Abstract
Our previous report demonstrated that RSK2 plays an important role in cell proliferation and transformation induced by tumor promoters such as epidermal growth factor mediated through the N-terminal kinase domain of RSK2 in JB6 Cl41 mouse skin epidermal cells in vitro. However, no direct evidence has been reported regarding the relationship of RSK2 activity and human skin cancer. To elucidate the relationship of RSK2 activity and human skin cancer, we examined the effect of knocking down RSK2 expression on epidermal growth factor-induced anchorage-independent transformation in the premalignant HaCaT human skin keratinocyte cell line and on soft agar colony growth of SK-MEL-28 malignant melanoma cells. We found that the phosphorylated protein levels of RSK2 were enhanced in cancer tissues compared with normal tissues in a human skin cancer tissue array. We found that UVB stimulation induced increased in not only the total and phosphorylated protein levels of ERKs and RSK2 but also the nuclear localization and gene expression of RSK2. RSK2 knockdown inhibited proliferation and anchorage-independent transformation of HaCaT cells and soft agar colony growth of malignant melanoma cells. Moreover, RSK2–/– mouse embryonic fibroblast (MEF) showed enhanced sub-G1 accumulation induced by UVB stimulation compared with RSK2+/+ MEFs, indicating that RSK2 might play an important role in tolerance against stress associated with ultraviolet. Importantly, activated RSK2 protein levels were highly abundant in human skin cancer tissues compared with matched skin normal tissues. Taken together, our results demonstrated that RSK2 plays a key role in neoplastic transformation of human skin cells and in skin cancer growth.
Introduction
The mitogen-activated protein (MAP) kinases are key regulators of cell proliferation and oncogenesis (1,2). The MAP kinase extracellular signal-regulated protein kinases (ERKs) mediate their activation signals through the phosphorylation of 90-kDa ribosomal S6 kinases (p90RSKs) (3,4), which are a family of serine/threonine kinases that respond to many growth factors, peptide hormones and neurotransmitters (5,6). RSK2 is a member of the p90RSK family and is activated by ERKs and phosphoinositide-dependent kinase 1 (PDK1)(3,4), but not p38 kinases (7). When activated, RSK2 is translocated to the nucleus and phosphorylates various nuclear proteins, including c-Fos, Elk-1, histones, cyclic AMP responsive element binding (CREB) protein (8–13), activating transcription factor 4 (ATF4) (14), p53 (15), NFAT3 (16) and ATF1 (17). Therefore, the RSK2 protein is likely to mediate a variety of cellular processes, including proliferation and transformation as well as cell cycle. However, direct evidence linking RSK2 to human skin cancer development has not yet been fully obtained.
The Ras-ERKs-RSK2 pathway regulates cell proliferation, survival, growth and motility (18,19) and tumorigenesis (13). RSK2 is a direct substrate kinase of ERKs and is located between ERKs and its own target transcription factors (7,17). Furthermore, many human solid cancers exhibit an activated Ras mutation (RasG12V; constitutively active Ras) with the highest incidence observed in colon cancer (45%), pancreatic cancer (90%), non-small-cell lung cancer (35%) and melanomas (15%) (20). Furthermore, our previous study demonstrated that the total RSK2 protein level was significantly higher in human skin cancer tissues and cancer cells compared with normal skin tissues and premalignant cell lines (7). We also demonstrated that the N-terminal kinase domain of RSK2 plays an important role in transducing the RSK2 activation signal to its substrates (7). RSK2 requires activation of its C-terminal domain by its upstream kinases, ERKs, in order to phosphorylate and activate its substrates (7,17). For example, a recombinant RSK2 protein purified from Escherichia coli cannot phosphorylate the RSK2 substrate, NFAT3, but phosphorylation of RSK2 by ERKs at the linker region of RSK2 gives RSK2 the ability to phosphorylate its substrates through its N-terminal kinase domain. This suggests that ERKs/RSK2 signaling plays an important role in RSK2-mediated cell proliferation and cell transformation (7,17). Importantly, our study demonstrated that coexpression of RasG12V and RSK2 in NIH3T3 cells increased foci formation compared with RasG12V alone and si-RNA knockdown of RSK2 in RasG12V- or RasG12V/RSK2-coexpressing NIH3T3 cells completely blocked foci formation (13). This indicated that the Ras/ERKs/RSK2 signaling pathway plays a key role in cell transformation. However, the role of RSK2 activation and signaling in human skin cancer has not yet been fully elucidated.
Ultraviolet (UV) light is a well-known environmental carcinogen and is highly associated with skin carcinogenesis (21). Squamous cell carcinomas (SCCs) and basal cell carcinomas (BCCs), account for ~80% and 16% of all skin cancers, respectively. They occur predominantly on UV-exposed areas of the body and have been linked with chronic exposure to UV (22). Studies in various skin cell lines have demonstrated that epidermal growth factor (EGF) receptors (23), MAP kinases (24) and phosphatidylinositol 3-kinase (PI3-K) (25) are specific signaling molecules in ultraviolet B (UVB)-induced skin carcinogenesis. UVB treatment markedly enhances ERKs and p38 kinase signaling (26) and inhibition of ERKs and p38 kinase abrogated UVB-induced c-fos gene transcription and protein expression as well as AP-1 transactivation activity in human keratinocytes (21). Taken together, these results indicated that the Ras-ERKs-RSK2 signaling pathway might play an important role in human skin carcinogenesis. However, direct evidence linking RSK2 activity with human skin cancer development has not yet been reported. In this study, we found that UVB induced increased levels of total and phosphorylated ERKs, RSK2 and c-Fos in HaCaT cells and also caused nuclear accumulation of activated RSK2. Importantly, the activated RSK2 protein level was higher in human skin cancer tissues compared with normal tissues, demonstrating that activated RSK2 plays an important role in human skin cancer development and progression.
Materials and methods
Reagents and antibodies
Chemical reagents, including Tris, NaCl and sodium dodecyl sulfate for molecular biology and buffer preparation were purchased from Sigma–Aldrich (St Louis, MO). Cell culture medium and other supplements were purchased from Life Science Technologies (Rockville, MD). Antibodies for western blot analysis were purchased from Cell Signaling Technology (Beverly, MA), Santa Cruz Biotechnology (Santa Cruz, CA) or Upstate Biotechnology (Charlottesville, VA).
Cell culture and transfection
HaCaT, a premalignant human skin keratinocyte cell line, and SK-MEL-28, a malignant melanoma (MM) cell line, were cultured with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) and antibiotics at 37°C in a 5% CO2 incubator. N/TERT1, a normal skin keratinocyte cell line, and SCC-13, a human skin squamous cell carcinoma cell line, were cultured in keratinocyte–serum-free medium (Life Science Technologies) supplemented with human recombinant epidermal growth factor and bovine pituitary extract as described in the manufacturer’s suggested protocols.
