Abstract
Denial of the appropriate cell-matrix interaction in epithelial cells induces apoptosis and is called ‘anoikis’. Cancer cells are resistant to anoikis and it is believed that the resistance to anoikis helps promote tumor malignancy especially metastasis. We and others have demonstrated that the expression of tight junction protein claudin-1 is highly upregulated in colorectal cancer (CRC) and helps promote tumor progression and metastasis. However, molecular mechanism/s underlying claudin-1-dependent regulation of CRC progression remains poorly understood. In current study, we have determined that claudin-1 expression modulates anoikis in colon cancer cells to influence colon cancer invasion and thus metastasis. We have further provided data that claudin-1 modulates anoikis in a Src-Akt-Bcl-2-dependent manner. Importantly, claudin-1 physically associates with Src/p-Src in a multiprotein complex that also includes ZO-1, a PDZ-binding tight junction protein. Taken together, our data support the role of claudin-1 in the regulation of CRC progression and suggest that the regulation of anoikis may serve as a key regulatory mechanism in claudin-1-dependent regulation of CRC progression. Our findings are of direct clinical relevance and may open new therapeutic opportunity in colon cancer treatment and/or management.
Introduction
Epithelial cells exhibit an adhesion requirement for survival and undergo ‘anoikis’ when denied appropriate adherence. In contrast, a significant fraction of carcinoma cells remains viable even when they are deprived of normal contacts with the basement membrane. This capability enables intravascular transit of cancer cells and seeding at remote metastatic sites. Outcome from a series of studies indicate that resistance to anoikis or anchorage-independent survival is a hallmark of the tumorigenic ability of cancer cells and a critical prerequisite for the carcinoma progression (1). Importantly, treatments reversing the anoikis resistance of cancer cells suppress their ability to form primary tumors and to metastasize (2,3). In contrast, spontaneous acquisition of anoikis resistance is sufficient for non-malignant epithelial cells to acquire in vivo tumorigenicity (4). However, molecular mechanism/s that enable resistance to anoikis in colon cancer cells are not clearly understood. Furthermore, understanding the mechanism/s that may trigger anoikis in tumor cells is of potential interest in designing antitumor therapies.
We have previously reported that in human colon cancer samples and cell lines, expression of claudin-1, a tight junction protein, is highly increased and positively correlates with the tumor growth and disease progression (5). Other groups have made similar observations (6,7). Importantly, in our further studies, increasing the expression of claudin-1 in colon cancer cells induced resistance to anoikis and was associated with increased metastasis in a mouse xenograft model. In contrast, suppression of claudin-1 expression in colon cancer cells increased anoikis, whereas decreased metastasis in vivo (5).
The Src family kinase, Src is highly expressed and frequently mutated in colorectal cancer (8). In the normal functioning of the epithelial cells, Src is recruited to the sites of cell-extra-cellular matrix (ECM) adhesions and plays important role in mediating the cellular responses to cell-extra-cellular matrix adhesion (9,10). Notably, deprivation of the appropriate cell-extra-cellular matrix adhesion induces anoikis and multiple lines of evidence connect Src activation with the protection against anoikis (11). In this regard, simple overexpression of activated Src is sufficient to confer anoikis resistance in a variety of epithelial cells (12,13). A similar protective role of Akt phosphorylation and B-cell lymphoma-2 (Bcl-2) family of proteins in cell survival under stress conditions including anoikis is documented (14,15).
In the current study, we have confirmed that claudin-1 expression confers resistance to anoikis in colon cancer cells. We have further demonstrated that claudin-1-associated resistance to anoikis is dependent upon Src activation, which in turn modulates Akt phosphorylation and Bcl-2 expression. Furthermore, claudin-1 physically binds with Src/p-Src in a multiprotein complex that includes ZO-1, and loss of the association between claudin-1 and Src/p-Src decreases the resistance to anoikis. Taken together, our data uncovers a novel partnering between claudin-1 and Src in the regulation of colon cancer malignancy.
Materials and methods
Plasmids and reagents
Antibodies against claudin-1 and claudin-4 were purchased from Invitrogen Corp. (San Francisco, CA, USA). The anti-Bcl-2, anti-β-actin and anti-P-extracellular signal-regulated kinase (ERK) antibodies were from BD Biosciences (San Jose, CA, USA), Sigma (St. Louis, MO) and Santa Cruz biotechnology Inc. (Santa Cruz, CA, USA), respectively. The anti-cleaved caspase-3 (Asp175), anti-Src, anti-p-Src (Tyr416), anti-FAK, anti-p-FAK(Tyr576), anti-Paxillin, anti-p-Paxillin (Tyr 118), anti-Akt, p-Akt (S473), anti-ERK and anti-Bcl-xl antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). PP2 (529573) was purchased from Calbiochem Inc., USA) and the Src529Y construct was kindly provided by Dr Steve Hanks (Vanderbilt University Medical Center).
Cell culture and transfection
The generation and culture conditions for the SW480control, SW480claudin-1, SW620control and SW620siRNA cells, used in current studies, have been described previously (5). One day before transfection, cells were seeded in six-well cell-culture plates to provide a final density of 40–60% confluence (~3×105 cells/well) and were transfected using Effectene transfection reagent (Qiagen Inc.). Fold stimulation was calculated for each sample by dividing the normalized luciferase activity by the value obtained from the control transfection containing empty parental expression vectors (Plasmid cytomegalo Virus (pCMV)).
Anoikis
Anoikis was induced by plating cells on poly-HEMA-coated culture plates and apoptosis was determined using the Cell Death Detection ELISAPLUS kit (Roche Diagnostics Corp.) as described previously (5).
Immunoprecipitation
Cells were cotransfected with Green Fluorecent Protein (GFP), GFP-claudin-1, ΔPDZ-GFP-claudin-1 or ΔC-ter-GFP-claudin-1 constructs (Figure 5B). Forty-eight hours post-transfection, cells were harvested using lysis buffer containing 10mM Tris-HCl, pH 7.4, 10% glycerol, 150mM NaCl, 1mM ethylenediaminetetraacetic acid , 1mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid pH 8.0, 1% Nonidet P-40 and protease inhibitor mixture. Cleared cell lysates were incubated with 5 µg anti-GFP antibody bound to (50 µl) Dynabeads protein G (DYNAL) or mouse IgG (used as negative control). The beads were washed extensively with cold phosphate-buffered saline, and bound proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by immunoblot using anti-claudin-1, anti-p-Src, anti-Src, anti-ZO-1 or anti-GFP antibody.
