooke & Pate, 1985; Lu et al. 1993; Zhao & Kawai, 1994; Wang & Kawai, 1996; Galler et al. 2005) and pure myosin isoforms in solution were studied (Siemankowski et al. 1985; Weiss et al. 2001; Nyitrai et al. 2006; Iorga et al. 2007). Many of these studies successfully measured the rates of the two major transitions known to determine the duration of actomyosin interaction in sarcomeric isoforms, namely the rate of MgADP release from actomyosin (k–ADP) and the rate of actomyosin dissociation by MgATP (k+ATP), and suggested that k–ADP had a major role in defining V0.
This conclusion was also supported by a recent study in which the elementary event of force generation and shortening produced by a single motor domain of pure fast and slow skeletal myosin isoforms was studied (Capitanio et al. 2006). For the first time, two phases in the attached state of skeletal myosin were observed. Several lines of evidence supported the hypothesis that the duration of the first phase, which was very different for the slow and fast isoforms, was related to k–ADP. Interestingly, the second phase, which was very similar for both isoforms, was found likely to be related to k+ATP.
Thus, both kinetic analysis in solution and single-molecule analysis suggest that k–ADP is the rate constant of the cross-bridge cycle that mostly defines the unloaded shortening velocity (V0) of skeletal muscle myosin and may account for the main difference between the fast and slow myosin isoforms. Nevertheless, it was recently suggested that the relative contributions of k–ADP and k+ATP in defining shortening velocity could be different for the slow and fast isoforms at different temperatures (Nyitrai et al. 2006). The work of Nyitrai et al. (2006) has demonstrated that, at a low temperature (12°C), k+ATP plays a significant role in limiting the velocity of fast isoforms, and the hypothesis that, at physiological temperatures, k–ADP might limit V0 for both the isoforms was only suggested by extrapolation of the experimental data. The suggestion that k–ADP and k+ATP can have different relative roles in limiting V0 for the slow and fast isoforms is also supported by the study by Iorga et al. (2007).
In the present study, we have used an in vitro motility assay (IVMA) approach to investigate the effect of MgATP and MgADP concentrations on actin sliding velocity (Vf) of pure slow and fast skeletal myosin isoforms at different temperatures in order to investigate the kinetics mechanism underlying the functional differences between slow and fast myosin isoforms. The IVMA is a simplified model of muscle contraction that enables analysis of the molecular events that couple MgATP hydrolysis to mechanical work and enables this analysis to be performed on pure myosin isoforms over a wide range of temperatures (Homsher et al. 1992; Yamashita et al. 1994; Rossi et al. 2005). It has been suggested that MgADP binding/release can occur in two steps, because an isomerization step can precede MgADP release (Sleep & Hutton, 1980; Nyitrai & Geeves, 2004; Nyitrai et al. 2006; Albet-Torres et al. 2009; Mansson, 2010). While the analysis of actomyosin kinetics in solution might miss the isomerization step, the determination of k–ADP by IVMA is free from such uncertainty.
Our results suggest that, in the experimental conditions of IVMA, at physiological temperature and low ionic strength, k–ADP is the major determinant of the difference between slow and fast skeletal myosins in the actin sliding velocity.
Methods
Ethical approval
The experimental protocol was approved by the local Animal Ethics Committee, and all the experimental procedures conformed to the UK Animals (Scientific Procedures) Act, 1986.
Muscle sampling and protein preparation
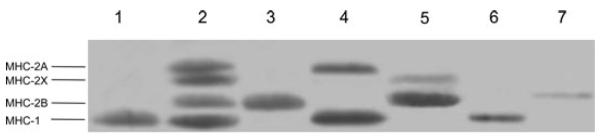
Gastrocnemius and soleus muscles were dissected from 3- (n = 5) and 6-month-old Wistar rats (n = 6), respectively. Rats were anaesthetized with ether and then decapitated. Muscle samples were characterized for myosin heavy chain (MHC) isoform composition by 8% SDS-PAGE, as described by Pellegrino et al. (2003). The gels were silver stained, so that a minor MHC band can be detected when its density is 1% of the major band (Bottinelli et al. 1994). Whole gastrocnemius muscles contained 80% of MHC-2B and 20% of MHC-2X (Fig. 1); however, the superficial portion of the muscles, which was used as a source of myosin, showed a pure MHC-2B content (Fig. 1). Soleus muscles contained 80% of MHC-1 and 20% of MHC-2A (Fig. 1). Muscles were placed in cold skinning solution and divided into several fibre bundles that were stored at –20°C in skinning solution with 50% glycerol for up to 2 weeks.
Figure 1. Examples of myosin heavy chain (MHC) electrophoretic separation in muscle samples and single fibres.
The distribution of MHC isoforms in soleus (line 4), gastrocnemius (line 5), the superficial portion of gastrocnemius (line 3) and a mixed sample composed of soleus and diaphragm muscles (line 2). Examples of the MHC composition of single fibres are shown in line 1 (pure type 1 fibre), line 6 (pure type 1 fibre) and line 7 (pure type 2B fibre). Gels were silver stained.
