Abstract
Epidemiological data and studies in rodent models strongly support the role of estrogens in the development of breast cancers. Oxidative stress has been implicated in this carcinogenic process. We have recently demonstrated that antioxidants vitamin C or butylated hydroxyanisole (BHA) severely inhibit 17β-estradiol (E2)-induced breast tumor development in female ACI rats. The objective of this study was to characterize the mechanism of antioxidant-mediated prevention of breast cancer. Female August Copenhagen Irish (ACI) rats were treated with E2, vitamin C, vitamin C + E2, BHA and BHA + E2 for up to 8 months. Superoxide dismutase 3 (SOD3) was suppressed in E2-exposed mammary tissues and in mammary tumors of rats treated with E2. This suppression was overcome by co-treatment of rats with E2 and vitamin C or BHA. 8-Hydroxydeoxyguanosine (8-OHdG) levels determined as a marker of oxidative DNA damage were higher in E2-exposed mammary tissues and in mammary tumors compared with age-matched controls. Vitamin C or BHA treatment significantly decreased E2-mediated increase in 8-OHdG levels in the mammary tissues and in MCF-10A cells. Increased DNA damage, colony and mammosphere formation, and migration in SOD3 knocked down MCF-10A cells, and nuclear translocation of SOD3 in vitamin C-treated mammary tissues and in MCF-10A cells suggest protective role of SOD3 against DNA damage and mammary carcinogenesis. Our studies further demonstrate that SOD3, but not SOD2 and SOD1, is induced by antioxidants and is regulated through NRF2. SOD3 may thus be an important gene in defense against oxidative stress and in the prevention of estrogen-mediated breast cancer.
Introduction
Long-term estrogen use has been associated with the initiation and development of breast cancer (1–5). The mechanisms of E2-induced breast carcinogenesis are, however, not clearly understood. In E2-induced breast carcinogenesis, oxidative stress produced by redox cycling between catechol estrogens and estrogen quinones is implicated to play an important role (6,7). Moreover, E2-quinones can also react with DNA to form depurinating adducts that are more likely to result in DNA mutations (6–8). During one electron oxidation of catechol estrogens to estrogen quinones, superoxide radicals are produced, that may be converted to hydrogen peroxide if sufficient superoxide dismutase (SOD) enzymes are available. Hydrogen peroxide is subsequently removed by cellular catalase and peroxidases (9). The SODs represent the major cellular defense system against superoxide radicals. In mammalian tissue, three isoforms of SODs have been identified: the cytoplasmic CuZnSOD (SOD1), the mitochondrial MnSOD (SOD2) and the extracellular SOD (EC-SOD or SOD3). SOD3 is mainly secreted into the extracellular space, but a smaller proportion is also found in plasma and other extracellular fluids (10–12).
Among all SODs, SOD3 is the least studied enzyme, but recent data support an important role for SOD3 in maintaining oxidative homeostasis in extracellular matrix and in nucleus as well (10,13). Disruption of the SOD3 gene in mice does not produce obvious pathologies under normal conditions, but these mice are more prone to environmental stressors (14,15). Moreover, very little is known about the regulation and potential of SOD3 in the prevention of E2-induced DNA damage and breast cancer. Transcription factor, nuclear factor erythroid 2-related factor 2 (NRF2), is the master regulator of the antioxidant response (16–19). NRF2-regulated phase II enzymes protect against the development of cancer by catalyzing reactions that convert highly reactive carcinogenic chemicals to less reactive products (16–19). Presence of an antioxidant responsive element (ARE) to which NRF2 binds has been reported in 5’-untranslated region of SOD3 gene (20). We have recently reported for the first time that two known prototypic antioxidants vitamin C (Vit C) and BHA can prevent E2-induced breast cancer in female ACI rats (2,5). This study was designed to examine the effects of antioxidants Vit C and BHA on SOD3 expression and role of SOD3 in prevention of oxidative DNA damage during breast carcinogenesis in an ACI rat model of breast cancer (21–24). In this study, we demonstrate that SOD3 plays an important role in the prevention of E2-induced oxidative DNA damage and breast carcinogenesis by antioxidants. This is the first direct evidence for the role of SOD3 in antioxidant-mediated prevention of DNA damage and regulation of SOD3 through NRF2.
Materials and methods
Treatment of animals
Female ACI rats (4 weeks of age; Harlan Sprague Dawley, Indianapolis, IN) were housed under controlled temperature, humidity and lighting conditions. After 1-week acclimatization period, rats were divided into following different groups: (i) Control, (ii) E2, (iii) BHA, (iv) BHA + E2, (v) Vit C and (vi) Vit C + E2. Rats were implanted subcutaneously with 3mg E2 pellets. E2 pellets were prepared in 17mg cholesterol as a binder as described previously (25,26). Control, Vit C and BHA groups received 17mg cholesterol pellet only. Vitamin C (1%) was administered in drinking water. BHA (0.7%) was fed to animals through phytoestrogen-free AIN76A diet (Dyets, Bethlehem, PA). Water was given ad libitum to all the animals. Each of the six treatment groups were divided into four subgroups, containing at least 10 rats in each subgroup. Each subgroup underwent treatments as described above for 7, 15, 120 or 240 days, respectively. At the end of the experimental time period, animals were anesthetized using isoflurane and euthanized. Mammary (both tumor and normal), uterus, lung, liver, spleen, heart, brain, thymus and kidney tissues were removed and snap frozen in liquid nitrogen for future analyses. Animal protocols used in this study were approved by the Institutional Animal Care and Use Committee.
Cell culture
Non-tumorigenic human breast epithelial cell line MCF-10A was purchased from American Type Culture Collection (ATCC, Manassas, VA). MCF-10A cells were grown in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (50:50) media (Mediatech, Herndon, VA). Twenty-four hours before treatment, cells were washed twice with phosphate-buffered saline (PBS) and then grown in phenol red-free DMEM/F12 (50:50) media supplemented with 5% charcoal-dextran stripped horse serum. Cells were treated with E2 (10 and 50nM), Vit C (250 µM and 1mM), BHA (250 µM), Vit C + E2 and BHA + E2 for 3 to 48h.
RNA interference
Small interfering RNAs (siRNAs) for NRF2, SOD3 and scrambled siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and Ambion (Austin, TX). MCF-10A cells were transfected with siSOD3 (5 nmol/l) or siNRF2 (20 nmol/l) using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA). Scrambled siRNA- (20 nmol/l) transfected MCF-10A cells were used as a negative control as described recently (24).
Real-time PCR analysis
Total RNA was isolated from ACI rat tissues using RNeasy lipid tissue kit (Qiagen, Valencia, CA) according to the supplier’s protocols. Five microgram total RNA was reverse transcribed using the superscript II reverse transcription system (Invitrogen). Real-time PCR was performed using rat-specific SOD3 QuantiTect primers using iCycler iQ5 system (Bio-Rad Laboratories, Hercules, CA). Data were analyzed from at least five different animals from each group. The expression of cyclophilin, a housekeeping gene, was used for quantification (27).