MTS assay
To estimate proliferation, HaCaT, N/TERT1, SCC-13 and SK-MEL-28 cells (1×103 cells/well) stably expressing sh-mock or sh-RSK2 were seeded into 96-well plates. After culturing for 2h, 20 µl of the CellTiter 96® Aqueous One Solution (Promega, Madison, WI) were added to each well and cells were then incubated for 1h at 37°C and 5% CO2. To stop the reaction, 25 µl of a 10% sodium dodecyl sulfate solution were added and absorbance was measured at 492 and 690nm. The MTS assay was conducted at 24h intervals for 96h.
Western blotting
Samples containing equal amounts of protein were resolved by the appropriate percentage sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes. The membranes were incubated in blocking buffer and then probed with specific primary antibodies against appropriate target proteins. The western blots were visualized using an enhanced chemiluminescence detection system (Amersham Biosciences Corp., Piscataway, NJ).
Anchorage-independent soft agar assay
EGF-induced cell transformation was investigated in pKLO-sh-mock- or pKLO-sh-RSK2-infected HaCaT normal human skin keratinocytes. Colony growth of cancer cells was assessed using pKLO.1-sh-GFP scramble (psh-mock) or pKLO.1-sh-RSK2 (psh-RSK2) infected SK-MEL-28 MM cells. In brief, HaCaT cells (8×103) were exposed to EGF (10ng/ml) in 1ml of 0.3% Basal Medium Eagle top agar containing 10% FBS. SK-MEL-28 cells (8×103) were cultured in 1ml of 0.3% Dulbecco’s modified Eagle’s medium top agar containing 10% FBS. The cultures were maintained in a 37°C, 5% CO2 incubator for 14 days and the cell colonies were scored using a microscope and the Image-Pro PLUS (v.6.2) computer software program (Media Cybernetics, Silver Spring, MD) as described by Colburn et al. (27).
Nuclear localization of RSK2
To separate the nuclear and cytosolic fractions of HaCaT cells after UVB stimulation, cells (4×106) were plated into 100mm dishes, cultured, starved overnight, stimulated with UVB (4 kJ/m2) and then harvested at the indicated time points. The cytosolic and nuclear fractions were extracted using NE-PER® Nuclear and Cytoplasmic Extraction Reagent (PIERCE, Rockford, IL) following the manufacturer’s suggested protocol.
Quantitative real-time PCR
HaCaT cells (4×106) were plated into 100mm dishes, cultured, starved overnight, stimulated with UVB (4 kJ/m2) and then harvested at the indicated time points. Total RNAs were extracted using Trizol Reagent (Invitrogen, Carlsbad, CA) and RSK2 gene expression was analyzed with 50ng of total RNA, an RSK2-specific real-time primer set (RPS6KA3 Cat no.: HS00177936_m1) and a glyceraldehyde 3-phosphate dehydrogenase-specific real-time primer set (Hs99999905_m1), by quantitative one-step real-time PCR using the TaqMan RNA-to-CT 1-step kit (Applied Biosystems, Foster City, CA) following the manufacturer’s suggested protocols. The CT values of RSK2 gene expression were normalized with the CT values of glyceraldehyde 3-phosphate dehydrogenase as an internal control to monitor equal RNA utilization.
Immunocytofluorescence assay
HaCaT cells (2×104 cells/well) were seeded in 2-chamber slides and cultured for 24h, starved overnight and then stimulated with UVB (4 kJ/m2). The cells were fixed with 4% formalin at the indicated time point after UVB stimulation. The cells were permeabilized with 0.5% triton X-100 in 1× phosphate-buffered saline (PBS) for 5min at room temperature, blocked with 3% bovine serum albumin in 1× PBS for 1h at 37°C and hybridized with phospho-RSK2 (T577) primary and Alex 488-conjugated secondary antibodies. The cells were mounted with Fluoro-Gel II with 4′,6-diamidino-2-phenylindole (DAPI) (Electron Microscopy Science, Hatfield, PA) and nuclear localization of activated RSK2 was observed under a laser scanning confocal microscope (NIKON C1si Confocal Spectral Image System, NIKON Instruments Co., Melville, NY; ×200). Fluorescence intensity was measured using the Image J computer software program (v.1.45) and normalized with nuclear DAPI intensity as described previously (7).
Tissue array
A human skin tissue array (SK801) was purchased from US Biomax (Rockville, MD) and tissue array analysis was conducted according to the manufacturer’s suggested protocols. The tissue array includes matched normal tissues (H1–H7), which were biopsied from the adjacent tissue of each cancer tissue (A1–A7) from seven individual patients. Briefly, the slide was baked at 60°C for 2h, deparaffinized and rehydrated. Antigens were then unmasked by submerging the slide into boiling sodium citrate buffer (10mM, pH 6.0) for 10min. The slide was blocked with 3% BSA in 1× PBS/0.03% Triton X-100 in a humidified chamber for 1h at room temperature, and then hybridized with a phospho-RSK2 (T577) antibody (1:200 dilution in 1× PBS/0.03% Triton X-100) at 4°C in a humidified chamber overnight. The slide was washed, hybridized with the secondary antibody-conjugated with Alex 488 (1:1000) for 1.5h at room temperature in the dark. Optical sections containing the activated RSK2 protein levels were captured by laser scanning confocal microscopy (NIKON Instruments Co., Melville, NY). Each image was converted and incorporated into a TIF file. The fluorescence intensity from a whole frame of each photo was individually measured using the Image J computer software program (v.1.45) and normalized with nuclear DAPI intensity as described previously (7).
