Abstract
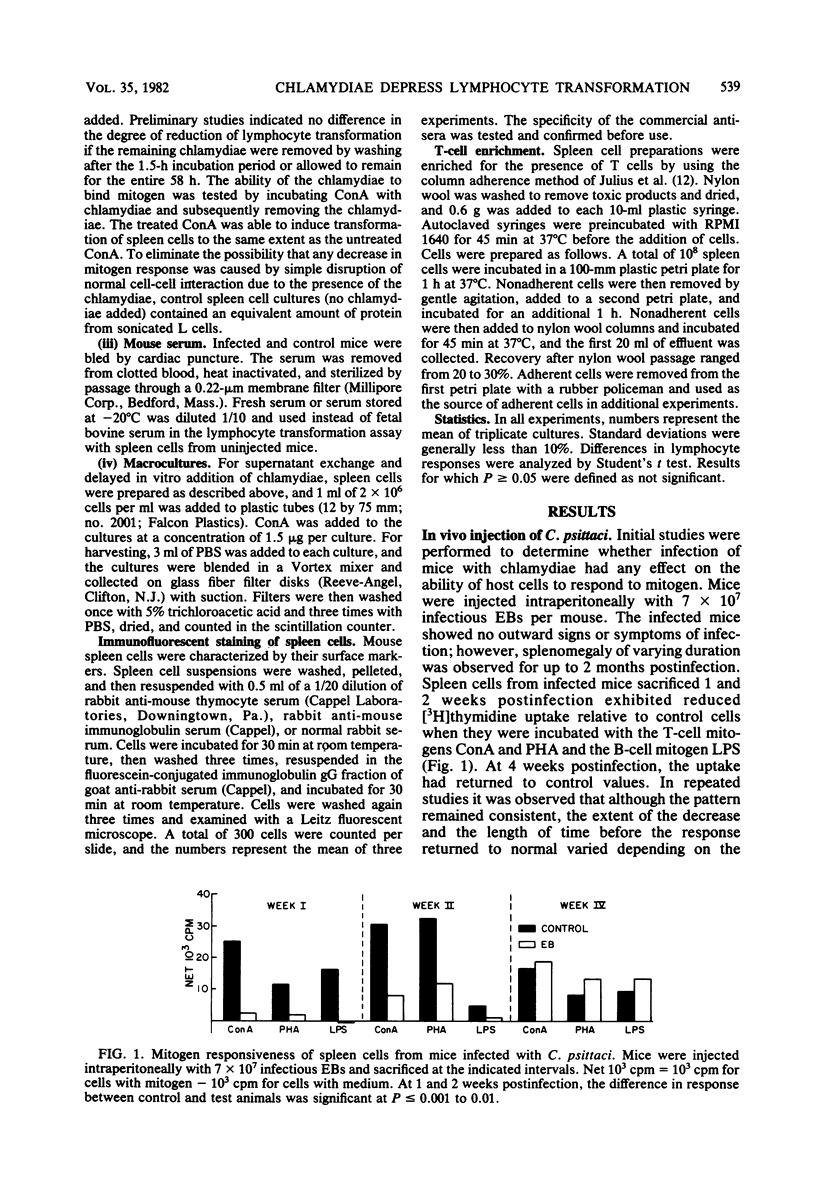
After intraperitoneal injection of mice with infectious, inactivated, or envelope preparations of the elementary body of Chlamydia psittaci, lymphocyte transformation of spleen cells to the mitogens concanavalin A, phytohemagglutinin, and lipopolysaccharide was significantly reduced 1 and 2 weeks postinjection. Lymphocyte response returned to the control values by 4 weeks. Similarly, transformation of cells by chlamydial antigen was not detected until 4 weeks postinjection. Injection of the noninfectious intracellular reticulate body, in contrast, had little effect on transformation of cells to concanavalin A. When control spleen cells were incubated with infectious or inactivated elementary bodies in vitro, response to all three mitogens was also reduced. The sooner the organisms were added after the addition of mitogen, the greater the reduction in transformation. Incubation with elementary body envelopes and reticulate bodies had no effect on lymphocyte transformation of the spleen cells to concanavalin A. The relationship between the observed ability to reduce the response in the in vitro assay of lymphocyte transformation and the actual in vivo establishment of infection is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmann G. B., Sachs D. H., Hodes R. J. Requirement for an Ia-bearing accessory cell in Con A-induced T cell proliferation. J Immunol. 1978 Nov;121(5):1981–1989. [PubMed] [Google Scholar]
- Andersson J., Grönvik K. O., Larsson E. L., Coutinho A. Studies on T lymphocyte activation. I. Requirements for the mitogen-dependent production of T cell growth factors. Eur J Immunol. 1979 Aug;9(8):581–587. doi: 10.1002/eji.1830090802. [DOI] [PubMed] [Google Scholar]
- Arnstein P. Observations on chemotherapy and immunization of birds against psittacosis. Am J Ophthalmol. 1967 May;63(5 Suppl):1260–1263. doi: 10.1016/0002-9394(67)94107-4. [DOI] [PubMed] [Google Scholar]
- Brownridge E., Wyrick P. B. Interaction of Chlamydia psittaci reticulate bodies with mouse peritoneal macrophages. Infect Immun. 1979 Jun;24(3):697–700. doi: 10.1128/iai.24.3.697-700.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier L. H., Blyth W. A. Immunogenicity of experimental trachoma vaccines in baboons. I. Experimental methods, and preliminary tests with vaccines prepared in chick embryos and in HeLa cells. J Hyg (Lond) 1966 Dec;64(4):513–528. doi: 10.1017/s0022172400040821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir S. P., Hakomori S., Kenny G. E., Grayston J. T. Immunochemical studies on chlamydial group antigen (presence of a 2-keto-3-deoxycarbohydrate as immunodominant group). J Immunol. 1972 Jul;109(1):116–122. [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hanna L., Schmidt L., Sharp M., Stites D. P., Jawetz E. Human cell-mediated immune responses to chlamydial antigens. Infect Immun. 1979 Feb;23(2):412–417. doi: 10.1128/iai.23.2.412-417.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins B. E., Wyrick P. B. Response of C3H/HeJ and C3H/HeN mice and their peritoneal macrophages to the toxicity of Chlamydia psittaci elementary bodies. Infect Immun. 1978 Nov;22(2):620–622. doi: 10.1128/iai.22.2.620-622.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. J. Wheat germ agglutinin blockage of chlamydial attachment sites: antagonism by N-acetyl-D-glucosamine. Infect Immun. 1979 Sep;25(3):946–953. doi: 10.1128/iai.25.3.946-953.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis V. J., Thacker W. L., Mitchell S. H. Demonstration of chlamydial endotoxin-like activity. J Gen Microbiol. 1979 Sep;114(1):215–216. doi: 10.1099/00221287-114-1-215. [DOI] [PubMed] [Google Scholar]
- MEYER K. F., EDDIE B. Immunity against some bedsonia in man resulting from infection and in animals from infection or vaccination. Ann N Y Acad Sci. 1962 Mar 5;98:288–313. doi: 10.1111/j.1749-6632.1962.tb30553.x. [DOI] [PubMed] [Google Scholar]