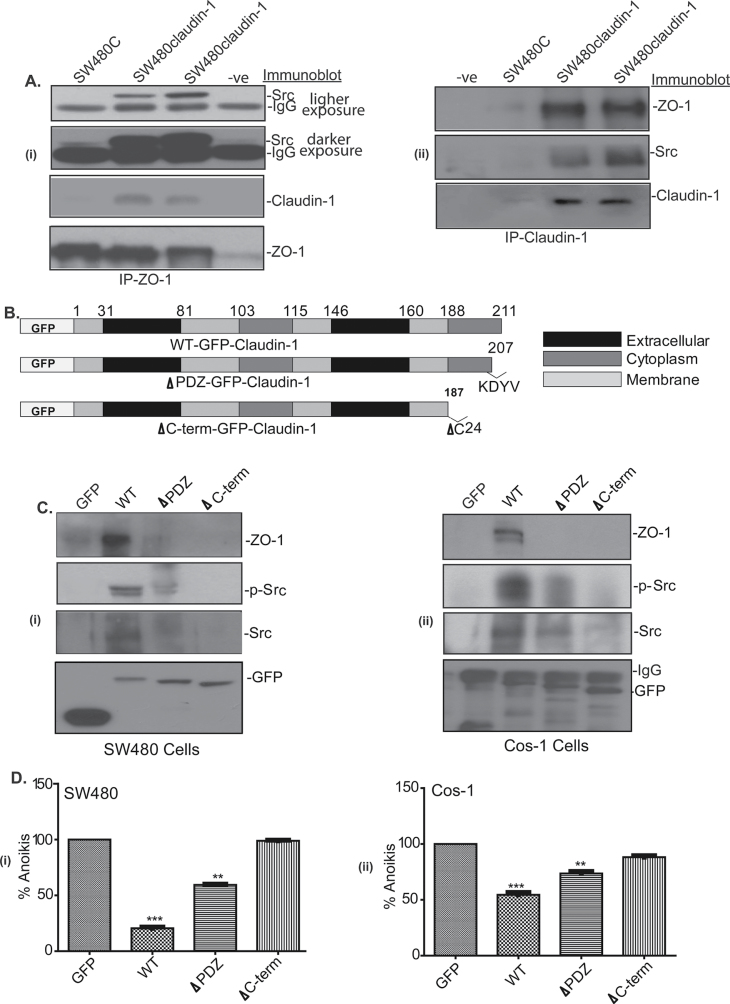
Fig. 5.
The C-terminal of claudin-1 is required for the association with Src and ZO-1. (A) Total cell lysate from SW480control (SW480C) and SW480claudin-1 cells was immunoprecipitated with (i) anti-ZO-1 antibody followed by immunoblotting with anti-ZO-1, anti-Src or anti-claudin-1 antibody and (ii) anti-claudin-1 antibody followed by immunoblotting with anti-ZO-1, anti-Src or anti-claudin-1 antibody. (B) Schematic representation of wild-type GFP-claudin-1, ΔPDZ-GFP-Claudin-1 (deletion of ‘KDYV’ PDZ-binding domain) and ΔC-ter-GFP-Claudin-1 (deletion of ‘ΔC24’ membrane domain). (C) SW480 cells (i) and COS-1 cells (ii) were transiently transfected with the respective GFP-claudin-1 mutant construct. Total cell lysates from transfected cells was subjected to immunoprecipitation using anti-GFP antibody followed by immunoblotting with Src, p-Src or ZO-1. (D) Anoikis in SW480 cells (i) and COS-1 cells (ii). Cells were transiently transfected with GFP-claudin-1 mutant constructs and Src. Values are mean ± standard deviation. Data representative of three or more experiments.
Immunoblot and immunofluorescence analyses
These analyses were performed as described previously (5).
Statistical analysis
PRISM software was used for statistical analysis. Student’s t-test, χ 2-test and analysis of variance were used to determine statistical significance as applicable, and differences were considered statistically significant at P < 0.05.
Results
Claudin-1 expression confers resistance to anoikis to colon cancer cells
To determine the effect of claudin-1 expression upon anoikis, we used SW480control (low endogenous claudin-1 expression), SW480claudin-1 (stably overexpressing claudin-1), SW620control (robust endogenous claudin-1 expression) and SW620siRNA (claudin-1 expression is stably silenced) cells. Notably, SW480control and SW620siRNA cells do not metastasize, whereas SW620control and SW480claudin-1 cells are highly metastatic in vivo when injected in athymic mice (5). To induce anoikis, cells were cultured (×1 106/ml) upon poly-HEMA-coated culture dishes as described in section ‘Materials and methods’. Samples were collected at 0, 3, 8 and 24h postplating where 0h represents the time immediately after plating the cells on poly-HEMA-dishes.
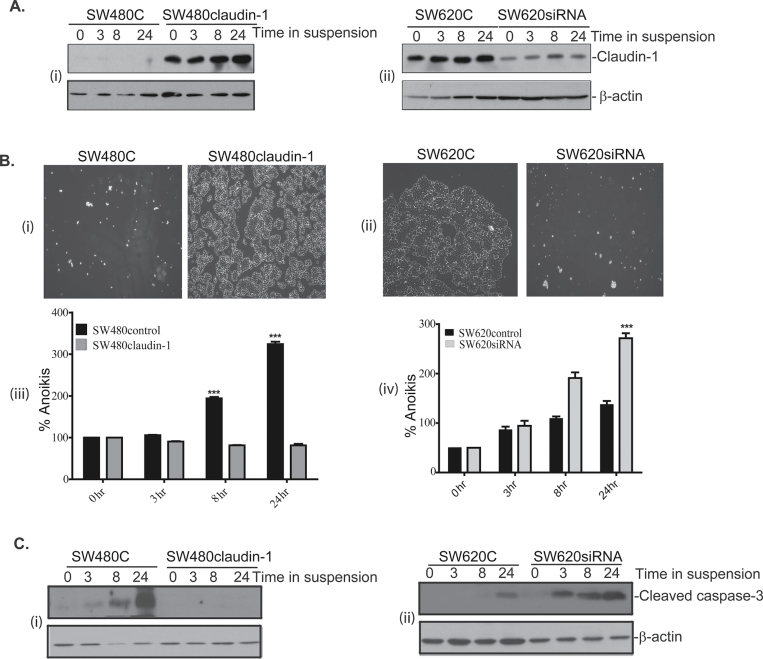
Immunoblot analysis using total cell lysates confirmed the expected claudin-1 expression levels in the cell lines under study during the culture on poly-HEMA-coated dishes (Figure 1A, i–ii). To determine the resistance to anoikis, we first assessed cell survival following culture in poly-HEMA-coated dishes. Cells were gently collected 24h after culture on poly-HEMA and were then replated upon regular culture dishes for 6h. The 6-h time-period (for the replating study) was chosen based on our determination that it is enough for SW480 or SW620 cells to adhere but not to multiply (unpublished data). Only healthy/surviving cells were expected to adhere. About 6h postreplating, attached cells were washed (×1 phosphate-buffered saline) to remove the dead, floating or loosely attached cells and were then photographed. As shown in Figure. 1B (i–ii), number of cells that remained attached were several fold higher in SW620control and SW480claudin-1 cells (with higher claudin-1 expression) compared with the SW480control and SW620siRNA cells (with low claudin-1 expression). These data suggested a positive correlation between claudin-1 expression and the resistance to anoikis in above cells. To confirm, we determined percentage of cells undergoing anoikis following culture on poly-HEMA. For this, we used the cell-death enzyme-linked immunosorbent assay as described previously (5). Results demonstrated progressively increased anoikis with the time of culture on poly-HEMA and it was significantly higher in SW480control and SW620siRNA cells compared with the SW620control and/or SW480claudin-1 cells after 3, 8 and 24h of culture on poly-HEMA-coated dishes (3.5–6 fold; Figure 1B, iii–iv). Consistently, there was also an increase in the expression of cleaved caspase-3 in low claudin-1 expressing cells compared with the high claudin-1 expressing cells under conditions of anokis, particularly after 8 and 24h of culture on poly-HEMA (Figure 1C, i–ii).