Results
UVB activates ERKs-RSK2 signaling
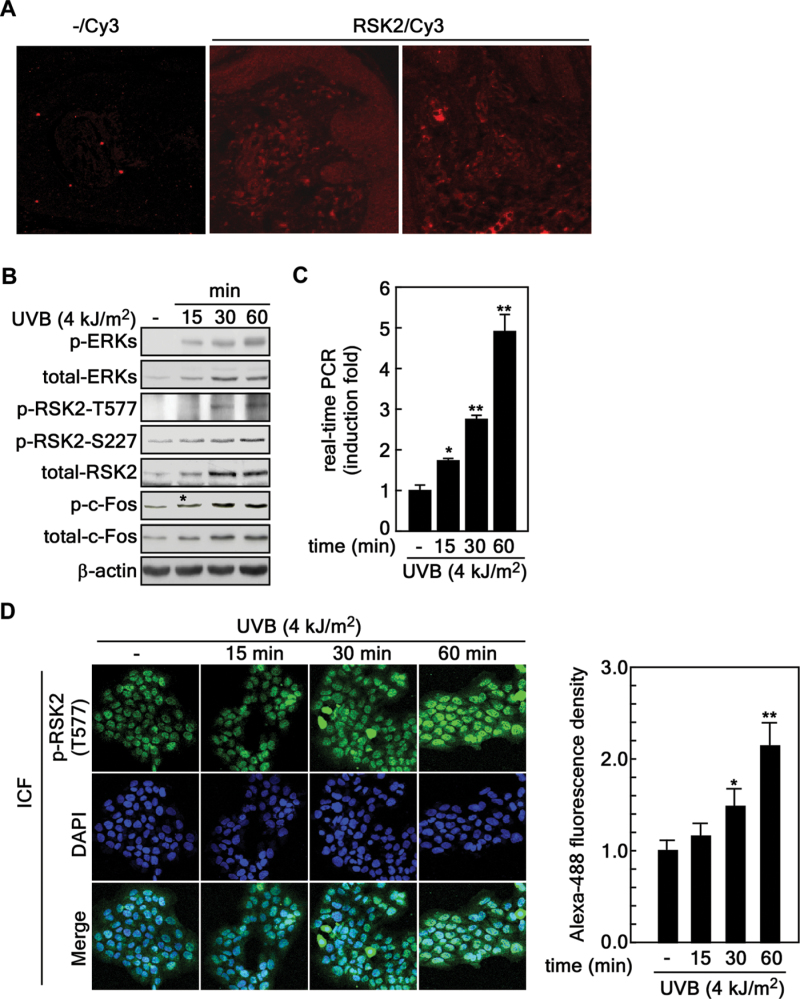
The MAP kinase signaling pathway not only promotes cell proliferation but also mediates cell survival and is upregulated in various cancer cells (20). Our previous study demonstrated that EGF, a tumor promoter, induced cell transformation through Ras-ERKs-RSK2 signaling (13). UV irradiation is the most common environmental carcinogen. It acts as a tumor initiator by inducing mutations in tumor suppressor proteins and functions as a tumor promoter by enhancing cellular signaling through the MAP kinase signaling pathways including ERKs (26). However, ERKs-RSK2 signaling in human skin cancer induced by UVB is not yet fully understood. To determine whether ERKs-RSK2 signaling is activated by UVB, we conducted immunofluorescence assessment of total RSK2 proteins in normal human skin tissues using a specific antibody. We found that RSK2 was expressed in epidermis and dermis of normal human skin tissues (Figure 1A). Notably, we found that phosphorylation of ERKs, RSK2 and c-Fos was increased by exposure to UVB (4 kJ/m2) in HaCaT human skin keratinocytes (Figure 1B). Unexpectedly, the total protein levels of ERKs, RSK2 and c-Fos were also enhanced by UVB stimulation in HaCaT human skin keratinocytes (Figure 1B). We used real-time PCR to confirm that the elevated RSK2 protein level resulted from increased rsk2 gene expression (Figure 1C). Importantly, we found that UVB treatment induced nuclear localization of activated RSK2 [i.e. phosphorylated RSK2 (Thr577)] in a time-dependent manner (Figure 1D). These results demonstrated that ERKs-RSK2 signaling has an important role in UV-induced human skin carcinogenesis.
Fig. 1.
UVB activates ERKs-RSK2 signaling. (A) Immunofluorescence of total RSK2 proteins in normal human skin tissues. Normal human skin tissues were used to visualize total RSK2 proteins using an RSK2-specific primary antibody and an Alexa568-conjugated secondary antibody. Optical sections showing total RSK2 protein levels were captured by laser scanning confocal microscopy as described in Materials and methods. (B) Activation profile of ERKs-RSK2 signaling induced by UVB exposure. HaCaT human skin keratinocytes (4×106) were seeded in 100mm culture dishes, incubated for 24h, starved overnight, and then stimulated with UVB (4 kJ/m2). The cells were harvested and proteins extracted. Activation of ERKs-RSK2 signaling was analyzed by western blotting using specific antibodies as indicated. β-Actin was used as an internal control to monitor equal protein loading. (C) UVB stimulates increased rsk2 mRNA expression. HaCaT human skin keratinocytes (4×106) were seeded, cultured for 24h, starved overnight and stimulated with UVB (4 kJ/m2). Total RNA was extracted at the indicated time point and rsk2 mRNA levels were analyzed by real-time RT–PCR as described in Materials and methods. Data are represented as means ± SD of values obtained from two independent triplicate experiments and significant differences were calculated using the Student’s t-test (*P < 0.05, **P < 0.001). (D) UVB induces nuclear localization of RSK2. HaCaT human skin keratinocytes (2×104) were seeded into 2-chamber slides, cultured for 24h, and then serum-starved overnight. Cells were stimulated with UVB (4 kJ/m2), fixed with 4% formalin for 15min and permeabilized with 0.5% triton X-100 in 1× PBS. Nuclear localization of RSK2 was observed by fluorescence microscope (×200) using a phospho-RSK2 (T577) primary antibody and an Alexa 488-conjugated secondary antibody. The fluorescence intensity was measured by the Image J computer program (v.1.45) and normalized by fluorescence of DAPI intensity. Data are represented as means ± SD of values obtained from 20 randomly selected cells and significant differences were calculated using the Student’s t-test (*P < 0.05, **P < 0.005).