Western blot analysis
Approximately 50mg ACI rat tissues were homogenized in a tissue protein extraction buffer (T-PER; Thermo Scientific, Rockford, IL). MCF-10A cell lysates were prepared in RIPA buffer with protease inhibitor cocktail (Sigma–Aldrich, St Louis, MO). Nuclear and cytoplasmic fractions were prepared from mammary tumors, mammary tissues and MCF-10A cells using NE-PER nuclear and cytoplasmic extraction kit (Thermo Scientific) according to supplier’s protocol. The Pierce BCA Protein Assay kit was used to determine protein concentrations (Pierce, Rockford, IL). Eighty microgram total protein from ACI rat tissues or 30 µg protein from MCF-10A cells was size fractionated on a 12% sodium dodecyl sulfate–polyacrylamide gel, and transferred onto a polyvinylidene difluoride membrane (Millipore Corp., Billerica, MA) under standard conditions (27,28). SOD1, SOD2, SOD3 and NRF2 primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) were used individually for immunodetection. Chemiluminescent detection was performed using the BM Chemiluminescence Detection kit (Roche, Indianapolis, IN) and Alpha Innotech FluorChem HD2 (Alpha Innotech, San Leandro, CA) gel documentation system. Membranes were reprobed with α-Tubulin antibody (Santa Cruz Biotechnology) for total or cytoplasmic protein fractions and Lamin B antibody (Santa Cruz Biotechnology) for nuclear protein fractions using the methods described above. Intensities of the bands were quantified and normalized using AlphaEase FC StandAlone software (version 6.0.0.14; Alpha Innotech).
SOD3 activity assay
SOD3 enzymatic activity was determined using in-gel activity staining assay as described previously (29).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were performed with MCF-10A cells using ChIP Assay Kit (USB Corporation, Cleveland, OH) as described previously (24). Immunoprecipitation was done using NRF2 antibody (Santa Cruz Biotechnology); ChIP without any antibody served as a negative control. Primers spanning SOD3 ARE used for the end point real-time PCR amplification were as follows: forward primer 5′-GTCCTCTTCCGGCAGCTT-3′ and reverse primer 5′-GAACTGGTGCACGTGGATG-3′. Amplification of input chromatin before immunoprecipitation at a dilution of 1:50 was used as a positive control. Agarose gel electrophoresis and Ct (cycle threshold) values for the amplified products for ChIP DNA and input DNA samples were used to analyze the results.
Immunohistochemical analysis
Immunohistochemical analysis with tissue sections of 4–5 μm thickness were carried out as described earlier (23). Anti-NRF2 or anti-SOD3 antibodies (Santa Cruz Biotechnology) were used for immunostaining. Tissue sections from at least three different animals in each group were used for immunostaining. Images were obtained at 100× using a Leica DMI 3000B microscope (Leica Microsystems Inc, Bannockburn, IL).
8-OHdG estimation
8-OHdG, an accepted marker of oxidative stress-mediated DNA damage, was estimated in E2-exposed ACI rat mammary tissues, mammary tumors and MCF-10A cells in the presence or absence of Vit C and BHA using Oxiselect oxidative DNA damage ELISA kit (Cell Biolabs, San Diego, CA) as described previously (24).
Colony formation assay
Five hundred viable siSOD3 or scrambled siRNA-transfected MCF-10A cells after 48h of transfection or -untransfected MCF-10A cells were seeded in 6-well plates and allowed to grow for 24h in phenol red-free complete media. The cells were then incubated in the presence or absence of E2 for 72h, washed in PBS and incubated for an additional 8 days in complete medium. The colonies obtained were washed with PBS and fixed in 10% formalin for 10min and again washed twice with PBS followed by staining with crystal violet (0.1% wt/vol solution in 10% ethanol). The colonies were counted, photographed and compared with respective untreated cells. Each treatment was done in triplicate.
Mammosphere formation assay
Mammosphere formation assay was carried out in ultra low attachment plates (Corning, Lowell, MA). Briefly, 5000 viable siSOD3 or scrambled-transfected MCF-10A cells after 48h of transfection or -untransfected MCF-10A cells were seeded into a 24-well plate. Cells were grown in serum-free DMEM/F12 (50:50) medium supplemented with 1× B27 (Invitrogen), 20ng/ml epidermal growth factor (Invitrogen), 20ng/ml basic fibroblast growth factor (Invitrogen), 1 µg/ml hydrocortisone (BD Biosciences, Bedford, MA), 5 µg/ml insulin (Invitrogen), penicillin/streptomycin (Lonza, Walkersville, MD) and 4 µg/ml heparin calcium salt (Thermo Scientific) at 37°C under 5% CO2 in the presence or absence of E2 (10nM). After 6–8 days of incubation, mammospheres were viewed under the microscope and photographed. Three replicate wells from a 24-well plate were used for each experimental condition.
Cell migration assay
Cell migration assay was used to study the metastatic potential of the cells. Briefly, a confluent monolayer of siSOD3 or scrambled-transfected MCF-10A cells or -untransfected MCF-10A cells was established and then a scratch was made through the monolayer, using a standard 200 µl plastic pipet tip, washed twice with PBS, and replaced in phenol red-free complete media. The cells were then incubated in the presence or absence of E2. Cells migrate into the scratch area as single cells from the confluent sides. After 12h of E2 treatment, the width of the scratch gap was viewed under the microscope and photographed. Three replicate wells from a 6-well plate were used for each experimental condition.
Statistical analyses
Statistical analyses were performed by using Sigma Plot 11.0 (Systat Software, San Jose, CA) and IBM SPSS Statistics 19 software (IBM Inc, Armonk, NY). One-way analysis of variance and least significant difference post hoc analysis was used to calculate P values for comparisons of SOD3, SOD2 and SOD1 protein expression and 8-OHdG levels among all groups of treated animals and MCF-10A cells. The unpaired t-test analysis was used to calculate P values for comparisons of SOD3 mRNA levels and SOD3 enzymatic activity between treated animals and respective age-matched controls. Fisher’s exact test was used to compare tumor incidence between two treatment groups. A P value <0.05 was considered significant.
Results
Estrogen treatment decreases, whereas antioxidants increase SOD3 expression
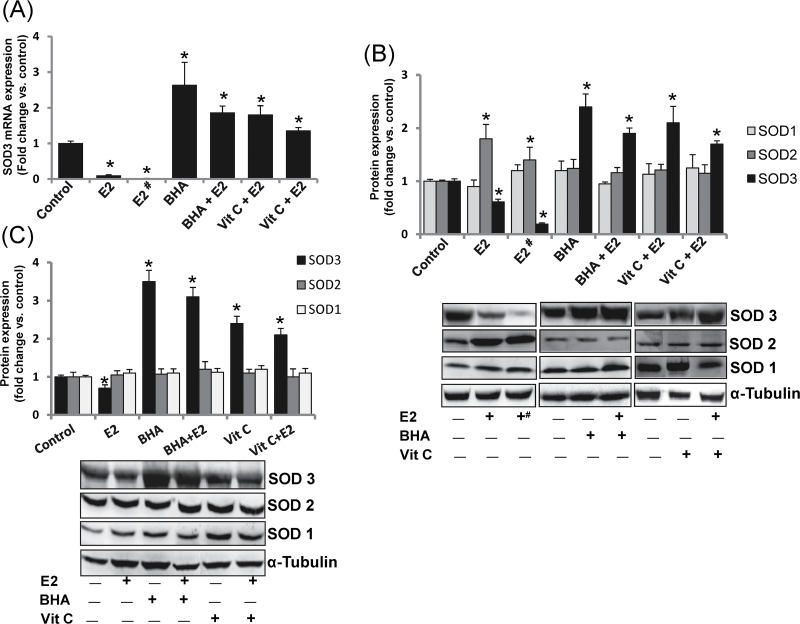
To investigate the effect of E2 on expression of antioxidant gene SOD3, we estimated the mRNA levels of SOD3 in mammary tissues and mammary tumors of female ACI rats treated with Vit C or BHA in the presence or absence of E2 for 8 months. SOD3 mRNA levels were significantly decreased in E2-treated mammary tissues and mammary tumors and significantly increased with antioxidant treatment compared with control mammary tissues (Figure 1A). The effect of Vit C and BHA on protein expression levels of all the three members of the SOD family (SOD1, SOD2 and SOD3) in the rat mammary tissues was determined. SOD3 protein expression was significantly decreased in mammary tissues and in mammary tumors of rats treated with E2 for 240 days and its expression was reverted and significantly increased upon treatment with Vit C or BHA alone or in combination with E2 (Figure 1B). The fold changes in SOD3 protein expression in mammary tissue of E2, BHA, BHA + E2, Vit C and Vit C + E2 treatment groups were 0.61, 2.4, 1.94, 2.11 and 1.73, respectively, after 240 days of treatment compared with sham-operated age-matched controls (Figure 1B and Table I). The fold change in SOD3 protein expression in mammary tumors was 0.19 compared with sham-operated age-matched controls (Figure 1B and Table I). Protein expression of SOD1 remained unchanged in mammary tumors and mammary tissues of rats treated with E2, BHA or Vit C compared with control mammary tissues (Figure 1B). However, SOD2 protein expression significantly increased in E2-treated mammary tissues and mammary tumors but co-treatment with antioxidants reverted it back to the base levels (Figure 1B). We also observed decreased protein expression of SOD3 in MCF-10A cells after 24h of E2 treatment and significant increase in its expression after treatment with antioxidants BHA or Vit C (Figure 1C). The protein expression of SOD1 and SOD2 remained unchanged in MCF-10A cells that underwent the same treatment (Figure 1C).