On each day of the experiment, fibre bundles were removed from the freezer and used for myosin extraction. Pure type 2B myosin isoform was prepared from the bulk superficial portion of gastrocnemius muscles according a procedure previously described in detail (Canepari et al. 2000). Pure myosin isoform 1 was prepared from single fibres containing only MHC-1 isoform. Briefly, single fibres (at least 8 mm long) were manually dissected from muscle samples, chemically skinned for 1 h in skinning solution containing 1% Triton X-100, and cut into two segments. The shorter segment (about 2 mm long) was dissolved in standard buffer (Laemmli et al. 1970) and used for MHC isoform identification on an 8% SDS-PAGE gel using a procedure previously described (Pellegrino et al. 2003). The longer segment (at least 6 mm long) was used for myosin extraction. In order to obtain sufficient pure type 1 myosin to perform IVMA experiments with different MgATP concentrations at different temperatures, at least 100 pure fibres containing the MHC-1 isoform were pooled before myosin extraction (Canepari et al. 2000). Extracted myosin was used to prepare heavy meromyosin (HMM) by proteolytic digestion with α-chymotrypsin according to a modification of the method of Margossian & Lowey (1982) previously described in detail (Canepari et al. 2000).
In vitro motility assay
The IVMA was performed according to Canepari et al. (1999, 2000) in the temperature range 20–35°C. A water–glycol ethylene solution from a thermostat (Thermo Haake DC10 Newington, NH, USA) was circulated through a coiled pipe created on the microscope stage and in a jacket of Perspex surrounding the microscope objective in order to maintain the desired temperature in the flow cell. The flow cell temperature was continuously monitored in all experiments by a thermometer probe (Delta Ohm HD8601P Thermometer) placed outside but very closely in contact with the flow cell.
The composition of the experimental buffer (AB) was as follows: Mops, 25 mM (pH 7.4 at 25°C); KCl, 25 mM; MgCl2, 4 mM; EGTA, 1 mM; DTT, 1 mM; glucose oxidase, 200 μg ml−1;catalase, 36 μg ml−1;glucose, 5 mg ml−1;and ATP, 2 mM. The concentration of HMM solution for all the experiments was 0.3 mg ml−1. The sliding velocity of the actin filaments (Vf) was measured at 20, 25 and 35°C in the absence and in the presence of 2 mM MgADP, varying MgATP concentrations in the range 0.01–2.00 mM. The [MgCl2] and [KCl] were changed to maintain the basic conditions above and the ionic strength. To ensure that at very low MgATP concentrations Vf was not affected by MgATP consumption, some experiments at low MgATP concentrations were performed in the presence of a MgATP regenerating system (creatine phosphate, 2 mM; and creatine phosphokinase, 100 units ml−1) in the AB buffer. No differences were found in Vf values. Moreover, no differences were observed between Vf values determined at the beginning and at the end of experiments lasting several minutes, indicating that no change in MgATP concentration occurred.
The compositions of solutions were determined using a computer program based on that developed by Fabiato & Fabiato (1979).
At each temperature and [MgATP], for each myosin sample, the velocities of at least 50 filaments were measured using purpose-designed software, and their distribution was characterized according to parametric statistics (Canepari et al. 1999, 2000).
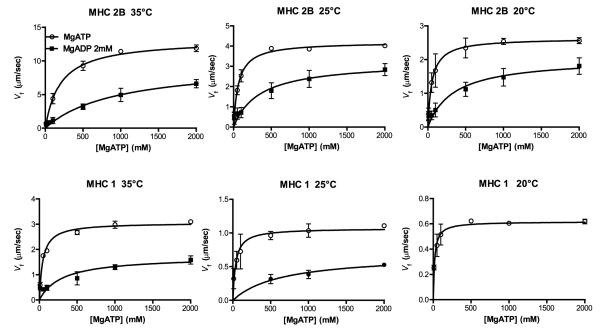
The relations between Vf and [MgATP] in the presence and in the absence of 2 mM MgADP (Fig. 2) represent the average of at least three data sets obtained with three different myosin samples. In turn, each data point of each sample was obtained from the average Vf of at least 50 filaments. Consequently, each data point in the curves reported in Fig. 2 is representative of ~150 filaments.
Figure 2. Michaelis–Menten relations between actin sliding velocity (Vf) and MgATP.
The Vf is shown at different MgATP concentrations in the absence (open circles) and presence of 2 mM MgADP (filled circles) for pure type 2B (MHC-2B) and type 1 myosin isoforms (MHC-1) at 20, 25 and 35°C. The values of Vf recorded at 20°C for slow myosin in the presence of 2 mM MgADP are not reported (see Methods). Each data point in the figure represents the mean ± SEM of at least three curves obtained with different muscle samples in the same experimental conditions (see Methods). Each data point of each relation was obtained from the average Vf of at least 50 filaments.
The values of Vf recorded at 20°C for slow myosin in the presence of 2 mM MgADP were very low and close to the sensitivity threshold of our system; therefore, these data are not reported.
Equations
The data points of the relations between Vf and [ATP] were fitted with the following Michaelis–Menten relation:
| (1) |
This enabled the calculation of Km, i.e. the concentration of MgATP at which the velocity was half-maximum, and Vmax, the velocity at infinite [MgATP].
