Abstract
Two human T-lymphoblastoid cell lines, CCRF/CEM and Molt 4, produced beta interferon (IFN-beta) upon infection with Sendai virus. Molt 4, but not CCRF/CEM, spontaneously produced up to 300 U of IFN-gamma per ml, apparently not contaminated with IFN-alpha or -beta. Phytohemagglutinin, a T-cell mitogen, did not stimulate IFN production in these lines. A third T-lymphoblastoid line, CCRF/HSB2, produced no IFN either spontaneously or after infection with Sendai virus or treatment with phytohemagglutinin. The Molt 4 cells contained an mRNA which could be translated by oocytes to give IFN-gamma. Molt 4 cells therefore provide a convenient source of human IFN-gamma and its mRNA for experimental purposes.
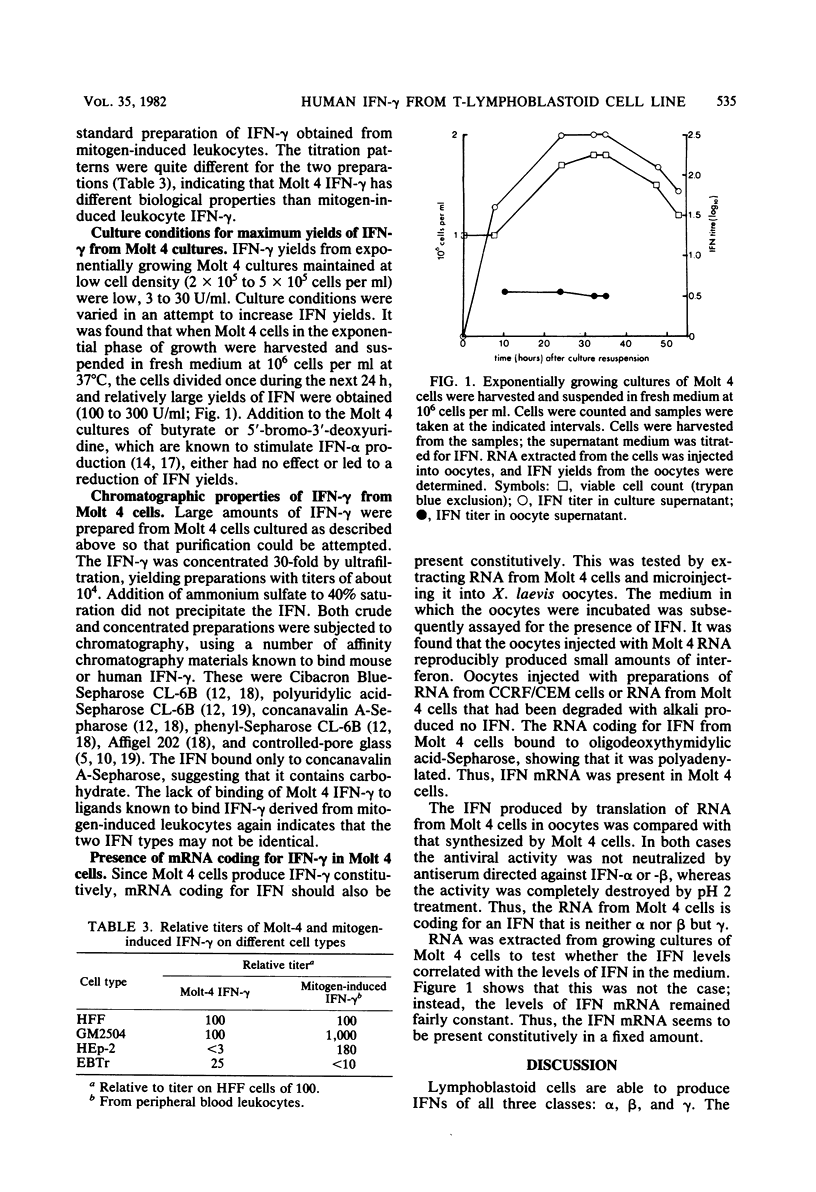
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. J., Johnston M. D., Westmacott L. M., Burke D. C. Department of Biological Sciences, University of Warwick, Coventry, CV47AL, England. J Gen Virol. 1974 Dec;25(3):381–390. doi: 10.1099/0022-1317-25-3-381. [DOI] [PubMed] [Google Scholar]
- Attallah A. M., Fleisher T., Khalil R., Noguchi P. D., Urritia-Shaw A. Proliferative and functional aspects of interferon-treated human normal and neoplastic T and B cells. Br J Cancer. 1980 Sep;42(3):423–429. doi: 10.1038/bjc.1980.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman A., Morser J. Export of proteins from oocytes of Xenopus laevis. Cell. 1979 Jul;17(3):517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Yip Y. K., Vilcek J. Characteristics of human lymphoblastoid (Namalva) interferon. J Gen Virol. 1978 Jan;38(1):51–59. doi: 10.1099/0022-1317-38-1-51. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Meurs E., Aujean O., Vaquero C., Stefanos S., Falcoff E. Antiviral response and induction of specific proteins in cells treated with immune T (type II) interferon analogous to that from viral interferon (type I)-treated cells. Virology. 1980 Jul 15;104(1):195–204. doi: 10.1016/0042-6822(80)90377-3. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Georgiades J. A., Stanton G. J., Dianzani F., Johnson H. M. Large-scale production and physicochemical characterization of human immune interferon. Infect Immun. 1979 Oct;26(1):36–41. doi: 10.1128/iai.26.1.36-41.