Fig. 1.
Antioxidants reverse E2-mediated decrease in SOD3 mRNA and protein expression. (A) SOD3 mRNA expression in E2-exposed mammary tumors and in mammary tissues of rats treated with E2, BHA or Vit C for 240 days. (B) SOD3, SOD2 and SOD1 protein expression in E2-exposed mammary tumors and in mammary tissues of rats treated with E2, BHA or Vit C for 240 days. The bar graph represents fold change in SOD3, SOD2 and SOD1 protein expression (mean ± SEM) in mammary tumors and in mammary tissues from at least three different animals compared with age-matched control mammary tissues. (C) Protein expression of SOD3, SOD2 and SOD1 in MCF-10A cells treated with E2 (10nM), BHA (250 µM) or Vit C (1mM) for 24h. The bar graph represents fold change in SOD3, SOD2 and SOD1 protein expression (mean ± SEM) in MCF-10A cells from at least three different replicates from three individual experiments compared with vehicle-treated MCF-10A cells. The symbols ‘E2#’ or ‘+#’ indicate E2-induced mammary tumor tissues and the symbol ‘*’ indicates P value <0.05 compared with respective controls.
Table I.
SOD3 enzyme activity, protein expression, 8-OHdG levels and tumor incidence in female ACI rats after different treatments
| Treatment | SOD3 enzyme activity (U/mg protein) | SOD3 protein expression (fold change versus control) | 8-OHdG levels (fold change versus control) | Tumor incidence (%) |
|---|---|---|---|---|
| Control | 3.45±0.18 | 1.00 | 1.00 | 0 |
| E2 | 2.12±0.31* | 0.61* | 1.79* | 82 |
| Tumor | 1.07±0.44* | 0.19* | 2.98* | |
| BHA | 6.54±0.45* | 2.4* | 0.89 | 0 |
| BHA + E2 | 5.72±0.28*,** | 1.94*,** | 1.23** | 24** |
| Vit C | 6.75±0.31* | 2.11* | 0.95 | 0 |
| Vit C + E2 | 6.18±0.18*,** | 1.73*,** | 1.02** | 29** |
SOD3 enzyme activity, SOD3 protein expression and DNA 8-OHdG levels were measured in mammary tumors and in mammary tissues from control, E2-, BHA-, BHA + E2-, Vit C- and Vit C + E2-treated rats after 240 days of treatment. Column one lists the different treatments each group of animals received. Column two shows the SOD3 enzyme activity as an average of values obtained for at least five different animals ± SEM. Columns three and four show fold change in SOD3 protein expression and DNA 8-OHdG levels, respectively, compared with age-matched control tissue. The percentage tumor incidence in each treatment group after 240 days of treatment is listed in column five.
* P < 0.05 compared with respective age-matched control tissue.
**P < 0.05 compared with E2-treated group.
Estrogen-mediated decrease in SOD3 protein expression is time-dependent, whereas antioxidants constitutively increase its expression
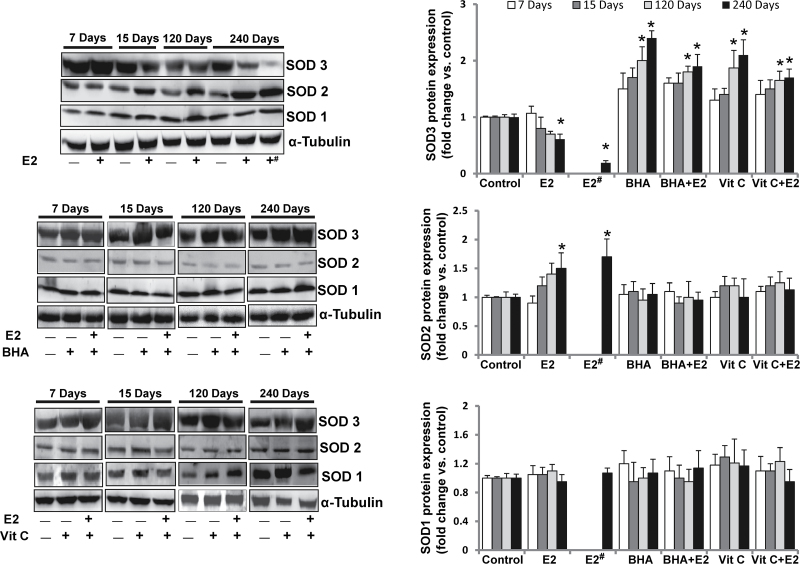
We have demonstrated that although E2 decreased SOD3 protein expression, Vit C or BHA significantly increased its expression in mammary tissues after 8 months of treatment (Figure 1B). To investigate the status of SOD3 during the course of breast carcinogenesis in ACI rats, we also examined the protein expression of SOD3 during early, preneoplastic and neoplastic stages, respectively (1,21,22). Western blot analysis demonstrated that protein expression of SOD3 after 7 days of E2 treatment did not change compared with respective control mammary tissue but its levels started decreasing after 15 days of treatment, this trend of decreased expression continued after 120 days and SOD3 protein expression significantly decreased after 240 days of E2 treatment (Figure 2). SOD3 protein expression was also significantly decreased in mammary tumors compared with sham-operated age-matched control mammary tissues (Table I). Time course studies with antioxidants revealed that the increase in mammary SOD3 protein expression started as early as after 7 days of treatment (Figure 2) and it increased significantly compared with sham-operated age-matched controls after 120 and 240 days of treatment (Figure 2).
Fig. 2.
E2 inhibits, whereas BHA and Vit C increase only SOD3 protein expression in a time-dependent fashion. Representative western blots are presented to show SOD3, SOD2 and SOD1 protein expression in mammary tumors and in mammary tissues of rats treated with E2, BHA or Vit C in presence or absence of E2 for 7, 15, 120 and 240 days. The bar graph represents SOD3, SOD2 and SOD1 protein fold change (mean ± SEM) in mammary tumors and in mammary tissues from at least three different animals compared with age-matched control mammary tissues. The symbols ‘E2#’ or ‘+#’ indicate E2-induced mammary tumor tissues and the symbol ‘*’ indicates P value <0.05 compared with respective age-matched control mammary tissue.