Assuming that the inhibition of Vf by MgADP follows the behaviour of a competitive enzyme inhibitor, we could calculate the inhibition constant (Ki) from the following equation:
| (2) |
where Kmt is Km in the presence of 2 mM MgADP.
According to a detachment-limited model of actin sliding velocity (Huxley, 1990), Vf is proportional to the myosin step size (d) divided by the duration of actin binding (ton), i.e. Vf = d/ton. The ton is the sum of the time required for MgADP release (1/k–ADP) from myosin plus the time required for MgATP to bind and induce dissociation from actin (1/k+ATP), as follows:
The MgATP-induced dissociation of the cross-bridge is a function of MgATP concentration. The [MgATP] dependence of ton depends upon whether MgATP binding or MgADP release defines the overall time of detachment. If we assume that MgADP release is the rate-limiting factor and that [MgADP] ≈ 0, then k+ATP is a linear function of [MgATP], that is k+ATP = k′+ATP [MgATP], where k′+ATP is the apparent second-order rate constant of MgATP binding to the cross-bridge. Then:
that is:
Dividing by k′+ATP, we obtain the following expression:
| (3) |
Thus, the plot of Vf versus [MgATP] will provide values of Vmax = k–ADP × d and Km = k–ADP/k′+ATP; hence, Vmax/Km = k′+ATP × d. From these relations, the values of k–ADP and k′+ATP can be determined (see Table 2).
Table 2.
Kinetic parameters calculated from the Michaelis–Menten relations
| Myosin isoform | 20°C | 25°C | 35°C | Ratio 35°C/25°C |
|---|---|---|---|---|
| MHC-2B | ||||
| k–ADP (s–1) | 263 | 420 | 1319 | 3.14 |
| k′+ATP (106 M–1 s–1) | 4.8 | 6.5 | 6.6 | 1.01 |
| MHC-1 | ||||
| k–ADP (s–1) | 62 | 107 | 306 | 2.86 |
| k′+ATP (106 M–1 s–1) | 3.3 | 2.9 | 6.7 | 2.3 |
The rate of MgADP release (k–ADP) and the rate of actomyosin dissociation induced by MgATP (k′+ATP) were calculated from Vmax (Vmax = k–ADP × d, where d = 10 nm) and from the ratio Vmax/Km(Vmax/Km = k′+ATP × d, where d = 10 nm), respectively (see Methods). Data were obtained on pure myosin 2B (MHC-2B) and pure myosin 1 (MHC-1) at 20, 25 and 35°C.
Data analysis
The relation between Vf and [MgATP] was analysed with a non-linear regression fitted with a Michaelis–Menten equation by a computer program (Prism 5.0; Graphpad Software Inc., La Jolla, CA, USA), which provided the values of Km and Vmax expressed as means±SEM. The statistical significance of the differences was assessed by two-way ANOVA for repeated measures followed by the Student–Neuman–Keuls post hoc test. A probability of less than 5% (P < 0.05) was considered to be significant.
The temperature dependence of the actin filament sliding velocity was analysed with Arrhenius plots. From the slopes of the linear regression lines, the kinetic energy that must be provided to start the reaction or activation energy (Ea) and the temperature coefficient (Q10) were calculated as described by Rossi et al. (2005).
Results
Effect of the concentration of nucleotides on actin sliding velocity
Actin sliding velocity was studied on pure slow (type 1) and pure fast (type 2B) myosin isoforms at MgATP concentrations of 0.01, 0.02, 0.1, 0.5, 1.0 and 2.0 mM (Fig. 2). Experiments were performed at 20, 25 and 35°C, in the absence and in the presence of 2 mM MgADP (Fig. 2). As expected, Vf was higher for the fast than for the slow isoform and increased with temperature (Fig. 2). The Vf increased with MgATP concentration, both in the presence and in the absence of MgADP. Velocities were lower in the presence of 2 mM MgADP at all MgATP concentrations (Fig. 2). Assuming that the relation between Vf and MgATP concentration followed the behaviour of a simple enzymatic reaction with MgATP as a substrate, we fitted the data with a Michaelis–Menten relation [eqn (1)] and calculated the following parameters: (a) the Michaelis constant (Km), which is the [MgATP] at which velocity is half-maximum; (b) Vmax, which is the maximal velocity at infinite MgATP concentration; and (c) the ratio Vmax/Km, which characterizes myosin enzyme kinetics at subsaturating MgATP concentrations.
The values of Km were lower for the slow than for the fast isoform at all temperatures, indicating that the velocity saturates at a lower MgATP concentration in the slow than in the fast isoform (Table 1). The Km increased with temperature in both isoforms. In the temperature range 25–35°C, the increase was much more evident for the fast (3.1-fold change) than for the slow isoform (1.25-fold change), indicating that MgATP binding to the slow isoform was little affected by temperature. The opposite was observed in the range 20–25°C, i.e. Km of the fast isoform had a lower temperature sensitivity (1.42-fold change) than that of the slow isoform (3.8-fold change).
Table 1.