RSK2 enhances tolerance against UVB-induced stress
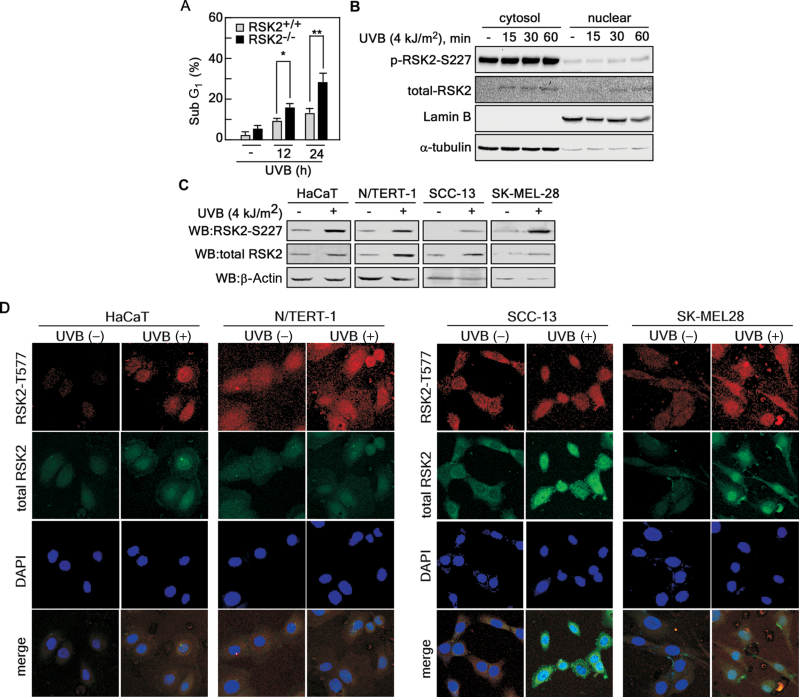
Our previous studies indicated that UV-activated RSK2 phosphorylates proapoptotic Bcl-2-associated death promoter (BAD) at Ser112 resulting in BAD inactivation and increased cell survival (28). At the same time, RSK2 also upregulates the transcription of antiapoptotic Bcl-2associated death promoter through CREB phosphorylation and activation (29). Because apoptosis is presumed to have a protective function against carcinogenesis, these effects suggest that RSK2 plays a role in enhancing tumorigenesis. To examine this hypothesis, we treated cells with UVB (4 kJ/m2) and found that the sub-G1 population was increased in RSK2-deficient (RSK2–/–) MEFs compared with RSK2 wild-type (RSK2+/+) MEFs (Figure 2A). Notably, nuclear localization of activated RSK2 and the total RSK2 protein level was increased by UVB treatment (Figure 2B). Moreover, we found that UVB enhanced the activated and total RSK2 protein levels in various human skin cells, including HaCaT, N/TERT-1, SCC-13 and SK-MEL-28 (Figure 2C). In addition, the activated and total RSK2 proteins were highly abundant in the nucleus following UVB treatment of HaCaT, N/TERT-1, SCC-13 and SK-MEL-28 cells (Figure 2D). These results demonstrated that RSK2 plays an important role for protection of cells from stress such as UV.
Fig. 2.
RSK2 enhances tolerance against UVB-induced stress. (A) RSK2 wild-type (RSK2+/+) and deficient (RSK2–/–) MEFs were treated with UVB (4 kJ/m2) and the sub-G1 population was analyzed by flow cytometry at the indicated time point. Data are represented as means ± SD of values obtained from an experiment with triplicate samples and significant differences were calculated using the Student’s t-test (*P < 0.05, **P < 0.005). (B) HaCaT human skin keratinocytes (4×106) were seeded in a 100mm dish, cultured for 24h, starved, stimulated with UVB (4 kJ/m2), and then harvested at the indicated time point. The cytosolic and nuclear proteins were extracted using NE/PER nuclear and cytoplasmic extraction reagents. Activated and total RSK2 protein levels were visualized using specific antibodies as indicated. Lamin B and α-tubulin were used as nuclear and cytoplasmic marker proteins, respectively. (C) UVB stimulation induces increased RSK2 protein levels in various skin cell lines. HaCaT, N/TERT-1, SCC-13 and SK-MEL-28 cells were seeded, starved, stimulated with UVB (4 kJ/m2) and cultured for 60min in a 37°C/5% CO2 incubator. The proteins were extracted and then activated and total RSK2 protein levels were visualized by western blotting using specific antibodies as indicated. β-Actin was used as an internal control to monitor equal protein loading. (D) HaCaT, N/TERT-1, SCC-13 and SK-MEL-28 cells (2×104) were seeded in 4-chamber slides, starved for 24h, stimulated with UVB and cultured for 60min in a 37°C/5% CO2 incubator. The cells were fixed, permeabilized, triple-stained with Alexa-488 to detect total RSK2, Alexa-568 to detect phosphor-RSK2 (T577) and DAPI for nuclear staining. Activated and total RSK2 protein levels were visualized under a laser scanning confocal microscope using specific antibodies as indicated.
RSK2 knockdown inhibits proliferation of normal human skin cells and skin cancer cells
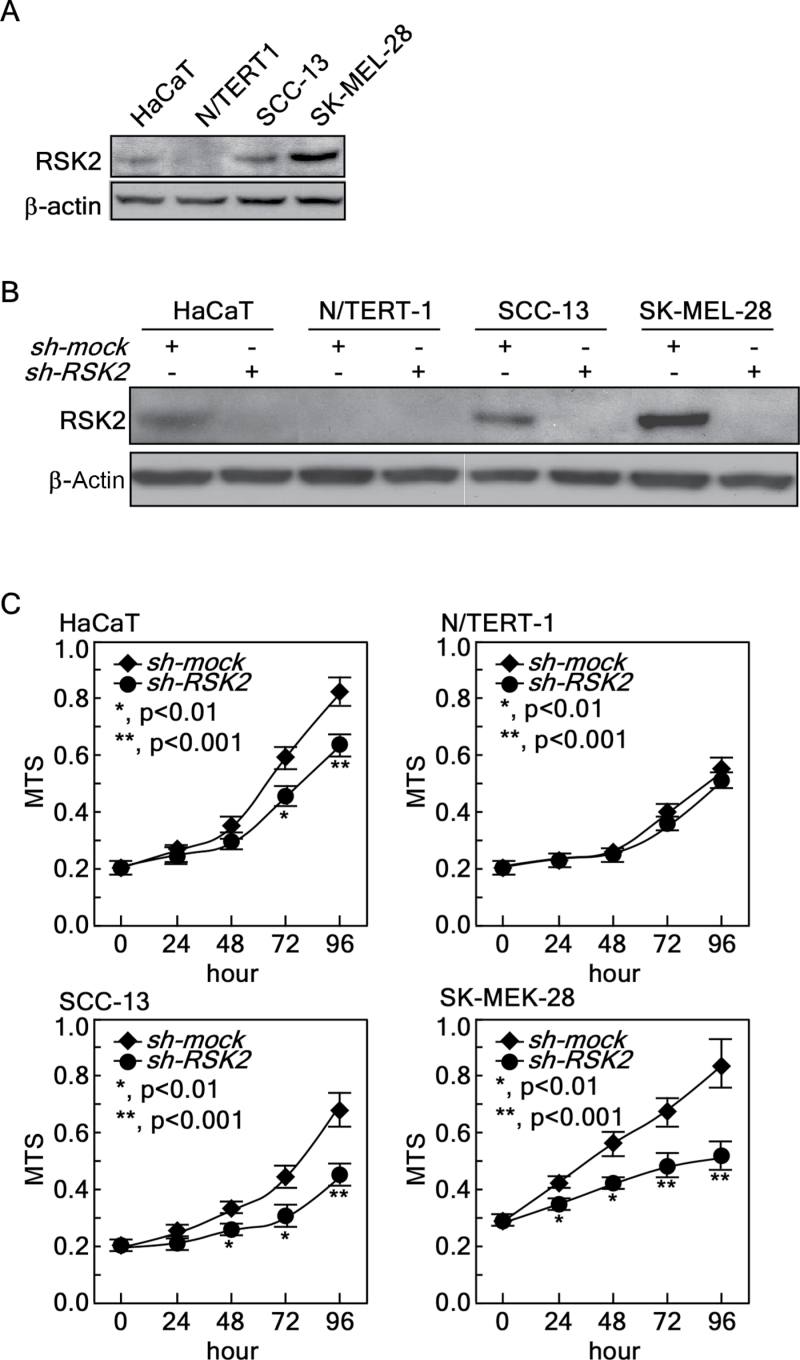