To examine whether antioxidant-mediated change in protein expression of SOD3 is tissue specific, we also analyzed SOD3 protein expression in different tissues of ACI rats treated with BHA or Vit C in the presence or absence of E2 for 240 days. Protein expression of SOD3 was detected in all the organs analyzed with variable levels of expression (Supplementary Figure 1, available at Carcinogenesis Online). Estrogen treatment significantly decreased protein expression of SOD3 in brain and uterus and BHA increased its expression in brain, heart, kidney and lung compared with respective age-matched control tissues, whereas Vit C treatment increased SOD3 protein expression in heart only (Supplementary Figure 1, available at Carcinogenesis Online).
Antioxidants do not increase SOD1 and SOD2 protein expression
E2, Vit C or BHA treatments did not alter SOD1 protein expression, whereas SOD2 protein expression was increased in E2-treated mammary tumors and mammary tissue compared with age-matched control mammary tissue after 240 days of treatment (Figure 1B). Protein expression of SOD1 and SOD2 in mammary tissues did not change with E2 or antioxidant treatments compared with respective age-matched control mammary tissues after 7, 15 and 120 days of treatment (Figure 2). Although SOD2 protein expression in mammary tissues and mammary tumors increased after 240 days of E2 treatment, the protein expression of SOD1 did not change (Figure 2). Antioxidant treatments did not increase SOD2 levels compared with controls (Figure 2).
E2 decreases and antioxidants increase SOD3 enzyme activity
SOD3 enzyme activity was evaluated in protein samples from the mammary tumors and mammary tissues of rats treated with E2, BHA, BHA + E2, Vit C and Vit C + E2 for 240 days and from age-matched controls. SOD3 activity was significantly decreased in mammary tumors and mammary tissue of rats treated with E2 compared with age-matched control mammary tissue (Table I). Consistent with protein expression data, a significant increase in SOD3 enzyme activity was detected in mammary tissues of rats treated with BHA, BHA + E2, Vit C or Vit C + E2 for 240 days compared with age-matched control mammary tissues (Table I).
Antioxidants upregulate SOD3 via NRF2-dependent pathway
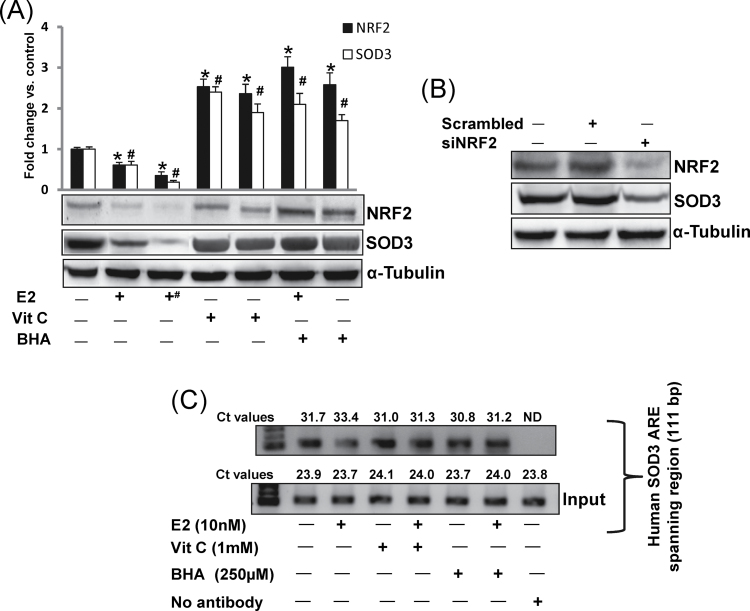
NRF2 is a known regulator of the antioxidant response (16–18,30). We examined whether antioxidants BHA and Vit C upregulate SOD3 expression in mammary tissues in a NRF2-dependent fashion. We observed significant decrease in NRF2 protein expression in mammary tumors and mammary tissues of rats treated with E2 for 240 days (Figure 3A). Vitamin C or BHA treatment significantly increased NRF2 protein expression (Figure 3A). In a similar manner, a significant decrease in SOD3 protein expression in E2-treated mammary tissues and mammary tumors, and increase in SOD3 protein expression in Vit C and BHA-treated mammary tissues was demonstrated (Figure 3A). A decrease in SOD3 protein expression was also observed after silencing of NRF2 in MCF-10A cells (Figure 3B). To further confirm whether inhibition of SOD3 after E2 treatment and its upregulation after antioxidant treatment was through differential binding of NRF2 to SOD3 promoter ARE, we carried out ChIP assay using MCF-10A cells. Following chromatin immunoprecipitation using anti-NRF2 antibody, DNA was recovered and subjected to real-time PCR analysis using PCR primers flanking the ARE of the human SOD3 gene promoter. Estrogen treatment inhibited the binding of NRF2 to the SOD3 gene promoter as shown by increase in Ct values, whereas antioxidants Vit C and BHA reversed E2-mediated inhibition by enhancing NRF2 binding to the SOD3 promoter as shown by decrease in Ct values compared with control (Figure 3C).
Fig. 3.
Antioxidants upregulate SOD3 via NRF2-dependent pathway. (A) Representative western blots are presented to show NRF2 and SOD3 protein expression in E2-exposed mammary tumors and in mammary tissues of rats treated with E2, Vit C or BHA for 240 days. The bar graph represents NRF2 and SOD3 protein fold change (mean ± SEM) in mammary tumors and in mammary tissues from at least three different animals compared with age-matched control mammary tissues. The symbol ‘+#’ indicates E2-induced mammary tumor tissues. The symbol ‘*’ indicates P value <0.05 for NRF2 protein expression compared with respective control mammary tissues, whereas the symbol ‘#’ indicates P value <0.05 for SOD3 protein expression compared with respective control mammary tissues. (B) MCF-10A cells were transfected with 20 nmol/l of scrambled siRNA or siNRF2 for 48h and western blot analyses were carried out using NRF2 antibody. Same membrane was reprobed with SOD3 and α-tubulin antibody. (C) MCF-10A cells were treated with Vit C (1mM) or BHA (250 μM) in the presence or absence of E2 (10nM), and vehicle for 3h, fixed with formaldehyde, cross-linked and chromatin sheared. The chromatin was immunoprecipitated with NRF2 antibody. NRF2 binding to ARE region of SOD3 promoter was analyzed by real-time PCR with specific primers for the human SOD3 ARE region. The ARE region of the SOD3 promoter was also amplified from 3 μl of purified soluble chromatin before immunoprecipitation to show input DNA. Representative ChIP agarose gels and real-time PCR Ct values from three independent experiments are shown (ND = not detected).