Biochemical parameters calculated from the Michaelis–Menten relations
| 20°C |
25°C |
35°C |
|||||
|---|---|---|---|---|---|---|---|
| Myosin isoform | Mean | SEM | Mean | SEM | Mean | SEM | Ratio 35°C/25°C |
| MHC-2B | |||||||
| Km(μM) | 54.16*‡ | 8.94 | 64.43*‡ | 4.87 | 199.80‡ | 21.52 | 3.10 |
| Kmt (μM) | 342 | 100 | 343 | 112 | 949.60 | 268 | 2.77 |
| Ki (μM) | 376.58 | – | 462.58 | – | 532.90 | – | 1.15 |
| Vmax (μm s–1) | 2.63†‡ | 0.08 | 4.20†‡ | 0.07 | 13.19†‡ | 0.33 | 3.14 |
| Vmax/Km (106 nm M–1 s–1) | 48 | – | 65 | – | 66 | – | 1.01 |
| Vmaxinh (μm s–1) | 2.04 | 1.69 | 3.23†‡ | 0.31 | 9.69†‡ | 1.22 | 2.99 |
| MHC-1 | |||||||
| Km (μM) | 18.62*‡ | 3.79 | 36.51‡ | 7.15 | 45.52‡ | 5.06 | 1.25 |
| Kmt (μM) | – | – | 647 | 253 | 302 | 112 | 0.47 |
| Ki (μM) | – | – | 119.60 | – | 354.80 | – | 2.97 |
| Vmax (μm s–1) | 0.62†‡ | 0.02 | 1.07†‡ | 0.03 | 3.06†‡ | 0.06 | 2.86 |
| Vmax/Km (106 nm M–1 s–1) | 33 | – | 29 | – | 67 | – | 2.3 |
| Vmaxinh (μm s–1) | – | – | 0.62†‡ | 0.12 | 1.725†‡ | 0.17 | 2.78 |
Values of Km ([MgATP] at which the velocity was half-maximum) and Vmax (velocity at infinite [MgATP]) were obtained from the relations between Vf and [MgATP]. Values of Kmt (Km in the presence of 2mM MgADP); Ki (inhibition constant) and Vmaxinh (Vmax in the presence of 2mM MgADP) were obtained from the same relation in the presence of 2 mM MgADP. Data were obtained on pure myosin 2B (MHC-2B) and pure myosin 1 (MHC-1) at 20, 25 and 35°C. Data at 20°C in the presence of 2 mM MgADP are not reported (see Methods). Values are expressed as means ± SEM.
Significantly different from 35°C for the samemyosin (P < 0.05);
significantly different from values of the same parameter at all other temperatures for the same myosin (P < 0.05); and
significantly different from the corresponding value for the other isoforms (P < 0.05).
Values of Vmax were three- to fourfold higher for the fast than for the slow isoform at all temperatures (Table 1), consistent with Vf values. In the range 25–35°C, Q10 values for Vmax were 2.86 and 3.14 and activation energies were 77.73 and 76.58 kJ mol−1 for the slow and fast isoform, respectively, indicating similar temperature sensitivity of the two isoforms, consistent with what was previously observed (Rossi et al. 2005).
Following the addition of 2 mM MgADP, the substrate concentration dependence of Vf was shifted towards higher MgATP concentrations. Fitting the data with the Michaelis–Menten equation, we could determine Kmt (Km in the presence of 2 mM MgADP). Values of Kmt were, at all temperatures and for both isoforms, higher that the corresponding values of Km (Table 1). Assuming that the inhibition of Vf by MgADP followed the behaviour of a competitive enzyme inhibitor, we could also calculate the inhibition constant [eqn (2)]. The values of Ki were lower for the slow than for the fast isoform at all temperatures (Table 1). The temperature dependence of Ki was greater for the slow (2.97-fold change between 25 and 35°C) than for the fast isoform (1.15-fold change in the same temperature range; Table 1). The analysis of the Vf dependence on [MgATP] for the slow isoform in the presence of MgADP at 20°C was not performed because Vf dropped to a very low value that prevented reliable measurements.
From the Michaelis–Menten equation in the presence of 2 mM MgADP, we could also calculate Vmaxinh (Vmax in presence of 2 mM MgADP). Values of Vmaxinh were, at all temperatures and for both isoforms, lower than the corresponding Vmax values, and Q10 values for Vmaxinh were similar as those for Vmax, suggesting that MgADP does not affect temperature sensitivity of velocity (Table 1).
Rate of actomyosin dissociation by MgATP (k′+ATP) and rate of MgADP release from actomyosin complex (k–ADP)
The values of k–ADP and k′+ATP were calculated from Vmax = k–ADP × d and Vmax/Km = k′+ATP × d [eqn (3)], assuming a step size d = 10 nm (Table 2). The value of d = 10 nm is at the upper end of the 5–10 nm range of measured step size (Hooft et al. 2007). It was considered to be identical for the slow and fast isoforms and therefore not responsible for the differences in shortening velocities among the isoforms (Palmiter et al. 1999; Capitanio et al. 2006).