Fig. 1.
Claudin-1 expression confers resistance to anoikis. (A) Expression of claudin-1 in SW480control (SW480C) and SW480claudin-1 cells (i) or SW620control and SW620siRNA cells (ii) plated on poly-HEMA-coated culture dishes for 0, 3, 8 and 24h. (B) SW480control and SW480claudin-1 cells (i) or SW620control and SW620siRNA cells (ii) were plated on poly-HEMA-coated culture dishes for 24h. Thereafter, cells were collected in the same culture medium by gentle pipetting and replated on regular culture dishes. About 6h after replating, floating cells were removed by washing with ×1 phosphate-buffered saline and attached cells were photographed. Images representative of at least three individual experiments, (iii and iv). Anoikis was determined in cells cultured on poly-HEMA-coated dishes, as above, using a cell-death enzyme-linked immunosorbent assay. Values for respective control cells were considered 100%. Each bar represents the mean ± standard deviation of three different experiments. ***P < 0.001 compared with 0h control. (C) Western blot analysis demonstrating changes in the expressions of cleaved-caspase-3 in SW480control and SW480claudin-1 cells (i) or SW620control and SW620siRNA cells (ii).
Taken together, outcome from the above studies supported a causal role of claudin-1 expression to the resistance to anoikis in colon cancer cells.
Claudin-1-dependent resistance to anoikis correlates with increased expressions of phospho-Src and phospho-Akt
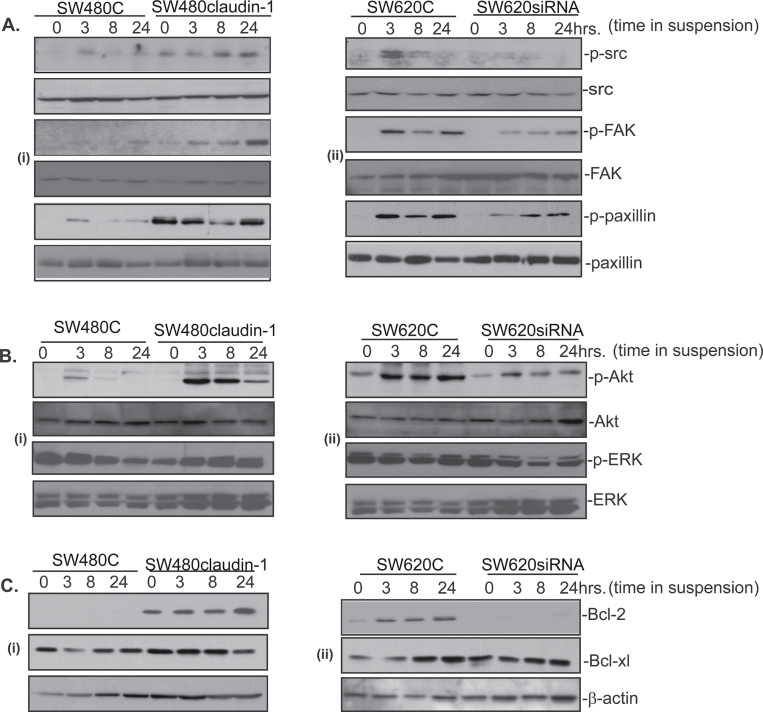
Src-tyrosine kinase is known to promote cell proliferation and resistance to anoikis (16–18). Therefore, we determined whether Src activation also helps regulate claudin-1-dependent resistance to anoikis. We also examined potential activation of FAK and Paxillin because of their known association with Src activation and accepted roles in the regulation of anoikis (19,20). Immunoblot analysis was performed using cell lysates from cells grown on poly-HEMA-coated culture dishes. Manipulation of claudin-1 expression or the culture under detached conditions did not affect the steady-state levels of Src, FAK or Paxillin expression. However, further analysis using phospho-specific antibodies showed sharp upregulation of the phosphorylation for Src (Tyr416), FAK (Tyr397), as well as Paxillin (Tyr118) in cells expressing high levels of claudin-1 (SW620control and SW480claudin-1) compared with the low claudin-1 expressing cells (SW480control and SW620siRNA) where a transient increase at 3h was observed following culture on poly-HEMA (Figure 2A, i–ii). Graphic quantitation of the differences in the expression/phosphorylation of above proteins is presented in the Supplementary Figure 1, available at Carcinogenesis Online. Taken together, we observed a positive correlation between claudin-1 expression, resistance to anoikis and Src phosphorylation along with the phosphorylation of other focal adhesion proteins.
Fig. 2.
Genetic modulation of claudin-1 expression in colon cancer cells affects survival signaling pathway/s in cells subjected to anoikis. Western blot analysis demonstrating changes in the expressions of (A) phospho-Src, phospho-FAK, phospho-Paxillin in SW480control (SW480C) and SW480claudin-1 cells (i) or SW620control and SW620siRNA cells (ii). (B) phospho-Akt and phospho-ERK 1/2 proteins in SW480control and SW480claudin-1 cells (i) or SW620control and SW620siRNA cells (ii). Total protein immunoblotting was used for normalization. (C) Expression of anti-apoptotic proteins Bcl-2 and Bcl-xl in SW480control and SW480claudin-1 cells (i) or SW620control and SW620siRNA cells (ii).
Expression of activated Src in epithelial cells induces transformation and activates signaling pathways associated with the proliferation and cell survival including MAP-ERK1/2 and PI-3 kinase (21,22). Importantly, activation of MEK or PI-3 kinase also protect from anoikis; however, their effects appear to be tissue/cell-specific (14,23). Therefore, we assessed whether upregulation of Src phosphorylation in claudin-1 high cells is associated with phosphorylation of ERK1/2 MAP kinase or Akt or both. However, neither manipulation of claudin-1 expression nor the culture on poly-HEMA had major effects upon MAP-ERK1/2 phosphorylation in the cell lines under study (Figure 2B, i–ii). In contrast, culture on poly-HEMA induced marked increases in Akt phosphorylation (used as the marker of PI-3 kinase activation) in both SW480claudin-1 and SW620control cells that expressed high claudin-1. A small increase in Akt phosphorylation was also observed in the SW480control and SW620siRNA cells at 3h post-poly-HEMA-culture. However, it was transient and decreased with the time cells were cultured on poly-HEMA-coated dishes (Figure 2B, i–ii).