To examine the direct effect of RSK2 on proliferation of human skin cells, we chose HaCaT cells, a premalignant human skin keratinocyte cell line, N/TERT-1 cells, a human skin keratinocyte cell line immortalized by telomerase (30), SCC-13 cells, a human skin epidermal squamous cell carcinoma cell line (31) and SK-MEL-28 MM cells. We first examined the RSK2 protein level in these cells. We found that N/TERT-1 expressed a very low level of RSK2. RSK2 was weakly expressed in HaCaT cells, moderately expressed in SCC-13 cells, but highly expressed in SK-MEL-28 cells (Figure 3A). We infected each cell type with sh-mock or sh-RSK2 for stable expression, and confirmed that sh-RSK2 almost totally blocked RSK2 expression in these cells (Figure 3B). To explore the effect of knocking down RSK2 expression on proliferation, we used the MTS assay and found that RSK2 knockdown inhibited proliferation of HaCaT, SCC-13 and SK-MEL-28 cells (Figure 3C). Interestingly, N/TERT-1 human skin keratinocytes were not significantly affected by RSK2 knockdown (Figure 3C). Importantly, the efficiency of proliferation inhibition induced by RSK2 knockdown corresponded with the endogenous RSK2 protein level (Figure 3C). These results demonstrated that RSK2 has an important role in the proliferation of human skin cells.
Fig. 3.
RSK2 knockdown inhibits normal human skin and cancer cell proliferation. (A) Endogenous RSK2 protein levels in normal human skin HaCaT, N/TERT-1 keratinocytes, human skin SCC-13 and SK-MEL-28 cancer cells. Cells were cultured with complete cell culture media and proteins were extracted. The RSK2 protein levels were analyzed by western blotting using RSK2-specific primary antibody and a horseradish peroxidase-conjugated secondary antibody. β-Actin was used as an internal control to monitor equal protein loading. (B) Endogenous RSK2 expression is silenced by infection with sh-RSK2 in HaCaT, N/TERT-1, SCC-13 and SK-MEL-28 cells. Knockdown efficiency was analyzed by western blotting using an RSK2-specific primary antibody and a horseradish peroxidase-conjugated secondary antibody. β-Actin was used as an internal control to monitor equal protein loading. (C) The cells from (B) were used for a proliferation assay. Cells (1×103) were seeded into 96-well plates and proliferation was measured by MTS assay at 24h intervals up to 96h. Data are represented as means ± SD of values obtained from six experiments and significant differences were calculated using the Student’s t-test (*P < 0.05, ** P < 0.005).
Knockdown of RSK2 suppresses EGF-induced anchorage- independent transformation and colony growth of skin cells
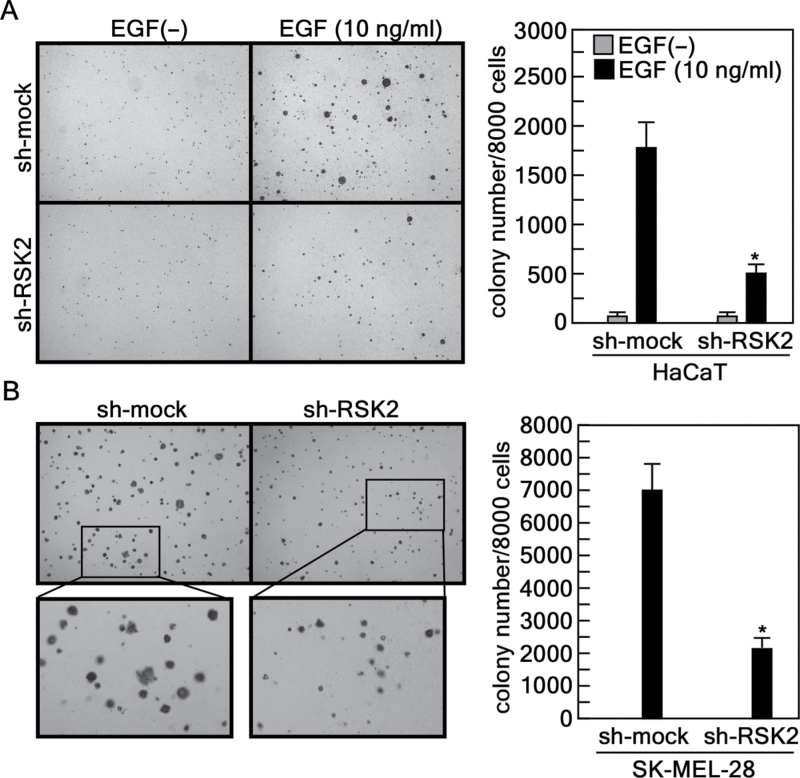
To examine whether RSK2 knockdown affects anchorage-independent cell transformation induced by EGF or cancer cell colony growth under anchorage-independent conditions, we conducted a soft agar assay with HaCaT (32) and SK-MEL-28 cells. We found that HaCaT cells expressing sh-mock formed colonies when stimulated by EGF (Figure 4A). However, colony formation was dramatically reduced by expression of sh-RSK2 (Figure 4A). Furthermore, RSK2 knockdown suppressed growth of SK-MEL-28 MM cells in soft agar compared with sh-mock control cells (Figure 4B). In addition, soft agar assay results indicated that sh-RSK2 inhibited the number of colonies formed as well as colony size (Figure 4). These results demonstrated that RSK2 also has an important role in cell transformation and cancer cell colony growth.
Fig. 4.