While the values of k–ADP were much lower for the slow than for the fast isoform at all temperatures, the values of k′+ATP were similar for both isoforms (Table 2). Moreover, k–ADP showed temperature dependence comparable to the temperature dependence of Vmax for both isoforms. In contrast, k′+ATP showed a temperature dependence that was different for the slow and the fast isoform and varied with the temperature range. The k′+ATP of the fast isoform varied much more than that of the slow isoform in the range 20–5°C, but did not vary with temperature in the range 25–35°C. In contrast, the k′+ATP of the slow isoform varied much more than that of the fast isoform in the range 25–35°C, but did not vary with temperature in the range 20–25°C.
Discussion
The in vitro motility assay approach was used to study the effect of MgATP and MgADP concentrations on the actin sliding velocity of pure slow and fast skeletal myosin isoforms at different temperatures. In IVMA, as the viscous force imposed on moving actin filaments is negligible, the filament velocity (Vf) is expected to be limited only by cross-bridge kinetics and, therefore, to be a good index of actomyosin kinetics in unloaded conditions. Moreover, a very significant correlation was found between V0 and Vf of actin on myosin extracted from the same fibres (Canepari et al. 1999; Pellegrino et al. 2003). The IVMA approach enables reliable study of the following factors: (a) the cross-bridge kinetics over a wide range of temperatures (20–35°C), because isolated myosin is stable up to physiological temperatures (Homsher et al. 1992; Yamashita et al. 1994; Rossi et al. 2005); and (b) the mechanical and the kinetic properties of pure myosin isoforms in the same experimental conditions that reproduce the actomyosin cycle as a whole.
Binding of MgATP to myosin
The value of Vf for slow and fast myosin depended on [MgATP] and increased with increasing temperature. The values of Q10 and Ea were consistent with those expected on the basis of previous studies on isolated myosin in similar temperature ranges (Homsher et al. 2003; Rossi et al. 2005), muscle fibres (Pate et al. 1994) and muscles (Ranatunga, 1984; Kawai et al. 2000).
The relation between Vf and MgATP followed classic michaelian behaviour. Values of Km (Table 1) for the fast skeletal isoforms were in good agreement with those for the shortening velocity of rabbit skeletal fibres in similar temperature ranges (Cooke & Pate, 1985; Pate et al. 1992) and with those for rabbit, rat and chicken pectoralis fast myosin in similar assay systems (Homsher et al. 1992; Yamashita et al. 1994; Regnier et al. 1998; Baker et al. 2002; Debold et al. 2008). The small differences among these studies may be explained by the differences in the skeletal muscle types (usually a mixture of fast isoforms), myosin preparation (myosin or the proteolitic fractions HMM or S1) and/or assay conditions (temperature, pH, ionic strength and the presence of MgATP back-up system). The values of Km related to pure type 1 skeletal myosin are reported here, for the first time, and are very similar to those found for rat α-cardiac myosin in the IVMA system (Yamashita et al. 1994). The much lower values of Km indicate that the Vf of the slow isoform is less sensitive to the decrease of substrate concentration than Vf of the fast isoform (Pate et al. 1992; Yamashita et al. 1994). As both decrease in force and a decrease in velocity of shortening occur in fatigue, the latter observation is consistent with the lower fatigability of slow compared with fast fibres. Moreover, as Km = k–ADP/k+ATP [see eqn (3)], Km can be considered to define the [MgATP] above which k–ADP plays a larger role than k+ATP in defining Vf. Therefore, the higher Km of fast compared with slow myosin suggests that k+ATP plays a more relevant role in defining Vf in fast than in slow myosin at subsaturating [MgATP].
Interestingly, the slow and fast isoforms showed a different temperature sensitivity, which also varied with the temperature range considered. In the range 25–35°C, the sensitivity to the decrease of substrate concentration did not change much on increasing temperature for the slow isoform (1.25-fold change), but increased significantly for the fast isoform (3.1-fold change). As Km = k–ADP/k+ATP, the larger effect of temperature on k+ATP of slow than fast myosin and the similar effect on k–ADP of both isoforms (Table 2) can account for the latter phenomenon. Vice versa, in the range 20–25°C, the temperature sensitivity of Km was high for the slow (3.8-fold change) and low for the fast isoform (1.42 fold change), consistent with the lack of sensitivity of k+ATP in the slow isoform and the increase in sensitivity of k+ATP of the fast isoform.
Binding of MgADP to myosin
In the presence of 2 mM MgADP, the substrate dependence of the velocity shifted towards higher [MgATP] for both isoforms. The value of Kmt was much higher than Km for both isoforms at all temperatures, suggesting a competition between MgADP and MgATP for the myosin binding site.
To better define the impact of MgADP on velocity, assuming a competitive inhibition of Vf by MgADP, the inhibition constant (Ki) was calculated [eqn (2)]. The values of Ki (Table 1) are consistent with the values for rat skeletal and cardiac myosin in a similar assay system (Yamashita et al. 1992), but somewhat higher than those obtained for the shortening velocity of rabbit fibres (Cooke & Pate, 1985) and for the actin filament velocity of rabbit HMM and of rabbit and chicken myosin (Baker et al. 2002; Homsher et al. 2003; Greenberg et al. 2009). The differences in the values of Ki among these studies may be attributed to the differences in muscle types, myosin preparations, ionic strengths, MgADP concentrations and the temperature of the assay conditions.
