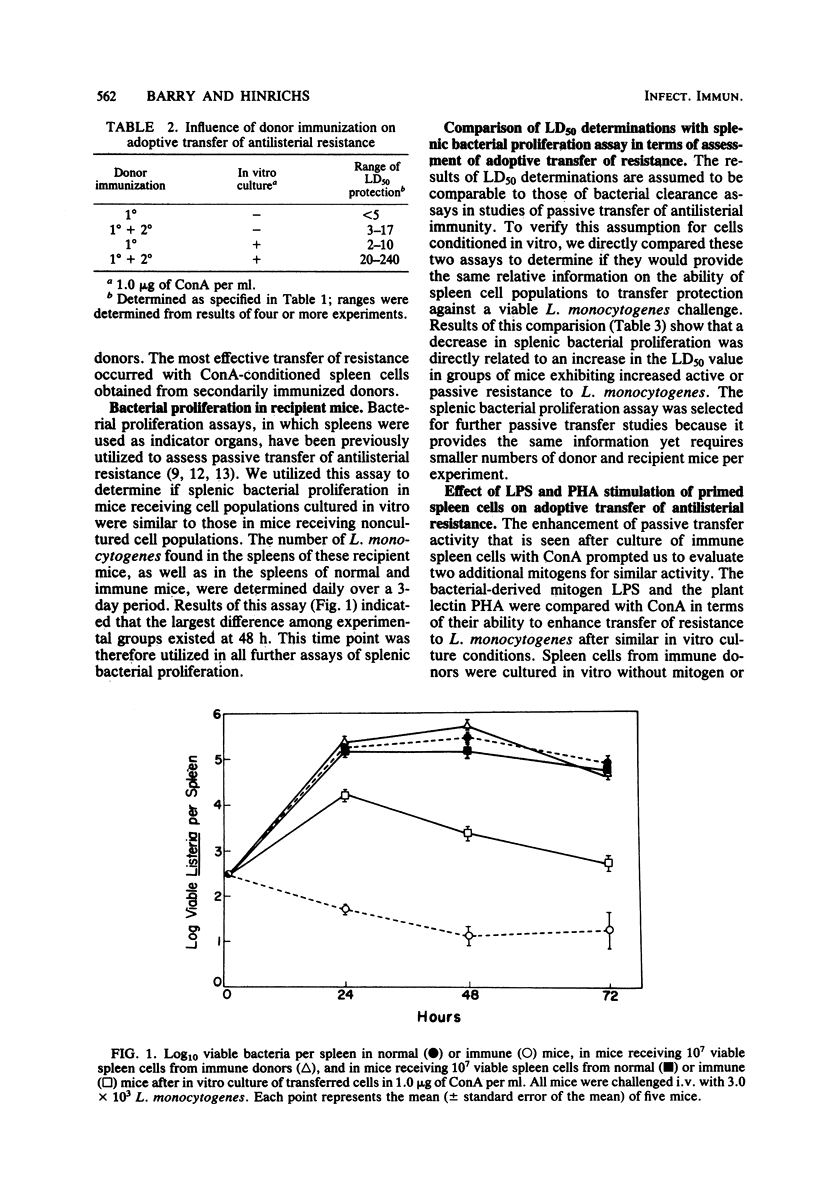
Abstract
In vitro incubation of spleen cells with T-cell mitogens has been shown to augment cytotoxic and cytolytic T-cell function. The plant lectins Concanavalin A and phytohemagglutinin were employed in a similar fashion to investigate their abilities to enhance cell-mediated immunity to the microbial pathogen Listeria monocytogenes. Spleen cells from immune mice were incubated in vitro with Concanavalin A or phytohemagglutinin before passive transfer into normal recipients. Results indicated a 10- to 100-fold enhancement in the ability of these cells stimulated in vitro to transfer antilisterial resistance, as assayed by changes in 50% lethal dose values and enumeration of splenic bacterial proliferation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Bonavida B. Concanavalin A-mediated activation of antigen-primed lymphocytes into secondary cytotoxic lymphocytes. J Exp Med. 1977 Feb 1;145(2):293–301. doi: 10.1084/jem.145.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M. Concanavalin A-mediated in vitro activation of lymphocytes primed against syngeneic SV40-induced tumor-associated antigens in mice into secondary effector cells capable of specifically preventing tumor growth. Cell Immunol. 1979 Aug;46(1):201–207. doi: 10.1016/0008-8749(79)90259-4. [DOI] [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Heininger D., Touton M., Chakravarty A. K., Clark W. R. Activation of cytotoxic function in T lymphocytes. J Immunol. 1976 Dec;117(6):2175–2180. [PubMed] [Google Scholar]
- Hinrichs D. J., Roberts C. M., Waxman F. J. Regulation of paralytic experimental allergic encephalomyelitis in rats: susceptibility to active and passive disease reinduction. J Immunol. 1981 May;126(5):1857–1862. [PubMed] [Google Scholar]
- Kearns R. J., Hinrichs D. J. Kinetics and maintenance of acquired resistance in mice to Listeria monocytogenes. Infect Immun. 1977 Jun;16(3):923–927. doi: 10.1128/iai.16.3.923-927.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Panitch H. S. Adoptive transfer of experimental allergic encephalomyelitis with activated spleen cells: comparison of in vitro activation by concanavalin a and myelin basic protein. Cell Immunol. 1980 Nov;56(1):163–171. doi: 10.1016/0008-8749(80)90091-x. [DOI] [PubMed] [Google Scholar]
- Panitch H. S., McFarlin D. E. Experimental allergic encephalomyelitis: enhancement of cell-mediated transfer by concanavalin A. J Immunol. 1977 Sep;119(3):1134–1137. [PubMed] [Google Scholar]
- Reme T. X., Dupuy d'Angeac A., Radal M., Serrou B. Use of concanavalin A to analyse the mechanism of memory cell differentiation into killer cells. Ann Immunol (Paris) 1979 Nov-Dec;130(6):827–840. [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Tartof D., Fitch F. W. Immunologically specific cytolytic activity induced in long-term mixed leukocyte culture cells by concanavalin A. J Immunol. 1977 Jan;118(1):35–42. [PubMed] [Google Scholar]
- Waterfield J. D., Waterfield E. M., Möller G. Lymphocyte-mediated cytotoxicity against tumor cells. I. Con A activated cytotoxic effector cells exhibit immunological specificity. Cell Immunol. 1975 Jun;17(2):392–404. doi: 10.1016/s0008-8749(75)80043-8. [DOI] [PubMed] [Google Scholar]