Knockdown of RSK2 suppresses EGF-induced anchorage-independent cell transformation and colony growth in soft agar. (A) Suppression of anchorage-independent transformation of HaCaT cells by knocking down endogenous RSK2. Cells stably expressing sh-mock or sh-RSK2 were cultured and subjected to an EGF-induced anchorage-independent cell transformation assay. Cells (8×103) were exposed to EGF (10ng/ml) in 1ml of 0.3% Basal Medium Eagle agar containing 10% FBS. The cultures were maintained in a 37°C, 5% CO2 incubator for 14 days and then colonies were counted using a microscope and the Image-Pro PLUS (v.6.2) computer software program. Data are represented as means ± SD of values from triplicate experiments and statistical significance was determined using the Student’s t-test (*P < 0.001). (B) Colony growth in soft agar is inhibited by knocking down endogenous RSK2. SK-MEL-28 cells stably expressing sh-mock or sh-RSK2 were tested for colony growth under anchorage-independent conditions. Cells (8×103) were mixed with 0.3% top agar in complete cell culture medium. The cultures were maintained in a 37°C, 5% CO2 incubator for 10 days and then colonies were counted using a microscope and the Image-Pro PLUS (v.6.2) computer software program. Data are represented as means ± SD of triplicate values and statistical significance was determined using the Student’s t-test (*P < 0.001).
Tissue array analysis of RSK2 in solid human skin cancer and normal skin tissues
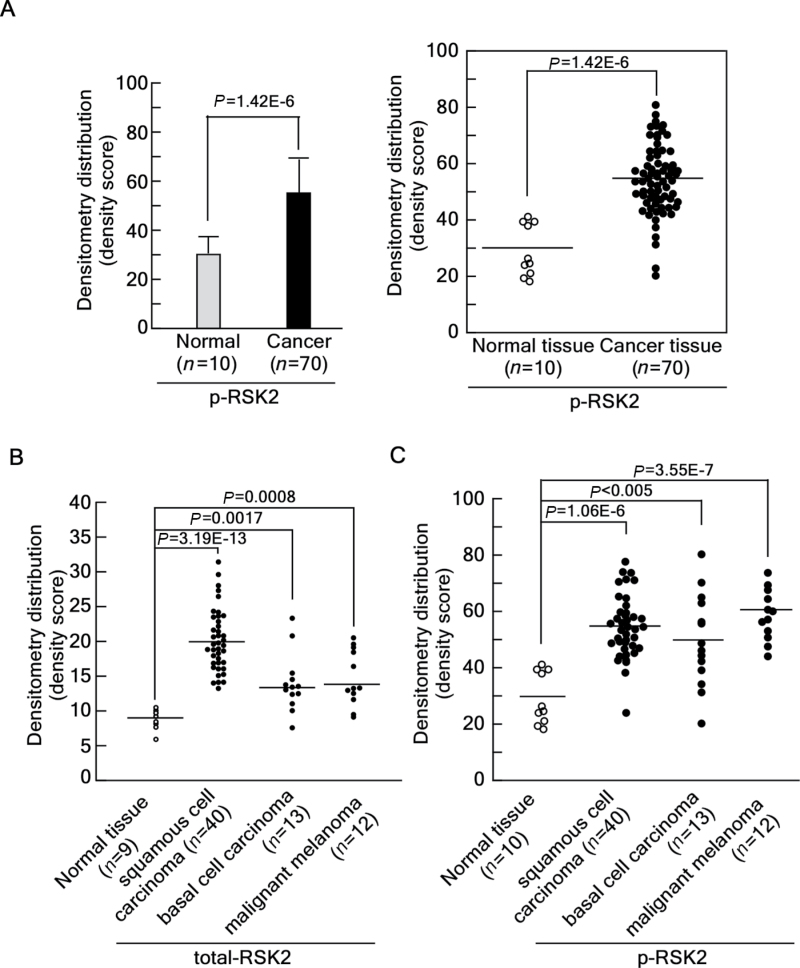
To examine the physiological relevance of RSK2 in human skin cancer, we analyzed a human skin cancer tissue array using 80 human skin core tissues, which included 70 human skin cancer tissues and 10 normal skin tissues. Our previous study demonstrated that human skin cancer tissues contain higher RSK2 protein levels compared with normal skin tissues (7). Using the same tissue array, we examined the expression of activated RSK2 using a phospho-RSK2 (T577)-specific antibody and an Alexa 488-conjugated secondary antibody. We assessed the level of activated RSK2 in tissue array specimens by measuring the fluorescence light intensity. We found that activated RSK2 was highly abundant in human skin cancer tissues at about 2-fold above normal tissue expression (Figure 5A, left panel). The light intensity distribution clearly showed that activated RSK2 protein levels are enhanced in human skin tissues compared with normal skin tissues (Figure 5A, right panel). To examine RSK2 (Thr577) more fully in different kinds of skin cancers, the human skin cancer array was divided into SCC (n = 40), BCC (n = 13) and MM (n = 12). We re-analyzed the total RSK2 protein levels from our previous tissue array results (7) and the activated RSK2 protein level. We found that total and activated RSK2 protein levels were most highly abundant in SCC (Figure 5B and 5C). Moreover, although the total protein levels were moderately enhanced in BCC and MM (Figure 5B), the activated RSK2 protein levels were dramatically increased in both BCC and MM (Figure 5C). The average total RSK2 levels were about 200% in SCC and 140% in BCC and MM compared with levels in normal skin tissues (Supplementary Figure 1A, available at Carcinogenesis Online). Furthermore, the average protein level of activated RSK2 was about 190% in SCC, 176% in BCC, and 206% in MM compared with the activated RSK2 protein level observed in normal skin tissues (Supplementary Figure 1B, available at Carcinogenesis Online). These results demonstrated that total and activated RSK2 protein levels correspond with human skin cancer development.
Fig. 5.
Tissue array analysis of RSK2 in solid human skin cancer and normal skin tissues. (A) Activated RSK2 protein levels are higher in cancer tissues compared with normal tissues. An analysis of activated RSK2 was performed using a human skin tissue array that included matched normal and cancer tissues. Phosphorylated RSK2 was detected using an antibody against phospho-RSK2 (T577) and an Alexa 488-conjugated secondary antibody as described in Materials and methods. The light intensity of each sample was estimated using the Image J computer program (v.1.45). The overall average density of all samples is shown (left panel) along with the density of each individual sample (right panel). The fluorescence intensity of the individual sample was obtained using the Image J computer software program (v.1.45). (B–C) The 70 core samples of skin cancer tissues were classified into subclasses of skin cancer categories, including SCC, BCC and MM. The fluorescence light intensity of the activated RSK2 protein and total RSK2 protein levels were re-evaluated. The average density of each subclass is shown in Supplementary Figure 1A (total RSK2) and 1B [phospho-RSK2 [T577]), available at Carcinogenesis Online along with the density of each individual sample for total RSK2 (B) and activated RSK2 (C). Significant differences in A–C were evaluated using the Student’s t-test as described previously (7).