Claudin-1-dependent resistance to anoikis associated with an increase in Bcl-2 expression
The Bcl-2 family of proteins plays central role in the regulation of cell survival under stress conditions including anoikis (15). Therefore, we further examined expressions of Bcl-2 and Bcl-xl, the two Bcl-2 family proteins often implicated in the resistance to anoikis, in the cells under study following culture under anoikis condition. All cell lines under study expressed Bcl-xl; however, its expression was not modulated either by manipulation of claudin-1 expression or by culture in poly-HEMA-coated dishes (Figure 2C, i–ii). In contrast, Bcl-2 expression was not detectable in the cells with low claudin-1 expression (SW480control and SW620SiRNA) at any time point of study. On the other hand, SW620control cells had detectable Bcl-2 expression, which increased with the time of culture on poly-HEMA and remained upregulated until the end of study. Similarly, Bcl-2 expression was high in SW480claudin-1 cells and remained upregulated throughout the study (Figure 2C, i–ii). Taken together, the increased expression of Bcl-2 correlated positively with high-claudin-1 expression and the resistance to anoikis.
Src helps mediate claudin-1-dependent resistance to anoikis
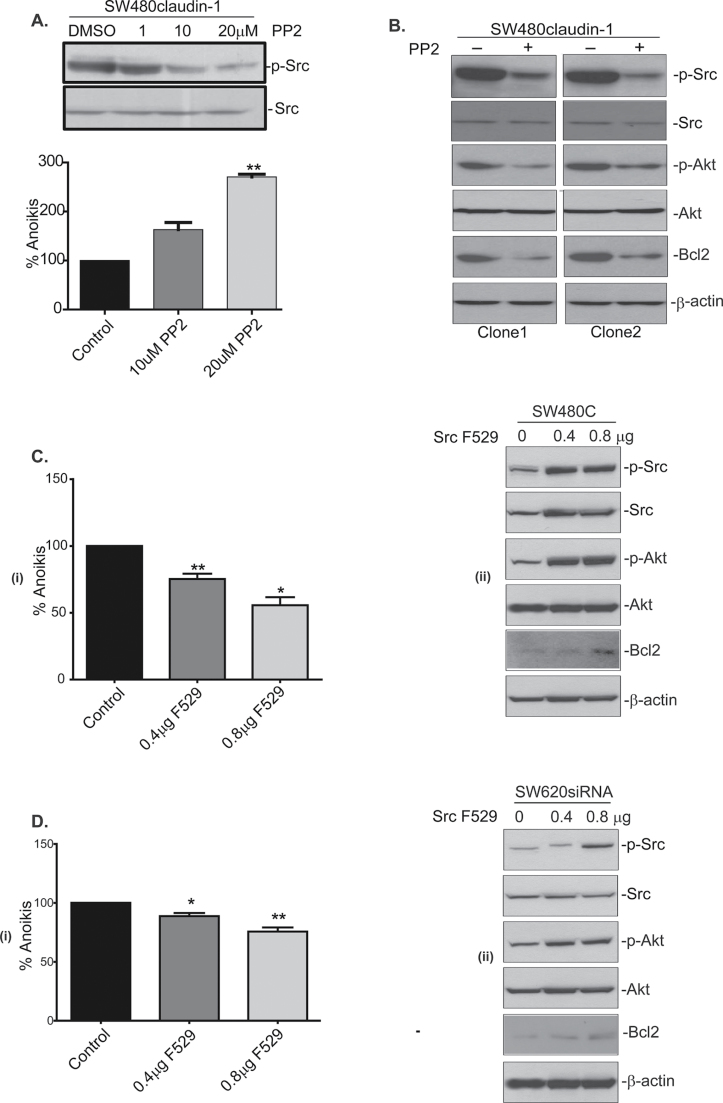
In further studies, we determined whether there is a causal association between claudin-1 overexpression, Src activation and resistance to anoikis. We used PP2, a widely used and highly specific pharmacological inhibitor of Src activation for this purpose. SW480claudin-1 cells were cultured on poly-HEMA-coated dishes in the presence or absence of PP2 (10 or 20 µM) for 24h. Immunoblot analysis confirmed effective inhibition of Src phosphorylation by PP2 treatment in a dose-dependent manner (Figure 3A). In addition, PP2 treatment resulted in a significant increase in anoikis (2.5-fold versus untreated control, P < 0.05) in SW480claudin-1 cells despite robust claudin-1 expression (Figure 3A). We further examined if PP2 treatment also affected the levels of phospho-Akt and/or Bcl-2 expression. Indeed, PP2 treatment inhibited the poly-HEMA-culture-dependent increases in the Akt phosphorylation and Bcl-2 expression in two different clones of SW480claudin-1 cells (Figure 3B). Taken together, our data suggested a key role of Src phosphorylation in claudin-1-dependent modulation of prosurvival signaling cascade and the resistance to anoikis.
Fig. 3.
Effect of the modulation of Src activation upon claudin-1-dependent resistance to anoikis. (A) Effect of PP2 treatment (10 or 20 µM) in SW480claudin-1 cells upon Src phosphorylation and anoikis using the cell-death enzyme-linked immunosorbent assay. Value for the control cells was considered 100%. Each bar represents the mean ± standard deviation of three experiments. (B) Effect of PP2 treatment in SW480claudin-1 cells (two different clones) on signaling/molecules associated with the resistance to anoikis. Akt and β-actin was used for the normalization of protein loading. Effect of the expression of the constitutively activated Src (F529) upon anoikis; expression of phospho-Akt and Bcl-2 in SW480control (SW480C) (C) and SW620siRNA cells (D). Apoptosis was measured using cell-death enzyme-linked immunosorbent assay (ii). Control cell values were considered 100% and are presented as mean ± standard deviation. Akt and β-actin was used for the normalization of protein loading. Results representative of at least three different experiments.
To further confirm the role of Src activation in claudin-1-dependent resistance to anoikis, we expressed constitutive active (CA) Src mutant in SW480control and SW620siRNA cells. As described before, these cells have low claudin-1 expression and are susceptible to anoikis when cultured upon poly-HEMA-coated dishes. Both cell types were subjected to transient transfection using progressively increasing concentration of F529 (CA-Src mutant). Control cells were transfected with empty vector. About 36h post-transfection, transfected cells were detached and replated on poly-HEMA-coated dishes for 24h. Resultant cells were used to prepare total lysates and/or measure anoikis. Immunoblot analysis confirmed expression of CA-Src as it resulted in increased Src phosphorylation (Figure 3C and 3D, ii). In our further analysis, we did observe a significant decrease in anoikis in both SW480control and SW620siRNA cells, which was proportional to the amount of CA-Src mutant DNA used for transfection {Figure 3C and D, i). Importantly, expression of CA-Src also increased the levels of p-Akt and Bcl-2 expression and thus supported causal association of p-Src with P-Akt and Bcl-2 expression in our cell model. Also, similar to the effect of the inhibition of Src-signaling (using PP2), inhibition of Akt signaling (using PI3K-specific inhibitor LY294002) ameliorated the effect of claudin-1 expression upon anoikis (Supplementary Figure 2, available at Carcinogenesis Online). Taken together, our data strongly supported a key role of Src-Akt-Bcl-2 activation in the regulation of claudin-1-dependent resistance to anoikis and associated prosurvival molecular events.