Antioxidant increases nuclear translocation of NRF2 and SOD3
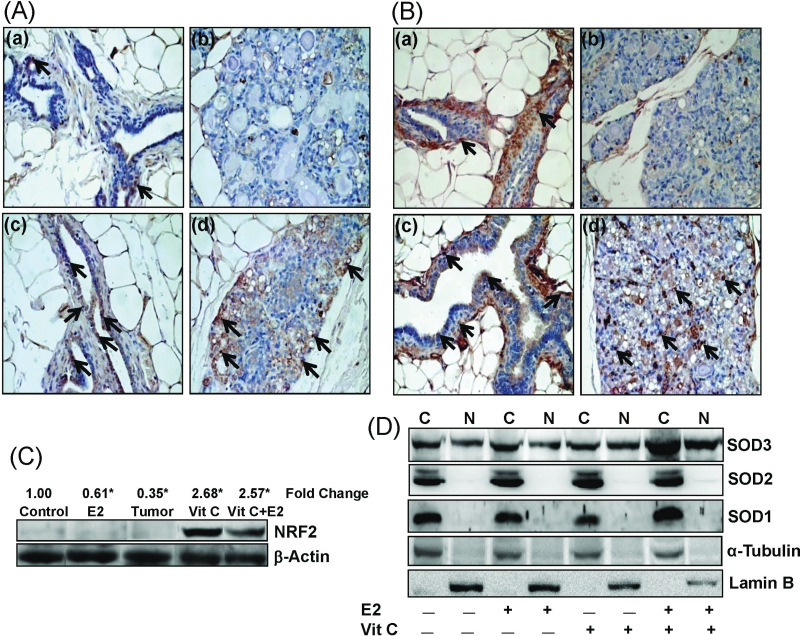
To examine the nuclear and cytoplasmic protein expression of NRF2 and SOD3, immunohistochemical analysis was carried out with control, E2, Vit C and Vit C + E2-treated mammary tissue sections from 240-days-treated rats. Nuclear as well as cytoplasmic expression of NRF2 was decreased in E2-treated mammary tissues (Figure 4A-b). Vitamin C treatment alone or in combination with E2 increased cytoplasmic as well as nuclear expression of NRF2 (Figure 4A-c, and d) compared with control mammary tissue (Figure 4A-a). Similarly, extracellular expression of SOD3 was decreased in E2-treated mammary tissues (Figure 4B-b), whereas Vit C treatment with or without E2 increased extracellular as well as nuclear expression of SOD3 (Figure 4B-c, and d) compared with age-matched control mammary tissues (Figure 4B-a).
Fig. 4.
Antioxidant increases NRF2 and SOD3 protein expression and nuclear translocation. (A and B) Formalin-fixed/paraffin-embedded mammary tissue sections from rats treated with E2, Vit C and Vit C + E2 for 240 days were immunostained with NRF2 (A) or SOD3 (B) antibody. Nuclear expression of NRF2 and SOD3 is shown in representative sections (arrows). (a) The mammary tissue of a representative control ACI rat, (b) E2-treated mammary tissue, (c) Vit C-treated mammary tissue and (d) Vit C + E2-treated mammary tissue. 100× magnification. (C) Nuclear fractions were prepared from tissues and used for western blot analysis. Intensities of the bands were quantified and normalized to β-actin. Representative western blot is presented to show increased nuclear translocation of NRF2 in Vit C and Vit C + E2-treated mammary tissues. Fold change in nuclear NRF2 expression compared with age-matched control mammary tissue is shown at the top. The symbol ‘*’ indicates P value <0.05 compared with control mammary tissue. (D) MCF-10A cells were treated with Vit C (1mM) in the presence or absence of E2 (10nM) for 3h. Nuclear and cytoplasmic fractions were prepared as described in Materials and methods. Representative western blots are presented to show the cytoplasmic and nuclear expression of SOD3, SOD2 and SOD1. C and N denote cytoplasmic and nuclear protein fractions, respectively.
To further determine the effect of Vit C on nuclear translocation of NRF2, we carried out western blot analyses of mammary tumors and mammary tissues of rats treated with E2, Vit C and Vit C + E2 for 240 days. Although nuclear translocation of NRF2 was significantly decreased in E2-treated mammary tissues and mammary tumors, Vit C and Vit C + E2 treatments significantly increased nuclear translocation of NRF2 (Figure 4C). Since SOD3 was the only SOD that was significantly increased after treatment with antioxidants, we examined whether nuclear translocation of SOD3 also increased after treatment with antioxidant Vit C. In western blot analysis, we observed that SOD3 was the only SOD that was translocated to the nucleus (Figure 4D). SOD1 and SOD2 proteins were present only in the cytoplasmic fractions and were absent in the nuclear fractions of the cells from all the treatment groups (Figure 4D). Moreover, Vit C + E2 treatment increased SOD3 protein expression in cytoplasmic as well as nuclear fractions (Figure 4D).
Role of SOD3 in protection against E2-mediated carcinogenic process
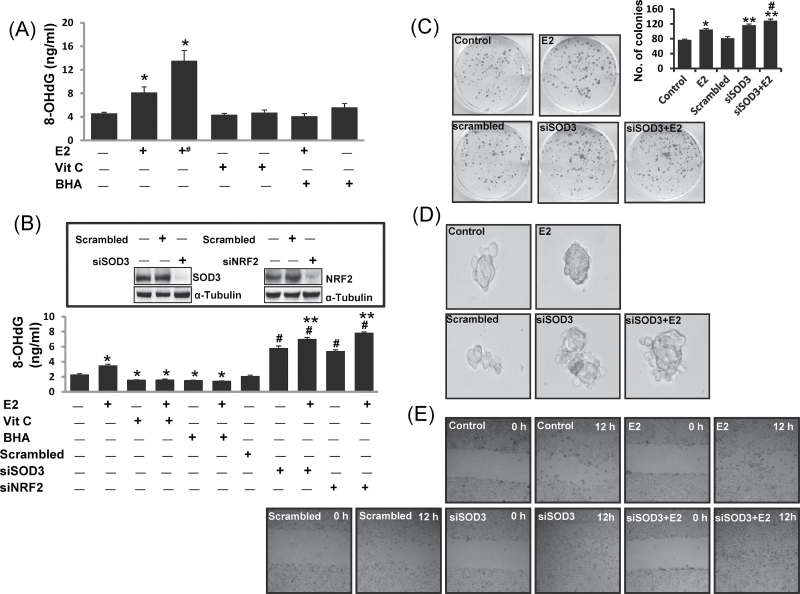
To demonstrate the protective role of SOD3 against E2-mediated carcinogenic process, we performed DNA damage, colony formation, mammosphere formation and cell migration assays after silencing SOD3 in MCF-10A cells. DNA 8-OHdG levels were quantified as a marker of oxidative DNA damage in mammary tissues as well as in MCF-10A cell line. 8-OHdG levels were also quantified in SOD3 as well as in NRF2 knocked down MCF-10A cells. About 2- and 3-fold increase (P <0.05) in 8-OHdG levels were demonstrated in E2-treated mammary tissue and mammary tumors, respectively, compared with control mammary tissue (Figure 5A and Table I). No significant difference in 8-OHdG levels was detected in mammary tissue DNA samples from Vit C-, Vit C + E2-, BHA- and BHA + E2-treated groups (Figure 5A). Treatment of MCF-10A cells with E2 for 48h significantly increased 8-OHdG levels compared with vehicle-treated controls (Figure 5B). However, 8-OHdG levels were significantly decreased after treatment of MCF-10A cells with Vit C, Vit C + E2, BHA or BHA + E2 for 48h relative to vehicle-treated cells (Figure 5B). Furthermore, a significant increase in 8-OHdG levels in siSOD3 or siNRF2-transfected MCF-10A cells compared with scrambled siRNA-transfected MCF-10A cells was demonstrated (Figure 5B). Moreover, treatment with E2 further increased 8-OHdG levels in siSOD3, and siNRF2-transfected MCF-10A cells compared with 8-OHdG levels in these cells in the absence of E2 treatment (Figure 5B). Estrogen treatment also increased the number of colonies; mammosphere formation and migration of MCF-10A cells compared with vehicle control (Figure 5C–E). Silencing of SOD3 in MCF-10A cells significantly increased the number of colonies formed in presence or absence of E2 compared with scrambled control (Figure 5C). Similarly, SOD3 silencing in MCF-10A increased the mammosphere formation as well as migration of the cells (Figure 5D and 5E).
Fig. 5.