Activated RSK2 protein abundance is higher in cancer tissues compared with matched normal tissues
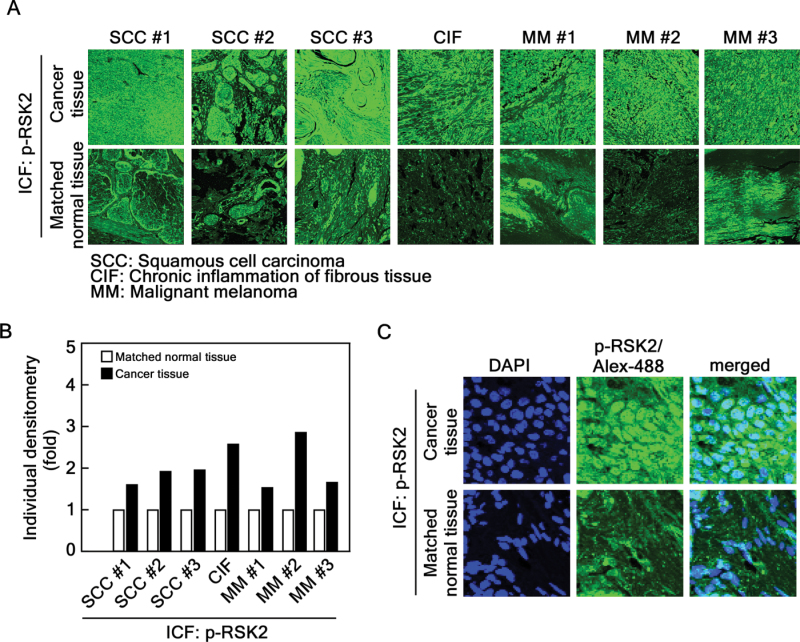
The tissue array panel includes seven matched normal tissues and cancer tissues biopsied from the same patient. Using these tissue samples, we compared the fluorescence intensity (Figure 6A) and found that three SCCs, one chronic inflammation of fibrous tissue and three MM tissues contained 2–3-fold higher activated RSK2 protein levels (Figure 6A and B). The activated RSK2 localization into the nucleus was observed in skin cancer tissue compared with normal skin tissue (Figure 6C). These results corresponded well with the total protein level analyzed from our previous publication (7). These results provide strong evidence showing that RSK2 is an important kinase in human skin cancer development.
Fig. 6.
The activated RSK2 protein level is higher in cancer tissues compared with matched normal tissues. (A) The levels of activated RSK2 proteins were compared with matched human normal and cancer tissues, including SCC, chronic inflammation of fibrous tissue and MM. Representative samples are shown. (B) The relative light intensity of individual activated RSK2 in cancer tissues compared with matched each normal tissue sample is shown. (C) Elevation of the activated RSK2 protein levels in skin cancer tissue. A matched human skin cancer tissue and normal tissue in (A) were magnified to confirm whether activated RSK2 protein levels were accumulated in nucleus skin cancer tissue. The nucleus was stained with DAPI, phosphorylated RSK2 (T577) was stained with Alexa-488 and nuclear localization of RSK2 was confirmed by merging of DAPI and Alexa-488 as indicated.
Discussion
Our previous studies demonstrated that RSK2, as a downstream kinase of the Ras-ERKs signaling pathway, plays an important role in proliferation and transformation of mouse skin epidermal JB6 Cl41 cells (15). EGF or 12-O-tetradecanoylphorbol 13-acetate stimulation enhances phosphorylation of ERKs and RSKs and the G1/S cell cycle transition in JB6 Cl41 cells (15). Our recent study demonstrated that EGF stimulation induces an interaction between RSK2 and caspase 8, and phosphorylation of caspase 8 at Thr263 results in ubiquitination-mediated caspase 8 degradation (33). The degradation of caspase 8 inhibits apoptosis induced by Fas in HeLa cells (33). Therefore, RSK2 mediates cell survival rather than cell death. These observations correspond well with our finding that RSK2 plays an important role in proliferation and cell transformation induced by tumor promoters such as EGF or 12-O-tetradecanoylphorbol 13-acetate in JB6 Cl41 mouse skin epidermal cells (13).
Increased epidermal growth factor receptor activation is observed within a short time after UV exposure of cultured keratinocytes or skin (34,35), resulting in activation of the ERKs signaling pathway (34). Our results indicated that UVB irradiation induces phosphorylation of ERKs and RSK2 at Thr577, which is a site phosphorylated by ERKs (Figure 1B), and also causes RSK2 nuclear localization (Figures 1D, 2B and D), resulting in phosphorylation of c-Fos (Figure 1B). The phosphorylation of c-Fos (Ser362) by RSK2 plays an important role in osteosarcoma development (36) through its effects on cell cycle progression (37). Our previous results demonstrated that ectopic expression of RSK2 enhances cell proliferation; and knockout of RSK2 attenuates proliferation and cell cycle progression in JB6 Cl41 mouse skin epidermal cells (13). Based on these results, we found that RSK2 knockdown suppressed proliferation of HaCaT, SCC-13 and SK-MEL-28 cells (Figure 3C). Similar findings in A431 human skin epidermoid carcinoma, SK-MEL-5 and SK-MEL-28 MM and HCT-116 human colon cancer cells were obtained by treatment with the chemical inhibitor kaempferol, which is reported to suppress RSK2 N-terminal kinase activity (7). RSK2 knockdown suppressed RasG12V-induced foci formation of JB6 Cl41 cells (13). We confirmed that knockdown of RSK2 attenuated anchorage-independent transformation of HaCaT cells induced by EGF (Figure 5A) and soft agar colony growth of SK-MEL-28 MM cells (Figure 5B). Therefore, our results demonstrated that RSK2 plays a key role in human skin cancer.