Claudin-1 associates with Src/p-Src in a multiprotein complex
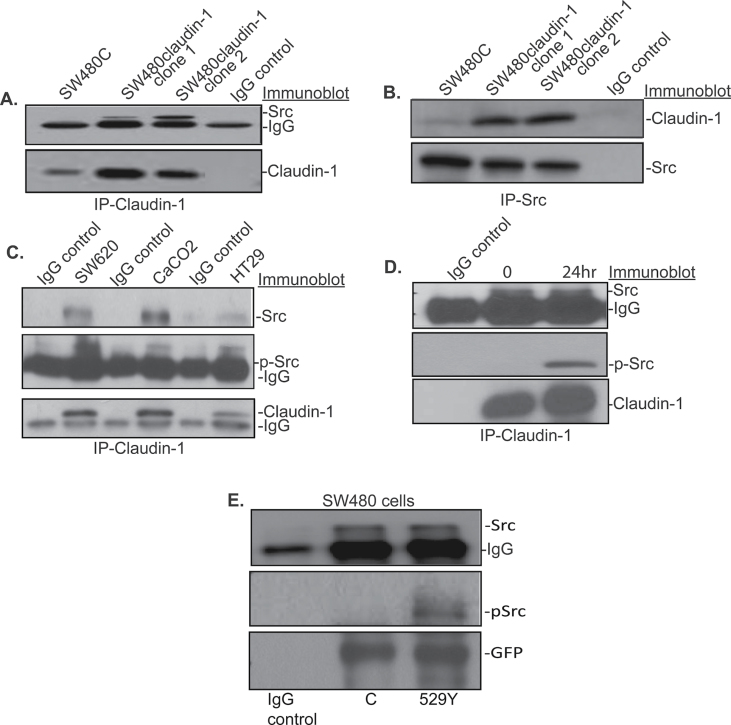
Since our studies demonstrated a key role of Src activation in the regulation of claudin-1-dependent resistance to anoikis, we further determined how claudin-1 expression influences Src phosphorylation. For this, we first explored if Src associates with claudin-1. We performed co-immunoprecipitation where total cell lysate from SW480control and SW480claudin-1 cells grown on poly-HEMA-coated dishes was immunoprecipitated using rabbit-anti-claudin-1 antibody. Immunoblotting of the resultant immunoprecipitate with anti-claudin-1 antibody confirmed effective pull down of claudin-1 protein. Further immunoblotting using a rabbit-anti-Src antibody detected Src protein in the same immunoprecipitate and thus suggested physical association of Src and claudin-1 (Figure 4A). To further confirm these findings, we immunoprecipitated same lysate using anti-Src antibody and then immunoblot with anti-Src and/or anti-claudin-1 antibodies. As shown in Figure 4B, Src pull down also brought down claudin-1 in claudin-1 overexpressing cells and thus supported physical interaction between the two.
Fig. 4.
Claudin-1 associates with Src. Total cell lysate from SW480control (SW480C) and SW480claudin-1 cells was immunoprecipitated using anti-claudin-1 (A) or anti-Src (B) antibody followed by immunoblotting with anti-Src and/or anti-claudin-1 antibody, respectively. (C) Total cell lysate from SW620, CaCo-2 and HT29 colon cancer cells was immunoprecipitated with anti-claudin-1 and immunoblotted with anti-Src, anti-p-Src or anti-claudin-1 antibody to determine association between endogenously expressed claudin-1 and Src. Data representative of three independent experiments. (D) Cells were collected after plating on poly-HEMA-coated plates for 0 and 24h and were used for immunoprecipitation using anti-claudin-1 antibody. These blots were immunoblotted with anti-Src, p-Src or claudin-1 antibody. (E) SW480 cells were cotransfected with Src wild type and GFP-claudin-1 or Src F529 (activated) and GFP-claudin-1 and were immunoprecipitated with GFP antibody. The association of Src or p-Src was monitored using immunoblotting.
We determined whether the observed physical interaction between claudin-1 and Src proteins is limited to the cells under study or a phenomenon common among claudin-1 expressing colon cancer cells. Immunoprecipitation was performed using anti-claudin-1 antibody and cell lysates (from cells plated on poly-HEMA-coated plates) from SW620, CaCo-2 and HT29 colon cancer cells that express variable amounts of endogenous claudin-1 protein. Immunoblot demonstrated co-existence of both, claudin-1 and Src in the resultant immunoprecipitate in all the cell lines tested (Figure 4C). We further determined presence of the phosphorylated Src in claudin-1 pull down in these cells. Claudin-1 associated with Src/p-Src in a complex. We then confirmed this association in SW480claudin-1 cell lysates from 0 and 24h of culture on poly-HEMA-coated dishes. As described before, Src phosphorylation in these cells increases upon culture on poly-HEMA (Figure 2A). Indeed, immunoblot analysis confirmed the presence of p-Src in claudin-1 pull downs in the above samples (Figure 4D). Furthermore, amount of p-Src in the claudin-1 pull down was several folds higher in samples from 24h post-poly-HEMA culture compared with the samples from 0h post-poly-HEMA culture despite similar levels of Src protein being pulled down in both samples. To further clarify this observation, we cotransfected SW480 cells with Src wild type and GFP-claudin-1 or the activated form of Src (SrcF529) and GFP-claudin-1. Resultant lysates were subjected to immunoprecipitation using anti-GFP antibody. Immunoblot analysis once again demonstrated presence of Src protein in claudin-1 pull down in all samples; however, a cotransfection with activated Src resulted in increased association of p-Src with GFP-claudin-1 as compared with wild-type Src (Figure 4E).
Claudin-1 is known to associate with ZO-1, whereas ZO-1 associates with Src (24,25). Therefore, in further studies, we determined whether claudin-1 associates with Src/p-Src in a multiprotein complex. In this regard, we examined the presence of ZO-1, a PDZ-domain protein implicated in cell proliferation and transformation in the claudin-1-Src/p-Src complex. To determine, we performed immunoprecipitation using anti-ZO-1 antibody followed by immunoblotting with anti-claudin-1 or anti-Src antibody. Immunoblotting with anti-ZO-1 antibody confirmed efficient pull down and immunoblotting with claudin-1 and Src antibodies confirmed the presence of claudin-1 and Src proteins in the ZO-1 pull down (Figure 5A, 5i). Conversely, immunoprecipitation with claudin-1 antibody pulled down ZO-1 and Src (Figure 5A, ii). Taken together, our data supported a novel interaction between claudin-1, ZO-1 and Src/p-Src and suggested that they form a multiprotein complex in colon cancer cells.