SOD3 protects against E2-induced oxidative DNA damage and mammary carcinogenesis. Female ACI rats were treated with E2, Vit C, Vit C + E2, BHA and BHA + E2 for 240 days as described in the Materials and methods. 8-OHdG levels were quantified in mammary tumors and in mammary tissues from rats as well as in MCF-10A cells treated with the above-mentioned chemicals. siSOD3- or siNRF2-transfected MCF-10A cells were also treated with E2 (50nM) for 48h and 8-OHdG levels were quantified. (A) Levels of 8-OHdG in mammary tumors and in mammary tissues from different treatment groups. These data are reported as an average of values obtained for at least six different animals ± SEM. The symbol ‘+#’ indicates 8-OHdG levels in E2-induced mammary tumor tissues. (B) Levels of 8-OHdG in MCF-10A cells treated with Vit C, and BHA in presence or absence of E2 and in SOD3 and NRF2 knocked down MCF-10A cells. Inhibition of SOD3 and NRF2 protein expression in siSOD3 or siNRF2-transfected MCF-10A cells is shown in the inset. The symbols ‘*’ and ‘#’ indicate P value <0.05 compared with control mammary tissues and scrambled-transfected MCF-10A cells, respectively, whereas the symbol ‘**’ indicates significant difference (P < 0.05) between siSOD3 and siSOD3 + E2 and between siNRF2 and siNRF2 + E2 groups. (C) Five hundred parental MCF-10A cells and scrambled-transfected or siSOD3-transfected MCF-10A cells were seeded in each well of 6-well plate and treated in presence or absence of E2 (10nM) for 72h. At the end of incubation period, colonies were photographed and counted. The bar graph is derived from the colonies counted and represent mean ± SEM. The symbols ‘*’ and ‘**’ indicate P value <0.05 compared with vehicle-treated MCF-10A cells and scrambled-transfected MCF-10A cells, respectively. (D) Parental MCF-10A cells and scrambled-transfected or siSOD3-transfected MCF-10A cells were seeded in suspension and treated in presence or absence of E2 (10nM) for 8 days. Pictures of mammosphere formed in suspension were taken by a microscope. (E) Photomicrographs demonstrating the results of the in vitro migration of parental MCF-10A cells, scrambled-transfected or siSOD3-transfected MCF-10A cells using the simple scratch technique. Cells were grown in monolayer, scratched and incubated in presence or absence of E2 (10nM) for 12h.
Discussion
Advances in the field of cancer prevention have defined some of the cellular and molecular events associated with the carcinogenic process (31–36). A large number of experimental, epidemiological and clinical data provide new approaches for cancer prevention (35,36). Induction of antioxidant enzyme(s) is one such approach that can be used to inhibit the overburden of oxidative stress in the carcinogenic process (1,2,5,37). Estrogens have been implicated in the development of breast cancer (25,33,34). However, the exact mechanisms by which estrogens exert their carcinogenic effects remain elusive. A growing body of clinical and epidemiological literature, our studies and those of others strongly support the role of estrogen metabolism-mediated oxidative stress in estrogen-induced breast carcinogenesis (1,2,5,25,33). Metabolic redox cycling between catechol estrogens and their corresponding quinones generates oxidative stress and potentially harmful superoxide radicals (31,32). Superoxide radicals thus produced may be converted to hydrogen peroxide if sufficient SOD enzymes are available. Out of the three SODs identified in mammalian tissues, SOD3 is the least studied enzyme, but recent data support an important role for SOD3 in maintaining oxidative homeostasis in extracellular matrix and other extracellular spaces as well as protection against oxidative damage to nuclear DNA and proteins (13,38). SOD3 contains a C-terminal, heparin-binding domain as well as one potential site for N-linked glycosylation (39,40). Once produced and secreted into the extracellular space, SOD3 binds to cell surfaces and extracellular matrix via heparin-binding domain (41). Ookawara et al. (2002) have suggested that the heparin-binding domain of SOD3 functions as the nuclear localization signal (13).
Very little information is available about the role of SOD3 in prevention of E2-induced breast cancer. We have recently reported for the first time that antioxidants Vit C and BHA can prevent E2-induced breast cancer in female ACI rats (2,5). In this study, we investigated the role of SOD3 in antioxidant-mediated prevention of oxidative DNA damage and subsequent breast carcinogenesis. We have demonstrated significant suppression of SOD3 mRNA and protein expression following long-term E2 treatment (Figure 1A and 1B) and this suppression was reversed upon co-treatment with Vit C or BHA (Figure 1A and 1B). Significant increase in SOD3 protein expression but not that of SOD1 or SOD2 following treatment with Vit C or BHA suggest that SOD3 might be the only SOD that is positively regulated by antioxidants in mammary tissues (Figure 1B). Furthermore, decrease in E2-mediated oxidative stress and inhibition of breast cancer by antioxidants Vit C or BHA (Table I) further support the importance of antioxidant enzyme SOD3 in prevention of E2-induced breast cancer (2,5). SOD2 protein expression was increased in E2-treated mammary tissue and mammary tumors after 240 days of treatment, but its expression remained unchanged after Vit C or BHA co-treatment with E2, compared with control mammary tissues (Figures 1B and 2). Expression of SOD2 has been shown to be regulated by specificity protein 1 (Sp1) and activating protein-2 (AP-2) transcription factors (42). These two transcription factors have opposite effects on SOD2 expression: although the Sp1 positively promotes transcription, the AP-2 proteins significantly repress its promoter activity (42,43). Estrogen treatment-mediated repression of AP-2 in breast cancer cells is known (44). Therefore, E2-mediated repression of AP-2 a negative regulator of SOD2 might be one of the possible reasons for overexpression of SOD2 in our study. Computer analysis reveals presence of Sp1 but absence of AP-2 binding regions in SOD3 promoter region. Moreover, we could detect NRF2 binding ARE regions only in SOD3 promoter and not in SOD2 promoter region. Overexpression of SOD2 in tumors has been shown to be associated with an increased frequency of tumor invasion and metastasis, whereas SOD3 has been shown to have protective characteristics against tumorigenesis (41,45,46). Thus, in our study overexpression of SOD2 and decreased expression of SOD3 in E2-treated mammary and mammary tumor tissues reveals that both overexpression of SOD2 and suppression of SOD3 may augment tumorigenesis induced by estrogen. Both Vit C and BHA specifically induced SOD3 and not SOD1 or SOD2 in mammary tissues as well as in MCF-10A cells (Figures 1B and 1C and 2). Therefore, we further investigated the role of SOD3 in prevention of oxidative DNA damage and mechanism of its regulation.
17β-Estradiol-mediated progressive decrease in SOD3 protein level was evident in the breast after 15 days of exposure and was significantly decreased both in the mammary tissues as well as in the mammary tumors after 240 days of E2 exposure (Figure 2). Vitamin C- or BHA-mediated increase in mammary SOD3 protein levels was evident as early as 7 days of treatment and significantly increased after 120 and 240 days of treatment compared with age-matched control mammary tissues (Figure 2). We have previously reported proliferative change in the breast as early as 7 or 15 days of E2 exposure, a progression from normal mammary tissue to proliferative tissue such as atypical ductal hyperplasia, later progressing to tumor formation and malignancy (1). During these early stages (7 and 15 days) and during preneoplastic stage (120 days), most of the changes including genetic instability responsible for the initiation of carcinogenesis occur in the mammary tissue (21,47). Our laboratory and others have previously reported that tumors appear in this rat model after 4–5 months of E2 treatment (1,21,22). In this study, time-dependent decrease in SOD3 protein expression after E2 treatment and corresponding increase after Vit C or BHA treatment indicates a possible protective role of SOD3 during mammary carcinogenesis. We have also observed a parallel increase in SOD3 enzymatic activity in mammary tissues following Vit C or BHA treatment for 240 days (Table I). Moreover, in E2-treated mammary tissues and mammary tumors, both SOD3 protein expression and activity were significantly decreased compared with controls (Figure 1B and Table I). Differential expression of SOD3 protein in some organs in response to E2, BHA and Vit C indicates an organ-specific regulation of this enzyme that needs to be further investigated (Supplementary Figure 1, available at Carcinogenesis Online).