Our previous results demonstrated that human skin cancer tissues contain higher total RSK2 protein levels compared with normal skin tissues (7). Using the same tissue array, we found that phospho-RSK2 protein levels were about 2-fold higher in skin cancer tissues compared with normal skin tissues (Figure 5A). Furthermore, we re-evaluated our previous and current tissue array results by dividing the samples into subclasses of skin cancer, SCC, BCC and MM (Figure 5B). We found that total and activated RSK2 levels were most increased in SCC compared with normal skin tissues (Figure 5B and C; Supplementary Figure 1, available at Carcinogenesis Online). Although the total protein levels in BCC and MM tissues were moderately enhanced (about 1.5-fold), the activated RSK2 protein levels were increased by about 2-fold in skin cancer tissues (Figure 5B and 5C; Supplementary Figure 1, available at Carcinogenesis Online) compared with normal tissues. Furthermore, additional results demonstrated that the activated RSK2 protein level was higher in cancer tissues compared with normal adjacent skin tissues in the same patient (Figure 6). Taken together, our results provide strong evidence of an association of total and activated RSK2 protein levels and human skin cancer development. Moreover, our results further suggest that identifying inhibitors against RSK2 activity might be an effective means to treat skin cancer in humans.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
The Hormel Foundation and National Institutes of Health (CA120388, R37CA081064, CA027502, ES016548); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2000961); the Research Fund (M-2012-B0002-00028 of The Catholic University of Korea).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- BCC
basal cell carcinoma
- DAPI
4′,6-diamidino-2-phenylindole
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated protein kinase
- FBS
fetal bovine serum
- MAP
mitogen-activated protein
- MM
malignant melanoma
- PBS
phosphate-buffered saline
- RSK2
ribosomal S6 kinase 2
- SCC
squamous cell carcinoma
- UV
ultraviolet;
- UVB
ultraviolet B
References
- 1. Sebolt-Leopold J.S. (2000). Development of anticancer drugs targeting the MAP kinase pathway. Oncogene 19 6594–6599 [DOI] [PubMed] [Google Scholar]
- 2. Roux P.P., et al. (2004). ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68 320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones S.W., et al. (1988). A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc. Natl. Acad. Sci. U.S.A. 85 3377–3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frödin M., et al. (2000). A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 19 2924–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frödin M., et al. (1999). Role and regulation of 90kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 151 65–77 [DOI] [PubMed] [Google Scholar]
- 6. Nebreda A.R., et al. (1999). Perspectives: signal transduction. Cell survival demands some Rsk. Science 286 1309–1310 [DOI] [PubMed] [Google Scholar]
- 7. Cho Y.Y., et al. (2009). A regulatory mechanism for RSK2 NH(2)-terminal kinase activity. Cancer Res. 69 4398–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xing J., et al. (1996). Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science 273 959–963 [DOI] [PubMed] [Google Scholar]
- 9. Sassone-Corsi P., et al. (1999). Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science 285 886–891 [DOI] [PubMed] [Google Scholar]
- 10. Blenis J. (1993). Signal transduction via the MAP kinases: proceed at your own RSK. Proc. Natl. Acad. Sci. U.S.A. 90 5889–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis R.J. (1995). Transcriptional regulation by MAP kinases. Mol. Reprod. Dev. 42 459–467 [DOI] [PubMed] [Google Scholar]
- 12. Ward G.E., et al. (1990). Identification of cell cycle-regulated phosphorylation sites on nuclear lamin C. Cell 61 561–577 [DOI] [PubMed] [Google Scholar]
- 13. Cho Y.Y., et al. (2007). Ribosomal S6 kinase 2 is a key regulator in tumor promoter induced cell transformation. Cancer Res. 67 8104–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang X., et al. (2004). ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology: implication for Coffin-Lowry Syndrome. Cell 117 387–398 [DOI] [PubMed] [Google Scholar]
- 15. Cho Y.Y., et al. (2005). The p53 protein is a novel substrate of ribosomal S6 kinase 2 and a critical intermediary for ribosomal S6 kinase 2 and histone H3 interaction. Cancer Res. 65 3596–3603 [DOI] [PubMed] [Google Scholar]
- 16. Cho Y.Y., et al. (2007). RSK2 mediates muscle cell differentiation through regulation of NFAT3. J. Biol. Chem. 282 8380–8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu K., et al. (2011). Eriodictyol inhibits RSK2-ATF1 signaling and suppresses EGF-induced neoplastic cell transformation. J. Biol. Chem. 286 2057–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pearson G., et al. (2001). ERK5 and ERK2 cooperate to regulate NF-kappaB and cell transformation. J. Biol. Chem. 276 7927–7931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schaeffer H.J., et al. (1999). Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19 2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sebolt-Leopold J.S., et al. (2004). Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer 4 937–947 [DOI] [PubMed] [Google Scholar]
- 21. Bowden G.T. (2004). Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer 4 23–35 [DOI] [PubMed] [Google Scholar]
- 22. Kwa R.E., et al. (1992). Biology of cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 26 1–26 [DOI] [PubMed] [Google Scholar]
- 23. Wan Y.S., et al. (2001). Ultraviolet irradiation activates PI 3-kinase/AKT survival pathway via EGF receptors in human skin in vivo. Int. J. Oncol. 18 461–466 [DOI] [PubMed] [Google Scholar]
- 24. Chen W., et al. (2001). Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene 20 3921–3926 [DOI] [PubMed] [Google Scholar]
- 25. Kabuyama Y., et al. (1998). Wavelength specific activation of PI 3-kinase by UVB irradiation. FEBS Lett. 441 297–301 [DOI] [PubMed] [Google Scholar]
- 26.Bode A.M., et al. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci. STKE. (2003);2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 27. Colburn N.H., et al. (1981). Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc. Natl. Acad. Sci. U.S.A. 78 6912–6916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. She Q.B., et al. (2002). Activation of JNK1, RSK2, and MSK1 is involved in serine 112 phosphorylation of Bad by ultraviolet B radiation. J. Biol. Chem. 277 24039–24048 [DOI] [PubMed] [Google Scholar]
- 29. Bonni A., et al. (1999). Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286 1358–1362 [DOI] [PubMed] [Google Scholar]
- 30. Dickson M.A., et al. (2000). Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20 1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubin A.L., et al. (1989). Coordination of keratinocyte programming in human SCC-13 squamous carcinoma and normal epidermal cells. J. Cell. Physiol. 138 208–214 [DOI] [PubMed] [Google Scholar]
- 32. Mizuno H., et al. (2006). Effects of MAP kinase inhibitors on epidermal growth factor-induced neoplastic transformation of human keratinocytes. Mol. Carcinog. 45 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peng C., et al. (2011). Phosphorylation of caspase-8 (Thr-263) by ribosomal S6 kinase 2 (RSK2) mediates caspase-8 ubiquitination and stability. J. Biol. Chem. 286 6946–6954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El-Abaseri T.B., et al. (2005). Chemoprevention of UV light-induced skin tumorigenesis by inhibition of the epidermal growth factor receptor. Cancer Res. 65 3958–3965 [DOI] [PubMed] [Google Scholar]
- 35. El-Abaseri T.B., et al. (2006). Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis 27 225–231 [DOI] [PubMed] [Google Scholar]
- 36. David J.P., et al. (2005). Essential role of RSK2 in c-Fos-dependent osteosarcoma development. J. Clin. Invest. 115 664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sunters A., et al. (1998). Control of cell cycle gene expression in bone development and during c-Fos-induced osteosarcoma formation. Dev. Genet. 22 386–397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.