Claudin-1 C-terminal domain is required for association with Src
We further examined molecular details of the association between claudin-1 and Src/p-Src. Of interest, claudin-1 protein contains PDZ-binding domain through which it interacts with ZO-1 (26). Since ZO-1 is part of the protein complex containing claudin-1 and Src/p-Src, we determined whether interaction between claudin-1 and Src/p-Src is dependent upon claudin-1 C-terminal. In this regard, we generated mutants of claudin-1 lacking either the complete C-terminal domain (Amino acids 188–211, ∆C-term-GFP-claudin-1) or only the PDZ-binding domain (207–211; ∆PDZ-GFP-claudin-1). All constructs were GFP-tagged in their 5ʹ for the ease of detection (Figure 5B). SW480 cells were transiently transfected with GFP-claudin-1 (full length) and Src, ∆PDZ-GFP-claudin-1 and Src, or ∆C-term-GFP-claudin-1 and Src. About 24h post-transfection, these cells were replated on poly-HEMA-coated plates. Cell extracts were prepared 24h postculture and subjected to immunoprecipitation using anti-GFP antibody. The resultant immunoprecipitate was resolved on sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to immunoblotting with anti-GFP, Src, p-Src or ZO-1 antibody. As expected, immunoblotting with anti-GFP antibody detected GFP band in the SW480 control cells due to the presence of GFP-vector construct and served as negative control (Figure 5C, 5i and ii, lane 1). In contrast, it detected respective size band in all other transfected cell lines where size of the band corresponded to the molecular weight of GFP and claudin-1 together and progressively decreased from the full-length-GFP-claudin-1 > ∆PDZ-GFP-claudin-1 > ∆C-term-GFP-claudin-1, as per our prediction (Figure 5C, 5i and ii, lanes 2–4). Further as expected, robust Src and/or p-Src, ZO-1 expressions were detected in the immunoprecipitate from the cells transfected with full-length claudin-1 cDNAs. However, this pull down of Src and/or p-Src was markedly decreased in the immunoprecipitate from cells transfected with the ∆PDZ-GFP-claudin-1 and full-length Src and was not detectable altogether in the cells that received ∆C-term-GFP-claudin-1 along with the full-length Src. We obtained similar findings when above study was done using COS-1 cells (Figure 5C). Taken together, results suggested that the C-terminal domain of claudin-1 is required for its association with Src/p-Src.
Loss of association with Src decreases the effect of claudin-1 expression upon resistance from anoikis
Cumulative outcome from the above studies supported a key role of Src activation in claudin-1-dependent resistance to anoikis. Therefore, we further determined whether loss of the association between claudin-1 and Src would also ameliorate the effect of claudin-1 expression upon resistance to anoikis. For this purpose, SW480 cells were cotransfected with GFP-claudin-1 (full length) and Src, ∆PDZ-GFP-claudin-1 and Src, or ∆C-term-GFP-claudin-1 and Src. About 48h post-transfection, cells were detached and plated on poly-HEMA-coated plates for 24h and then were subjected to the measurement of anoikis. Results demonstrated that overexpression of the full-length GFP-claudin-1 induced resistance to anoikis (i.e. decreased apoptosis, 4-fold compared with control, P < 0.001). However, we did not observe similar increase in the resistance to anoikis in SW480 cells overexpressing ∆PDZ-GFP-claudin-1 (1.8-fold, P < 0.05), whereas overexpression of the ∆C-term-GFP-claudin-1 failed to induce any increase in the resistance from anoikis compared with the control cells (Figure 5D, 5i). We obtained similar results upon cotransfection of COS-1 cells with claudin-1 full length or mutant constructs and Src followed by exposure to anoikis-inducing culture condition (Figure 5D, ii). Taken together, our data suggested that mutual partnering between claudin-1 and Src confers resistance to anoikis in colon cancer cells.
Discussion
Spread of the disease to distant vital organs is the primary cause of the death among colon cancer patients (27). Therefore, for effective disease management, it is important that we understand molecular mechanism/s that help promote colon cancer malignancy. In this regard, a marked increase in claudin-1 expression along with dysregulation of its cellular distribution in human colon cancer patient samples is reported by us and other groups (5,6,28). An increase in claudin-1 expression has also been observed in other malignant cancers of non-colonic origin (28). It is further noteworthy that claudin-1 expression in colon cancer correlates with the cancer progression in a positive manner and genetic manipulation of claudin-1 expression was sufficient to modulate metastatic potential of the colon cancer cells when subjected to the mouse model of colon cancer metastasis (5). To metastasize, tumor cells need to detach from the parent tumor mass and travel to the distant organs before colonizing. This is why resistance to anoikis is considered a key regulator of cancer malignancy (invasion and metastasis) including colon cancer (1). Outcomes from our current studies support such a notion as manipulation of claudin-1 expression in colon cancer cells exerts similar effects upon their metastatic ability and resistance to anoikis.
Our postulation that claudin-1 expression helps regulate colon cancer malignancy through the modulation of the resistance to anoikis gains support from our observation that claudin-1 partners with Src/p-Src in colon cancer cells. Notably, forced expression of activated Src induces neoplastic transformation of epithelial cells, and outcome from a series of studies supports specific role of the activated Src in the regulation of resistance to anoikis (23,29). Importantly, similar to our current findings, Windham et al. also observed low intrinsic Src expression in SW480 cells, and Src activation by enforced expression resulted in an increase in the resistance to anoikis. It should be noted that SW480 cells do not metastasize when subjected to the mouse model of colon cancer metastasis. However, overexpression of claudin-1 (SW480claudin-1 cells) induces metastatic ability in these cells (5). We now demonstrate that SW480claudin-1 cells are also resistant to anoikis and express higher levels of phospho-Src compared with the SW480 cells. Abrogation of either Src phosphorylation (using pharmacological inhibitor) or association of Src/p-Src with claudin-1 (using mutant claudin-1 constructs) had similar effects upon claudin-1-dependent resistance to anoikis. It was further notable that the association between claudin-1 and Src/p-Src was detected at all-time points during the culture of cells on poly-HEMA; however, the amount of claudin-1 bound to p-Src increased with the time of culture in poly-HEMA. This finding correlated with the fact that phosphorylation of Src increased with the time of culture on poly-HEMA-coated plates. Taken together, our data support a key role of activated Src in claudin-1-dependent resistance to anoikis in colon cancer cells.