The inhibition constant can be considered proportional to k–ADP/k′+ATP (Homsher et al. 2003). Thus, an elevated Ki for the fast isoform may be a result of a faster dissociation rate of MgADP, because k–ADP can be considered a diffusion-limited process and therefore similar for all myosin isoforms (Nyitrai et al. 2006). Higher k–ADP could account for the higher Vf of the fast isoform compared with the slow isoform, which is consistent with a major role of k–ADP in defining shortening velocity (Weiss et al. 2001). The temperature dependence of Ki was different for the two isoforms. The values of Ki increased about threefold for the slow isoform and about 1.15-fold in for the fast isoform between 25 and 35°C. These results seem to be in accordance with the results of Nytrai and colleagues that showed, in the range between 12 and 25°C, that KAD (KAD = k–ADP/k′+ATP) was almost temperature independent in rat fast S1 and increased with increasing temperature in rat slow S1 (Nyitrai et al. 2006; Bloemink et al. 2007; Iorga et al. 2007). On the basis of these results, a significant role of k–ADP in limiting the velocity of fast myosin isoform seems unlikely.
If we assume, however, that MgADP competes with MgATP for the nucleotide site on the myosin molecule in a competitive way (Siemankowski & White, 1984) then we would expect that high [MgATP] could remove the MgADP competition and produce the same Vmax values. Surprisingly, values of Vmaxinh (Vmax in the presence of 2 mM MgADP calculated from the Michaelis–Menten equation) were, at all temperatures and for both isoforms, lower than the corresponding values of Vmax (Table 1); therefore, the reliability of Ki and the suggestion regarding the role of k–ADP in the fast isoform might be questionable.
Cross-bridge kinetics
According to a detachment-limited model of actin sliding velocity, k′+ATP and k–ADP could be calculated [eqn (3)]. The values of k′+ATP were similar for the slow and fast isoforms (Table 2). The values of k′+ATP were similar to those obtained for fast isoforms in IVMA (Baker et al. 2002; Homsher et al. 2003; Jackson & Baker, 2009) and to those obtained for fast and slow isoforms in optical trap experiments (Capitanio et al. 2006). The values were also similar to those obtained from biochemical measurements in solution (Nyitrai et al. 2006). However, while k′+ATP determined in solution increased 2.5-fold between 12 and 25°C for both slow and fast isoforms (Nyitrai et al. 2006), in the present study the sensitivity of k′+ATP to temperature was different for the slow and the fast isoform and varied with the temperature range. In the range 25–35°C, k′+ATP of the slow isoform, but not of the fast isoform, increased with temperature, whereas in the range 20–25°C, k′+ATP of the fast isoform, but not of the slow isoform, increased with temperature (Table 2). The discrepancy could be due to the known variation in temperature sensitivity with the temperature ranges analysed (Ranatunga, 1998; Rossi et al. 2005) and to the different experimental conditions (i.e. ionic strength, 50 versus 100 mM).
Importantly, k′+ATP values were much higher than k–ADP values (Table 2). Therefore, the present results suggest that in the temperature range 20–35°C k′+ATP is unlikely to define Vf of slow and fast isoforms and its temperature sensitivity.
At all temperatures, the values for k–ADP were greater (fourfold) in the fast than in the slow isoform and increased with Vf between 20 and 35°C in both isoforms. The values of k–ADP are higher than those found for fast chicken myosin (291 s−1 at 25°C and 100 mM ionic strength; Hooft et al. 2007; Jackson & Baker, 2009), but similar to those determined by optical trap experiments for fast and slow isoforms in similar experimental conditions (42.8 and 1156 s−1 for the slow and the fast isoform, respectively; Capitanio et al. 2006). The data are also consistent with those obtained in solution (Nyitrai et al. 2006; Bloemink et al. 2007; Iorga et al. 2007) for the slow isoform. The data are not consistent with those obtained for fast isoform, which showed that k–ADP was faster than k′+ATP and temperature independent. At variance with what observed by Baker and co-workers (Baker et al. 2002; Hooft et al. 2007; Jackson & Baker, 2009), in our experimental conditions the values of k–ADP determined at low [MgATP] were not different from those determined at high [MgATP].
Discrepancies with Nyitrai et al. (2006) regarding the role of k–ADP can have several explanations. The different temperature range studied could account for the different temperature sensitivity of k–ADP. Moreover, the lower ionic strength in the present study could contribute to the lower values of k–ADP compared with k′+ATP for fast isoforms. In fact, k–ADP decreases twofold for slow myosin, but does not change for fast myosin with decreasing ionic strength from 200 to 30 mM, whereas over the same ionic strength range k′+ATP increases twofold more for slow than for fast isoform (Nyitrai et al. 2006). Finally, in IVMA an ‘MgADP state’, which could be inaccessible in solution, can be sensed. It has been suggested, in fact, that MgADP binding/release can occur in two steps, because an isomerization step can precede MgADP release (Sleep & Hutton, 1980; Nyitrai & Geeves, 2004; Nyitrai et al. 2006; Albet-Torres et al. 2009; Mansson, 2010). The MgADP state, which precedes isomerization, is inaccessible by simply adding MgADP to actomyosin in rigor, as in solution studies (Nyitrai et al. 2006), whereas it occurs normally in IVMA, because the whole actomyosin cycle takes place.