- Maizel A. L., Mehta S., Ford R. J. T-lymphocyte/monocyte interaction in response to phytohemagglutinin. Cell Immunol. 1979 Dec;48(2):383–397. doi: 10.1016/0008-8749(79)90133-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron Microscopic Observations on the Fine Structure of Cell Walls of Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1332–1337. doi: 10.1128/jb.104.3.1332-1337.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor-Withey K. S., Clayton C. E., Roelants G. E., Askonas B. A. Trypanosomiasis leads to extensive proliferation of B, T and null cells in spleen and bone marrow. Clin Exp Immunol. 1978 Dec;34(3):359–363. [PMC free article] [PubMed] [Google Scholar]
- Mehra V. L., Talwar G. P., Balakrishnan K., Bhutani L. K. Influence of chemotherapy and serum factors on the mitogenic response of peripheral leucocytes of leprosy patients to phytohaemagglutinin. Clin Exp Immunol. 1972 Oct;12(2):205–213. [PMC free article] [PubMed] [Google Scholar]
- Morrison W. I., Roelants G. E., Mayor-Withey K. S., Murray M. Susceptibility of inbred strains of mice to Trypanosoma congolense: correlation with changes in spleen lymphocyte populations. Clin Exp Immunol. 1978 Apr;32(1):25–40. [PMC free article] [PubMed] [Google Scholar]
- Murray E. S., Charbonnet L. T., MacDonald A. B. Immunity to chlamydial infections of the eye. I. The role of circulatory and secretory antibodies in resistance to reinfection with guinea pig inclusion conjunctivitis. J Immunol. 1973 Jun;110(6):1518–1525. [PubMed] [Google Scholar]
- Orenstein N. S., Mull J. D., Thompson S. E., 3rd Immunity to chlamydial infections of the eye. V. Passive transfer of antitrachoma antibodies to owl monkeys. Infect Immun. 1973 Apr;7(4):600–603. doi: 10.1128/iai.7.4.600-603.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page L. A. Stimulation of cell-mediated immunity of chlamydiosis in turkeys by inoculation of chlamydial bacterin. Am J Vet Res. 1978 Mar;39(3):473–480. [PubMed] [Google Scholar]
- Schramek S., Kazár J., Sádecký E. Serological cross-reactions of lipid A components of lipopolysaccharides isolated from Chlamydia psittaci and Coxiella burnetii. Acta Virol. 1980 May;24(3):224–224. [PubMed] [Google Scholar]
- Schwab J. H. Suppression of the immune response by microorganisms. Bacteriol Rev. 1975 Jun;39(2):121–143. doi: 10.1128/br.39.2.121-143.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- Tamura A., Tanaka A., Manire G. P. Separation of the polypeptides of Chlamydia and its cell walls by polyacrylamide gel electrophoresis. J Bacteriol. 1974 Apr;118(1):139–143. doi: 10.1128/jb.118.1.139-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg M. A., Israel E. Viral inhibition of lymphocyte mitogenesis. I. Evidence for the nonspecificity of the effect. J Immunol. 1980 Jan;124(1):64–70. [PubMed] [Google Scholar]
- Ware J. L., Folds J. D., Baseman J. B. Serum of rabbits infected with Treponema pallidum (Nichols) inhibits in vitro transformation of normal rabbit lymphocytes. Cell Immunol. 1979 Feb;42(2):363–372. doi: 10.1016/0008-8749(79)90201-6. [DOI] [PubMed] [Google Scholar]
- Watson R. R., MacDonald A. B., Murray E. S., Modabber F. Z. Immunity to chlamydial infections of the eye. 3. Presence and duration of delayed hypersensitivity to guinea pig inclusion conjuctivitis. J Immunol. 1973 Aug;111(2):618–623. [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A. Growth of Chlamydia psittaci in macrophages. Infect Immun. 1978 Mar;19(3):1054–1060. doi: 10.1128/iai.19.3.1054-1060.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyrick P. B., Brownridge E. A., Ivins B. E. Interaction of Chlamydia psittaci with mouse peritoneal macrophages. Infect Immun. 1978 Mar;19(3):1061–1067. doi: 10.1128/iai.19.3.1061-1067.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