Studies have uncovered multiple cellular targets that may help mediate Src-dependent regulation of the resistance to anoikis (30). In this regard, study by Coll et al. (31) proposed that v-Src induces resistance to anoikis in intestinal epithelial cells via upregulation of the expression of Bcl-xL, a Bcl-2 family protein with established role in cell survival and cancer progression (32). Interestingly, we did not observe major alteration in the expression of Bcl-xL, either under the condition of anoikis or upon manipulation of claudin-1 expression. However, we did observe upregulation of the expression of Bcl-2 in colon cancer cells that expressed high levels of claudin-1 and were resistant to anoikis compared with the low claudin-1 expressing cells. Bcl-2 is also a member of the Bcl-2 family of proteins and is implicated in cell survival and cancer (33). Importantly, use of PP2 to inhibit Src phosphorylation not only inhibited the claudin-1-dependent resistance to anoikis but also decreased Bcl-2 expression. Furthermore, Src activation is required for the activation of Akt to direct cell-survival signals in detached colon cancer cells (34). Role of the PI3K/Akt pathway in mediating cell survival is well established (35). In our current studies, culture under anoikis conditions induced specific increase in the expression of p-Akt in the high claudin-1 expressing and anoikis resistant cells. PP2 treatment in these cells inhibited the resistance to anoikis, as well as the anoikis-induced increases in p-Akt and Bcl-2 expressions. Notably, inhibition of the Akt signaling alone also ameliorated the effect of claudin-1 expression upon anoikis. Taken together, our data suggest that claudin-1 expression helps regulate the ability of colon cancer cells to survive under anoikis condition through the regulation of Src-Akt-Bcl-2 axis.
Yet another potential mechanism that may help regulate claudin-1-dependent resistance to anoikis is claudin-1-dependent regulation of the transcriptional repressor of E-cadherin, ZEB-1 (36). Notably, loss of E-cadherin expression at the site of cell–cell adhesion signifies epithelial cell transformation and metastasis (37–39). Src binds with E-cadherin and dysregulates its expression and cellular distribution and thereby disrupts the cell–cell adhesion, in turn, enabling cancer cells to detach from their original site. Dysregulation of E-cadherin in the colon cancer cell lines HT29 and SW480 prevents cellular adhesion, which can be restored by the use of PP2, a p-Src inhibitor (40). Claudin family members regulate E-cadherin expression in cancer cells (41), and we have recently reported that claudin-1 regulates E-cadherin expression in colon cancer cells through the regulation of ZEB-1 (36). Importantly, claudin-1-dependent regulation of ZEB-1 expression was dependent upon Akt activation. It should be noted that we did observe a robust increase in ZEB-1 expression combined with a sharp decrease in E-cadherin expression in the colon cancer cells that were resistant to anoikis and expressed high levels of claudin-1 (36). Thus, it is tempting to speculate that claudin-1-dependent regulation of E-cadherin helps assist in its role in the regulation of the resistance to anoikis, however remains to be determined and is part of our ongoing investigation. Apart, claudin-1 associates with the tetraspin CD-81 and integrin α2 in colon cancer cells (42,43). Tetraspanins associate with the integrins, which upon activation with appropriate ligands induce inside-out signaling and thus regulate cellular decision to survive and/or die under detached condition. Notably, integrins associate with Src itself in addition to the focal adhesion complex proteins FAK and Paxillin in a complex (44). Of interest, in our current study, we have also observed increased phosphorylation of focal adhesion kinases FAK and Paxillin in claudin-1 overexpressing cells. An interdependence among the integrin ligation and Src activation through direct/indirect activation of focal adhesion kinases is well recognized (45). Also, ZO-1 associates with the integrins (46,47). Thus, potential role of the multiprotein complexing, which in turn could induce conformational changes in Src protein and thus its activation is plausible and however remain to be further investigated.
Our findings suggest that claudin-1 associates with Src/p-Src in a multiprotein complex that includes ZO-1, a PDZ-domain-containing and tight-junction-associated protein. Notably, studies have identified Src as a component of the tight junction where G-alpha-mediated Src activation enhances paracellular permeability via currently unknown mechanism/s (48). G-alpha-12 binds to ZO-1 (48). Thus, it is suggested that ZO proteins serve as Src/Csk scaffolds and is potentially involved in the regulation of Src-dependent transformation. Src and ZO-1 also associate with each other in complexes with gap junction and desmosomal proteins (49). ZO-1 associates with claudin-1 (50). Thus, potential of a multiprotein complex can be postulated, which may help regulate diverse cellular functions during epithelial cell transformation including anoikis. Our postulation gains support from the recent findings that immunoprecipitation of claudin-2, yet another claudin family member, in Madin Darby canine kidney II cells immunoprecipitated a large multiprotein complex (~600-kDa), which contained claudin-1 (51). However, further studies that involve proteomic analysis are required to fully characterize the protein composition and functional scope of the potential protein complex involving claudin-1, Src/p-Src and ZO-1 identified in our current studies and is currently underway.
In summary, work presented in the current manuscript has revealed an important role of claudin-1 in the detachment-induced regulation of Src activation and protection against anoikis. The present findings thus suggest that claudin-1 might be causally involved in promoting local invasion, as well as distant metastasis via targeting the Src-mediated signaling and resistance to anoikis. Resistance to anoikis could predict a greater ability to form metastatic lesions. Indeed, overexpression of claudin-1 correlates with metastatic and invasion properties in colon carcinoma (5). Identification of molecular events that control anoikis in colon cancer cells has significant therapeutic implications.
Supplementary material
Supplementary Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (CA124977 to P.D., DK088902 to A.B.S).