Previous studies have shown epigenetic regulation of antioxidant gene SOD3 (48–51). NRF2-mediated regulation of several antioxidant enzymes has also been previously established (16–19). Presence of an ARE region to which NRF2 binds has been demonstrated in 5′-untranslated region of SOD3 gene (20). Therefore, we examined whether the regulation of SOD3 in E2-induced breast cancer is mediated through transcription factor NRF2 and have identified regulation of SOD3 via NRF2-dependent pathway. Decreased protein expression of NRF2 and SOD3 in E2-treated mammary tissues and mammary tumor tissues after 240 days of treatment and corresponding increased expression of these proteins after BHA or Vit C treatment suggested NRF2-mediated regulation of SOD3 (Figure 3A). Decreased protein expression of SOD3 in NRF2 knocked down MCF-10A cells further suggested NRF2-mediated regulation of SOD3 (Figure 3B). Results from ChIP assay with MCF-10A cells treated with BHA or Vit C alone or in combination of E2 further confirmed E2-mediated decreased and antioxidant-mediated increased binding of NRF2 to the ARE region of SOD3 promoter (Figure 3C). Collectively, our results provide direct evidence of NRF2-mediated regulation of SOD3 in E2-induced breast carcinogenesis.
During homeostatic conditions, NRF2 remains in bound form with Kelch-like ECH-associated protein 1 (Keap1) in cytoplasm (17,18). Keap1 regulates the nuclear translocation of NRF2 and thus activation of NRF2-regulated genes (17,18). Immunohistochemical and western blot studies using mammary tissues of ACI rats confirmed E2-mediated decrease in NRF2 protein expression in cytoplasm and nuclear localization of NRF2, and Vit C-mediated increased cytoplasmic expression as well as increased nuclear localization of NRF2 (Figure 4A and 4C). Estrogen is known to decrease NRF2 expression and function (52). Ansell et al. have shown that E2-liganded estrogen receptor-alpha represses NRF2-regulated transcription of phase II genes by repressing transcription of NRF2 (52). Furthermore, our unpublished in vivo data suggest that E2 treatment increases expression of microRNA-93 that binds to 3′-untranslated region of NRF2 and inhibits its protein expression, whereas antioxidant Vit C increases NRF2 protein expression through inhibition of microRNA-93. Vitamin C not only increased nuclear translocation of NRF2 and thus NRF2-regulated SOD3 protein expression but also increased translocation of SOD3 to the nucleus (Figure 4B and 4D). Presence of nuclear localization signals in SOD3 that helps it in its nuclear localization has been demonstrated previously (13). Our observation that SOD3 is the only SOD that was upregulated and translocated to nucleus after Vit C treatment further suggests the importance of SOD3 against nuclear oxidative stress and oxidative DNA damage (Figure 4D).
Estrogen treatment has been shown to induce oxidative stress and DNA damage in mammary tissue during tumorigenic process (1–3,5). We demonstrated significant increase in 8-OHdG levels in E2-treated mammary tissues and mammary tumors and a pattern of decreased 8-OHdG levels after antioxidant treatment compared with age-matched control mammary tissue (Figure 5A and Table I). These studies suggest a protective role of antioxidants against oxidative DNA damage. The 8-OHdG levels in mammary tissues after 240 days of BHA or Vit C treatment were inversely correlated with expression of SOD3 protein in mammary tissues and thus indicate a possible role of SOD3 in prevention of oxidative DNA damage in mammary tissues (Figures 1A, 12 and 5A). In vitro data with MCF-10A cells also demonstrated significant decrease (P < 0.05) in 8-OHdG levels upon Vit C or BHA treatments compared with vehicle-treated controls (Figure 5B). Significantly increased 8-OHdG levels (P < 0.05) in SOD3 knocked down MCF-10A cells compared with vehicle, scrambled or E2-treated parental MCF-10A cells further confirmed the role of SOD3 in prevention of estrogen-induced DNA damage (Figure 5B). Similarly, significant increase in 8-OHdG levels in NRF2 knocked down MCF-10A cells compared with vehicle, scrambled- or E2-treated parental MCF-10A cells confirmed the protective role of NRF2 against oxidative DNA damage (Figure 5B). Significant increased levels of 8-OHdG in siSOD3 or siNRF2-transfected cells that were treated with E2 compared with siSOD3- or siNRF2-transfected cells without E2 treatment confirmed that the increase in 8-OHdG levels was specific to E2-induced oxidative damage (Figure 5B). Increased colony formation, cell migration and mammosphere formation in SOD3 knocked down MCF-10A cells compared to scrambled- or vehicle-treated MCF-10A cells confirmed the anticarcinogenic role of SOD3 (Figure 5C–E). Overall, these findings support the role of SOD3 in prevention of mammary carcinogenesis. Our study provides the first direct evidence for the role of SOD3 in antioxidant-mediated prevention of DNA damage and regulation of SOD3 through NRF2. SOD3 may thus have a potential in the development of therapeutic strategies for the prevention of estrogen-induced breast neoplasia.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health Grant (CA 109551); University of Missouri Research Board Grant (HKB).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- 8-OHdG
8-Hydroxydeoxyguanosine
- AP-2
activating protein-2
- ARE
antioxidant responsive element
- BHA
butylated hydroxyanisole
- ChIP
chromatin immunoprecipitation
- DMEM
Dulbecco’s modified Eagle’s medium
- NRF2
nuclear factor erythroid 2-related factor 2
- PBS
phosphate-buffered saline
- siRNAs
small interfering RNAs
- SOD
superoxide dismutase
References
- 1. Mense S.M., et al. (2008). Estrogen-induced breast cancer: alterations in breast morphology and oxidative stress as a function of estrogen exposure. Toxicol. Appl. Pharmacol. 232 78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mense S.M., et al. (2009). Vitamin C and alpha-naphthoflavone prevent estrogen-induced mammary tumors and decrease oxidative stress in female ACI rats. Carcinogenesis 30 1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montano M.M., et al. (2007). Protective roles of quinone reductase and tamoxifen against estrogen-induced mammary tumorigenesis. Oncogene 26 3587–3590 [DOI] [PubMed] [Google Scholar]
- 4. Singh B., et al. (2010). Dietary quercetin exacerbates the development of estrogen-induced breast tumors in female ACI rats. Toxicol. Appl. Pharmacol. 247 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh B., et al. (2009). Antioxidant butylated hydroxyanisole inhibits estrogen-induced breast carcinogenesis in female ACI rats. J. Biochem. Mol. Toxicol. 23 202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liehr J.G., et al. (1986). Carcinogenicity of catechol estrogens in Syrian hamsters. J. Steroid Biochem. 24 353–356 [DOI] [PubMed] [Google Scholar]
- 7. Yager J.D. (2000). Endogenous estrogens as carcinogens through metabolic activation. J. Natl Cancer Inst. Monogr. 27 67–73 [DOI] [PubMed] [Google Scholar]
- 8. Cavalieri E.L., et al. (2010). Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 6 75–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zelko I.N., et al. (2002). Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med., 33 337–349 [DOI] [PubMed] [Google Scholar]
- 10. Karlsson K., et al. (1988). Extracellular superoxide dismutase in the vascular system of mammals. Biochem. J. 255 223–228 [PMC free article] [PubMed] [Google Scholar]
- 11. Karlsson K., et al. (1994). Turnover of extracellular-superoxide dismutase in tissues. Lab. Invest.; a Journal of Technical Methods and Pathology 70 705–710 [PubMed] [Google Scholar]