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson I., Lundgren E., Nilsson K., Strannegard O. A human neoplastic hematopoietic cell line producing a fibroblast type of interferon. Dev Biol Stand. 1979;42:193–197. [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Mizrahi A., O'Malley J. A., Carter W. A., Takatsuki A., Tamura G., Sulkowski E. Glycosylation of interferons. Effects of tunicamycin on human immune interferon. J Biol Chem. 1978 Nov 10;253(21):7612–7615. [PubMed] [Google Scholar]
- Morser J., Flint J., Meager A., Graves H., Baker P. N., Colman A., Burke D. C. Characterization of interferon messenger RNA from human lymphoblastoid cells. J Gen Virol. 1979 Jul;44(1):231–234. doi: 10.1099/0022-1317-44-1-231. [DOI] [PubMed] [Google Scholar]
- Morser J., Meager A., Colman A. Enhancement of interferon mRNA levels in butyric acid-treated Namalwa cells. FEBS Lett. 1980 Apr 7;112(2):203–206. doi: 10.1016/0014-5793(80)80180-3. [DOI] [PubMed] [Google Scholar]
- Ponzio N. M., Fitzgerald K. L., Vilcek J., Thorbecke G. J. Spontaneous production of T (type II) interferon by a murine reticulum-cell sarcoma. Ann N Y Acad Sci. 1980;350:157–167. doi: 10.1111/j.1749-6632.1980.tb20616.x. [DOI] [PubMed] [Google Scholar]
- Tovey M. G., Begon-Lours J., Gresser I., Morris A. G. Marked enhancement of interferon production in 5-bromodeoxyuridine treated human lymphoblastoid cells. Nature. 1977 Jun 2;267(5610):455–457. doi: 10.1038/267455a0. [DOI] [PubMed] [Google Scholar]
- Wietzerbin J., Stefanos S., Lucero M., Falcoff E., O'Malley J. A., Sulkowski E. Physico-chemical characterization and partial purification of mouse immune interferon. J Gen Virol. 1979 Sep;44(3):773–781. doi: 10.1099/0022-1317-44-3-773. [DOI] [PubMed] [Google Scholar]
- Wiranowska-Stewart M., Lin L. S., Braude I. A., Stewart W. E., 2nd Production, partial purification and characterization of human and murine interferons--type II. Mol Immunol. 1980 May;17(5):625–633. doi: 10.1016/0161-5890(80)90160-1. [DOI] [PubMed] [Google Scholar]
- de Ley M., van Damme J., Claeys H., Weening H., Heine J. W., Billiau A., Vermylen C., de Somer P. Interferon induced in human leukocytes by mitogens: production, partial purification and characterization. Eur J Immunol. 1980 Nov;10(11):877–883. doi: 10.1002/eji.1830101113. [DOI] [PubMed] [Google Scholar]