Supplementary Material
Acknowledgement
We would like to thank Dr Robert D. Beauchamp and Dr Josh Smith for critically reviewing the manuscript.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CA
constitutive active
- CRC
colorectal cancer
- ERK
extracellular signal-regulated kinase
References
- 1. Guadamillas M.C. et al (2011). Overcoming anoikis—pathways to anchorage-independent growth in cancer J. Cell Sci. 124 3189–3197. [DOI] [PubMed] [Google Scholar]
- 2. Rosen K. et al. (2000). Activated Ras prevents downregulation of Bcl-X(L) triggered by detachment from the extracellular matrix. A mechanism of Ras-induced resistance to anoikis in intestinal epithelial cells. J. Cell Biol. 149 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duxbury M.S. et al. (2004). EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene 23 1448–1456 [DOI] [PubMed] [Google Scholar]
- 4. Yoo B.H. et al (2012). Tumor suppressor protein kinase Chk2 is a mediator of anoikis of intestinal epithelial cells Int. J. Cancer 131 357–366 [DOI] [PubMed] [Google Scholar]
- 5. Dhawan P. et al. (2005). Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Invest. 115 1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dos Reis P.P. et al. (2008). Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer 113 3169–3180 [DOI] [PubMed] [Google Scholar]
- 7. Lee J.W. et al. (2005). Increased expressions of claudin-1 and claudin-7 during the progression of cervical neoplasia. Gynecol. Oncol. 97 53–59 [DOI] [PubMed] [Google Scholar]
- 8. Chen J. (2008). Is Src the key to understanding metastasis and developing new treatments for colon cancer? Nat. Clin. Pract. Gastroenterol. Hepatol. 5 306–307 [DOI] [PubMed] [Google Scholar]
- 9. Zhao J. et al. (2009). Migfilin interacts with Src and contributes to cell-matrix adhesion-mediated survival signaling. J. Biol. Chem. 284 34308–34320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Avizienyte E. et al. (2002). Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 4 632–638 [DOI] [PubMed] [Google Scholar]
- 11. Windham C et al (2002). Src activation regulates anoikis in human colon tumor cell lines Oncogene, 21 7797–7807. [DOI] [PubMed] [Google Scholar]
- 12. Demers M.J. et al. (2009). Intestinal epithelial cancer cell anoikis resistance: EGFR-mediated sustained activation of Src overrides Fak-dependent signaling to MEK/Erk and/or PI3-K/Akt-1. J. Cell. Biochem. 107 639–654 [DOI] [PubMed] [Google Scholar]
- 13. Bouchard V. et al. (2008). B1 integrin/Fak/Src signaling in intestinal epithelial crypt cell survival: integration of complex regulatory mechanisms. Apoptosis 13 531–542 [DOI] [PubMed] [Google Scholar]
- 14. Harada K. et al. (2011). Ephexin4 and EphA2 mediate resistance to anoikis through RhoG and phosphatidylinositol 3-kinase. Exp. Cell Res. 317 1701–1713 [DOI] [PubMed] [Google Scholar]
- 15. Nagaprashantha L.D. et al. (2011). The sensors and regulators of cell-matrix surveillance in anoikis resistance of tumors. Int. J. Cancer 128 743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei L. et al. (2004). Altered regulation of Src upon cell detachment protects human lung adenocarcinoma cells from anoikis. Oncogene 23 9052–9061 [DOI] [PubMed] [Google Scholar]
- 17. Reginato M.J. et al. (2005). Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol. Cell. Biol. 25 4591–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamaguchi H. et al. (2008). SRC directly phosphorylates Bif-1 and prevents its interaction with Bax and the initiation of anoikis. J. Biol. Chem. 283 19112–19118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zouq N.K. et al. (2009). FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J. Cell. Sci. 122(Pt 3)357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sachdev S. et al.(2009)Paxillin-Y118 phosphorylation contributes to the control of Src-induced anchorage-independent growth by FAK and adhesion. BMC Cancer 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manuel G.C. et al. (2006). PI3K and ERK 1-2 regulate early stages during head regeneration in hydra. Dev. Growth Differ. 48 129–138 [DOI] [PubMed] [Google Scholar]
- 22. Lei J. et al. (2011). Src kinase integrates PI3K/Akt and MAPK/ERK1/2 pathways in T3-induced Na-K-ATPase activity in adult rat alveolar cells. Am. J. Physiol. Lung Cell Mol. Physiol. 301 L765–L771 [DOI] [PubMed] [Google Scholar]
- 23. Haenssen K.K. et al (2010). ErbB2 requires integrin α5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells J. Cell Sci. 123 1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilleron J. et al. (2009). Connexin 43 gap junction plaque endocytosis implies molecular remodelling of ZO-1 and c-Src partners. Commun. Integr. Biol. 2 104–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nomme J. et al. (2011). The Src homology 3 domain is required for junctional adhesion molecule binding to the third PDZ domain of the scaffolding protein ZO-1. J. Biol. Chem. 286 43352–43360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Itoh M. et al. (1999). Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 147 1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayo S.C. et al. (2009). Current management of colorectal hepatic metastasis. Expert Rev. Gastroenterol. Hepatol. 3 131–144 [DOI] [PubMed] [Google Scholar]
- 28. Leotlela P.D. et al. (2007). Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene 26 3846–3856 [DOI] [PubMed] [Google Scholar]
- 29. Windham T.C. et al. (2002). Src activation regulates anoikis in human colon tumor cell lines. Oncogene 21 7797–7807 [DOI] [PubMed] [Google Scholar]
- 30. González-Mariscal L. et al. (2003). Tight junction proteins. Prog. Biophys. Mol. Biol. 81 1–44 [DOI] [PubMed] [Google Scholar]
- 31. Coll M.L. et al. (2002). Increased Bcl-xL expression mediates v-Src-induced resistance to anoikis in intestinal epithelial cells. Oncogene 21 2908–2913 [DOI] [PubMed] [Google Scholar]
- 32. Galante J.M. et al. (2009). ERK/BCL-2 pathway in the resistance of pancreatic cancer to anoikis. J. Surg. Res. 152 18–25 [DOI] [PubMed] [Google Scholar]
- 33. Youle R.J. et al. (2008). The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9 47–59 [DOI] [PubMed] [Google Scholar]
- 34. Beauséjour M. et al. (2012). Integrin/Fak/Src-mediated regulation of cell survival and anoikis in human intestinal epithelial crypt cells: selective engagement and roles of PI3-K isoform complexes. Apoptosis 17 566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahajan K. et al. (2012). PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. J. Cell. Physiol. 227 3178–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh A.B. et al. (2011). Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells. Gastroenterology 141 2140–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilmanns C. et al. (2012). Cooperate concept of metastasis: site-specific requirement of activated differentiation and dynamic deterioration. Cancer Metastasis Rev. 31 269–276 [DOI] [PubMed] [Google Scholar]
- 38. Baranwal S. et al. (2009). Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem. Biophys. Res. Commun. 384 6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wells A. et al. (2008). E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin. Exp. Metastasis 25 621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hikita T. et al. (2010). Purvalanol A, a CDK inhibitor, effectively suppresses Src-mediated transformation by inhibiting both CDKs and c-Src. Genes Cells 15 1051–1062 [DOI] [PubMed] [Google Scholar]
- 41. Lioni M. et al. (2007). Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am. J. Pathol. 170 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeisel M.B. et al. (2011). Hepatitis C virus entry into hepatocytes: molecular mechanisms and targets for antiviral therapies. J. Hepatol. 54 566–576 [DOI] [PubMed] [Google Scholar]
- 43. Ding L. et al. (2012). Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology 142 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mitra S.K. et al. (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18 516–523 [DOI] [PubMed] [Google Scholar]
- 45. Serrels A. et al. (2011). Src/FAK-mediated regulation of E-cadherin as a mechanism for controlling collective cell movement: insights from in vivo imaging. Cell Adh. Migr. 5 360–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caccavari F. et al. (2010). Integrin signaling and lung cancer. Cell Adh. Migr. 4 124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuomi S. et al.(2009)PKCepsilon regulation of an alpha5 integrin-ZO-1 complex controls lamellae formation in migrating cancer cells. Sci. Signal. 2ra32. [DOI] [PubMed] [Google Scholar]
- 48. Meyer T.N. et al. (2002). Zonula occludens-1 is a scaffolding protein for signaling molecules. Galpha(12) directly binds to the Src homology 3 domain and regulates paracellular permeability in epithelial cells. J. Biol. Chem. 277 24855–24858 [DOI] [PubMed] [Google Scholar]
- 49. Lie P.P. et al. (2010). Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int. J. Biochem. Cell Biol. 42 975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nunbhakdi-Craig V. et al. (2002). Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 158 967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Itallie C.M. et al. (2011). Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J. Biol. Chem. 286 3442–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.