In conclusion, the data presented here indicate that the rate of MgADP release from the actomyosin complex could define Vf in IVMA and therefore the difference between the fast and the slow myosin isoform. However, this conclusion applies to the conditions of IVMA (20–35°C) and low ionic strength (50 mM). We cannot exclude the possibility of a different relative role of k–ADP and k+ATP with different ionic strengths and at lower temperatures (Nyitrai et al. 2006; Iorga et al. 2007). Future experiments comparing the load dependence of the different isoforms could be useful to confirm and extend the present findings.
References
- Albet-Torres N, Bloemink MJ, Barman T, Candau R, Frölander K, Geeves MA, Golker K, Herrmann C, Lionne C, Piperio C, Schmitz S, Veigel C, Månsson A. Drug effect unveils inter-head cooperativity and strain-dependent ADP release in fast skeletal actomyosin. J Biol Chem. 2009;284:22926–22937. doi: 10.1074/jbc.M109.019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JE, Brosseau C, Joel PB, Warshaw DM. The biochemical kinetics underlying actin movement generated by one and many skeletal muscle myosin molecules. Biophys J. 2002;82:2134–2147. doi: 10.1016/S0006-3495(02)75560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemink MJ, Adamek N, Reggiani C, Geeves MA. Kinetic analysis of the slow skeletal myosin MHC-1 isoform from bovine masseter muscle. J Mol Biol. 2007;373:1184–1197. doi: 10.1016/j.jmb.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottinelli R, Betto R, Schiaffino S, Reggiani C. Unloaded shortening velocity and myosin heavy chain and alkali light chain isoform composition in rat skeletal muscle fibres. J Physiol. 1994;478:341–349. doi: 10.1113/jphysiol.1994.sp020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari M, Rossi R, Pellegrino MA, Bottinelli R, Schiaffino S, Reggiani C. Functional diversity between orthologous myosins with minimal sequence diversity. J Muscle Res Cell Motil. 2000;21:375–382. doi: 10.1023/a:1005640004495. [DOI] [PubMed] [Google Scholar]
- Canepari M, Rossi R, Pellegrino MA, Reggiani C, Bottinelli R. Speeds of actin translocation in vitro by myosins extracted from single rat muscle fibres of different types. Exp Physiol. 1999;84:803–806. [PubMed] [Google Scholar]
- Capitanio M, Canepari M, Cacciafesta P, Lombardi V, Cicchi R, Maffei M, Pavone FS, Bottinelli R. Two independent mechanical events in the interaction cycle of skeletal muscle myosin with actin. Proc Natl Acad Sci U S A. 2006;103:87–92. doi: 10.1073/pnas.0506830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debold EP, Beck SE, Warshaw DM. Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol Cell Physiol. 2008;295:C173–C179. doi: 10.1152/ajpcell.00172.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- Galler S, Wang BG, Kawai M. Elementary steps of the cross-bridge cycle in fast-twitch fiber types from rabbit skeletal muscles. Biophys J. 2005;89:3248–3260. doi: 10.1529/biophysj.104.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg MJ, Mealy TR, Watt JD, Jones M, Szczesna-Cordary D, Moore JR. The molecular effects of skeletal muscle myosin regulatory light chain phosphorylation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R265–R274. doi: 10.1152/ajpregu.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E, Nili M, Chen IY, Tobacman LS. Regulatory proteins alter nucleotide binding to acto-myosin of sliding filaments in motility assays. Biophys J. 2003;85:1046–1052. doi: 10.1016/S0006-3495(03)74543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsher E, Wang F, Sellers JR. Factors affecting movement of F-actin filaments propelled by skeletal muscle heavy meromyosin. Am J Physiol Cell Physiol. 1992;262:C714–C723. doi: 10.1152/ajpcell.1992.262.3.C714. [DOI] [PubMed] [Google Scholar]
- Hooft AM, Maki EJ, Cox KK, Baker JE. An accelerated state of myosin-based actin motility. Biochemistry. 2007;46:3513–3520. doi: 10.1021/bi0614840. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Sliding filaments and molecular motile systems. J Biol Chem. 1990;265:8347–8350. [PubMed] [Google Scholar]
- Iorga B, Adamek N, Geeves MA. The slow skeletal muscle isoform of myosin shows kinetic features common to smooth and non-muscle myosins. J Biol Chem. 2007;282:3559–3570. doi: 10.1074/jbc.M608191200. [DOI] [PubMed] [Google Scholar]
- Jackson DR, Jr, Baker JE. The energetics of allosteric regulation of ADP release from myosin heads. Phys Chem Chem Phys. 2009;11:4808–4814. doi: 10.1039/b900998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Kawaguchi K, Saito M, Ishiwata S. Temperature change does not affect force between single actin filaments and HMM from rabbit muscles. Biophys J. 2000;78:3112–3119. doi: 10.1016/S0006-3495(00)76848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK, Beguin F, Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970;47:69–85. doi: 10.1016/0022-2836(70)90402-x. [DOI] [PubMed] [Google Scholar]