- 12. Marklund S.L. (1982). Human copper-containing superoxide dismutase of high molecular weight. Proc. Natl. Acad. Sci. U.S.A. 79 7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ookawara T., et al. (2002). Nuclear translocation of extracellular superoxide dismutase. Biochem. Biophys. Res. Commun. 296 54–61 [DOI] [PubMed] [Google Scholar]
- 14. Ahmed M.N., et al. (2003). Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. Am. J. Respir. Crit. Care Med. 167 400–405 [DOI] [PubMed] [Google Scholar]
- 15. Folz R.J., et al. (1999). Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J. Clin. Invest. 103 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dhakshinamoorthy S., et al. (2000). Small maf (MafG and MafK) proteins negatively regulate antioxidant response element-mediated expression and antioxidant induction of the NAD(P)H:Quinone oxidoreductase1 gene. J. Biol. Chem. 275 40134–40141 [DOI] [PubMed] [Google Scholar]
- 17. Kensler T.W., et al. (2010). Nrf2: friend or foe for chemoprevention? Carcinogenesis 31 90–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kensler T.W., et al. (2007). Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47 89–116 [DOI] [PubMed] [Google Scholar]
- 19. Reddy N.M., et al. (2007). Genetic dissection of the Nrf2-dependent redox signaling-regulated transcriptional programs of cell proliferation and cytoprotection. Physiol. Genomics 32 74–81 [DOI] [PubMed] [Google Scholar]
- 20. Folz R.J., et al. (1994). Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics 22 162–171 [DOI] [PubMed] [Google Scholar]
- 21. Li J.J., et al. (2002). Ploidy differences between hormone- and chemical carcinogen-induced rat mammary neoplasms: comparison to invasive human ductal breast cancer. Mol. Carcinog. 33 56–65 [DOI] [PubMed] [Google Scholar]
- 22. Shull J.D., et al. (1997). Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis 18 1595–1601 [DOI] [PubMed] [Google Scholar]
- 23.Singh B., et al. Partial inhibition of estrogen-induced mammary carcinogenesis in rats by tamoxifen: balance between oxidant stress and estrogen responsiveness. PLoS ONE. (2011);6:e25125. doi: 10.1371/journal.pone.0025125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh B., et al. (2012). Induction of NAD(P)H-quinone oxidoreductase 1 by antioxidants in female ACI rats is associated with decrease in oxidative DNA damage and inhibition of estrogen-induced breast cancer. Carcinogenesis 33 156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bhat H.K., et al. (2003). Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc. Natl. Acad. Sci. U.S.A. 100 3913–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han X., et al. (1994). DNA single-strand breaks in kidneys of Syrian hamsters treated with steroidal estrogens: hormone-induced free radical damage preceding renal malignancy. Carcinogenesis 15 997–1000 [DOI] [PubMed] [Google Scholar]
- 27. Bhat H.K., et al. (2004). Suppression of calbindin D28K in estrogen-induced hamster renal tumors. J. Steroid Biochem. Mol. Biol. 92 391–398 [DOI] [PubMed] [Google Scholar]
- 28. Mense S.M., et al. (2008). Preferential induction of cytochrome P450 1A1 over cytochrome P450 1B1 in human breast epithelial cells following exposure to quercetin. J. Steroid Biochem. Mol. Biol. 110 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beauchamp C., et al. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44 276–287 [DOI] [PubMed] [Google Scholar]
- 30. Li W., et al. (2009). Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 48 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cavalieri E.L., et al. (1992). The approach to understanding aromatic hydrocarbon carcinogenesis. The central role of radical cations in metabolic activation. Pharmacol. Ther. 55 183–199 [DOI] [PubMed] [Google Scholar]
- 32. Cavalieri E.L., et al. (2002). Initiation of cancer and other diseases by catechol ortho-quinones: a unifying mechanism. Cell. Mol. Life Sci. 59 665–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cavalieri E.L., et al. (1997). Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl. Acad. Sci. U.S.A. 94 10937–10942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henderson B.E., et al. (2000). Hormonal carcinogenesis. Carcinogenesis 21 427–433 [DOI] [PubMed] [Google Scholar]
- 35. Kelloff G.J., et al. (1999). Cancer chemoprevention: progress and promise. Eur. J. Cancer 35 1755–1762 [DOI] [PubMed] [Google Scholar]
- 36. Kensler T.W. (1997). Chemoprevention by inducers of carcinogen detoxication enzymes. Environ. Health Perspect. 105(Suppl 4) 965–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wattenberg L.W. (1985). Chemoprevention of cancer. Cancer Res. 45 1–8 [PubMed] [Google Scholar]
- 38. Kim S.H., et al. (2005). Overexpression of extracellular superoxide dismutase (EC-SOD) in mouse skin plays a protective role in DMBA/TPA-induced tumor formation. Oncol. Res. 15 333–341 [DOI] [PubMed] [Google Scholar]
- 39. Fattman C.L., et al. (2003). Extracellular superoxide dismutase in biology and medicine. Free Radic. Biol. Med. 35 236–256 [DOI] [PubMed] [Google Scholar]
- 40. Folz R.J., et al. (1997). Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am. J. Respir. Cell Mol. Biol. 17 393–403 [DOI] [PubMed] [Google Scholar]
- 41. Teoh M.L., et al. (2009). Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer Res. 69 6355–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu Y., et al. (2002). Transcriptional regulation of the human manganese superoxide dismutase gene: the role of specificity protein 1 (Sp1) and activating protein-2 (AP-2). Biochem. J. 362(Pt 2)401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu C.H., et al. (2001). A family of AP-2 proteins down-regulate manganese superoxide dismutase expression. J. Biol. Chem. 276 14407–14413 [DOI] [PubMed] [Google Scholar]
- 44. Perissi V., et al. (2000). AP-2 transcription factors in the regulation of ERBB2 gene transcription by oestrogen. Oncogene 19 280–288 [DOI] [PubMed] [Google Scholar]
- 45. Connor K.M., et al. (2007). Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res. 67 10260–10267 [DOI] [PubMed] [Google Scholar]
- 46. Hempel N., et al. (2011). Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anticancer. Agents Med. Chem. 11 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Harvell D.M., et al. (2000). Rat strain-specific actions of 17beta-estradiol in the mammary gland: correlation between estrogen-induced lobuloalveolar hyperplasia and susceptibility to estrogen-induced mammary cancers. Proc. Natl. Acad. Sci. U.S.A. 97 2779–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hirsch S., et al. (2008). Methylation status in healthy subjects with normal and high serum folate concentration. Nutrition 24 1103–1109 [DOI] [PubMed] [Google Scholar]
- 49. Zelko I.N., et al. (2004). Sp1 and Sp3 transcription factors mediate trichostatin A-induced and basal expression of extracellular superoxide dismutase. Free Radic. Biol. Med. 37 1256–1271 [DOI] [PubMed] [Google Scholar]
- 50. Zelko I.N., et al. (2010). CpG methylation attenuates Sp1 and Sp3 binding to the human extracellular superoxide dismutase promoter and regulates its cell-specific expression. Free Radic. Biol. Med. 48 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zelko I.N., et al. (2011). Histone acetylation regulates cell-specific and interferon-gamma inducible EC-SOD expression in human pulmonary arteries. Am. J. Respir. Cell Mol. Biol. 45 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ansell P.J., et al. (2005). Repression of cancer protective genes by 17beta-estradiol: ligand-dependent interaction between human Nrf2 and estrogen receptor alpha. Mol. Cell. Endocrinol. 243 27–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.