- Lu Z, Moss RL, Walker JW. Tension transients initiated by photogeneration of MgADP in skinned skeletal muscle fibers. J Gen Physiol. 1993;101:867–888. doi: 10.1085/jgp.101.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson A. Actomyosin-ADP states, interhead cooperativity, and the force-velocity relation of skeletal muscle. Biophys J. 2010;98:1237–1246. doi: 10.1016/j.bpj.2009.12.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margossian SS, Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Nyitrai M, Geeves MA. Adenosine diphosphate and strain sensitivity in myosin motors. Philos Trans R Soc Lond B Biol Sci. 2004;359:1867–1877. doi: 10.1098/rstb.2004.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyitrai M, Rossi R, Adamek N, Pellegrino MA, Bottinelli R, Geeves MA. What limits the velocity of fast-skeletal muscle contraction in mammals? J Mol Biol. 2006;355:432–442. doi: 10.1016/j.jmb.2005.10.063. [DOI] [PubMed] [Google Scholar]
- Palmiter KA, Tyska MJ, Dupuis DE, Alpert NR, Warshaw DM. Kinetic differences at the single molecule level account for the functional diversity of rabbit cardiac myosin isoforms. J Physiol. 1999;519:669–678. doi: 10.1111/j.1469-7793.1999.0669n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate E, Lin M, Franks-Skiba K, Cooke R. Contraction of glycerinated rabbit slow-twitch muscle fibers as a function of MgATP concentration. Am J Physiol Cell Physiol. 1992;262:C1039–C1046. doi: 10.1152/ajpcell.1992.262.4.C1039. [DOI] [PubMed] [Google Scholar]
- Pate E, Wilson GJ, Bhimani M, Cooke R. Temperature dependence of the inhibitory effects of orthovanadate on shortening velocity in fast skeletal muscle. Biophys J. 1994;66:1554–1562. doi: 10.1016/S0006-3495(94)80947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino MA, Canepari M, Rossi R, D’Antona G, Reggiani C, Bottinelli R. Orthologous myosin isoforms and scaling of shortening velocity with body size in mouse, rat, rabbit and human muscles. J Physiol. 2003;546:677–689. doi: 10.1113/jphysiol.2002.027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga KW. The force–velocity relation of rat fast- and slow-twitch muscles examined at different temperatures. J Physiol. 1984;351:517–529. doi: 10.1113/jphysiol.1984.sp015260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranatunga KW. Temperature dependence of mechanical power output in mammalian (rat) skeletal muscle. Exp Physiol. 1998;83:371–376. doi: 10.1113/expphysiol.1998.sp004120. [DOI] [PubMed] [Google Scholar]
- Regnier M, Lee DM, Homsher E. ATP analogs and muscle contraction: mechanics and kinetics of nucleoside triphosphate binding and hydrolysis. Biophys J. 1998;74:3044–3058. doi: 10.1016/S0006-3495(98)78012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi R, Maffei M, Bottinelli R, Canepari M. Temperature dependence of speed of actin filaments propelled by slow and fast skeletal myosin isoforms. J Appl Physiol. 2005;99:2239–2245. doi: 10.1152/japplphysiol.00543.2005. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Siemankowski RF, White HD. Kinetics of the interaction between actin, ADP, and cardiac myosin-S1. J Biol Chem. 1984;259:5045–5053. [PubMed] [Google Scholar]
- Siemankowski RF, Wiseman MO, White HD. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985;82:658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleep JA, Hutton RL. Exchange between inorganic phosphate and adenosine 5′-triphosphate in the medium by actomyosin subfragment 1. Biochemistry. 1980;19:1276–1283. doi: 10.1021/bi00548a002. [DOI] [PubMed] [Google Scholar]
- Wang G, Kawai M. Effects of MgATP and MgADP on the cross-bridge kinetics of rabbit soleus slow-twitch muscle fibers. Biophys J. 1996;71:1450–1461. doi: 10.1016/S0006-3495(96)79346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Rossi R, Pellegrino MA, Bottinelli R, Geeves MA. Differing ADP release rates from myosin heavy chain isoforms define the shortening velocity of skeletal muscle fibers. J Biol Chem. 2001;276:45902–45908. doi: 10.1074/jbc.M107434200. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Sata M, Sugiura S, Momomura S, Serizawa T, Iizuka M. ADP inhibits the sliding velocity of fluorescent actin filaments on cardiac and skeletal myosins. Circ Res. 1994;74:1027–1033. doi: 10.1161/01.res.74.6.1027. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Sugiura S, Serizawa T, Sugimoto T, Iizuka M, Katayama E, Shimmen T. Sliding velocity of isolated rabbit cardiac myosin correlates with isozyme distribution. Am J Physiol Heart Circ Physiol. 1992;263:H464–H472. doi: 10.1152/ajpheart.1992.263.2.H464. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Kawai M. Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J. 1994;67:1655–1668. doi: 10.1016/S0006-3495(94)80638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]