Abstract
Long appreciated for their role as Type-2 effector cells, basophils have recently come into the spotlight for their role in the initiation of Type-2 immunity. Via an assortment of different activation pathways, basophils produce cytokines such as IL-4 that promote Th2 differentiation. Furthermore, recent studies using different experimental systems have shown that basophils can act as antigen presenting cells both in vitro and in vivo. In addition, basophils shape the Type-2 immune response by guiding antibody production and the memory response.
Introduction
Basophils were first described in humans by Paul Ehrlich in 1879, but it wasn’t until 100 years later that they were described in mice1,2. In both species, basophils primarily circulate in the blood stream, making up less than 1% of peripheral blood leukocytes3. As a result of their scarcity as well as a paucity of basophil specific markers, it was not until the development of IL-4 reporter mice and the incidental discovery that basophils constitutively expressed IL-4 reporters that basophils became easily identifiable4,5. These observations paved the way for recent studies identifying important roles for basophils in many aspects of the immune response, from activation to memory to the effector response.
Basophils are well known for their role as effector cells of the Type-2 immune response. They express high levels of FcεRI and thereby bind secreted IgE3,6. Upon crosslinking of antigen-specific IgE, basophils produce Type 2 cytokines such as IL-4 and IL-13 as well as degranulate, thereby releasing preformed mediators such as histamine and eicosanoids3,7. This ultimately leads to many of the characteristic symptoms of the Type 2 immune response. Similar pathways can also be induced by IgG1 crosslinking, which can lead to a specifically basophil-mediated anaphylaxis8. However, recent data have revealed a role for basophils not only in the effector response, but also in the initiation of the adaptive immune response.
Basophil Activation by Proteases
Cross-linking of FcεRI bound IgE is a well-known route to basophil activation, however recent evidence indicates that basophils can also be activated directly by some allergens and parasites9–13. One such method is via cysteine protease mediated activation11–13. The cysteine protease allergen papain, has been shown to directly activate basophils to produce cytokines such as IL-2, IL-4, IL-5 and IL-13 in the absence of antigen specific IgE12,13. Interestingly, activation with the protease allergen papain also leads to upregulation of MHC Class II and costimulatory molecules on the surface of basophils14. However, protease activated basophils do not appear to degranulate, indicating that there may be several distinct pathways of basophil activation; one leading to the stereotypical effector phenotype, while the other leads to a novel initiator phenotype13. These observations raise the question, what is the consequence of the initiator phenotype of basophils?
Induction of Th2 Mediated Immunity by Basophils
Although many cells can present antigen and produce cytokines involved in T cell activation and differentiation, dendritic cells clearly play a central role in the initiation of the Th1 and Th17 mediated immune responses. Th1 and Th17 differentiation requires a combination of cell surface and secreted signals for activation and differentiation of these effector cells from naïve CD4 T cells. Upon activation, dendritic cells become efficient antigen presenting cells, expressing high levels of MHC Class II on their surface and upregulating levels of necessary co-stimulatory molecules such as CD40 and CD86. At the same time, they also produce the cytokines that provide the necessary instructive signals for Th1 and Th17 differentiation. Thus, dendritic cells play a critical role in Th1 and Th17 activation and differentiation. It was long assumed that dendritic cells would play an analogous role in Th2 differentiation despite the fact that they do not produce the Th2 inducing cytokine IL-4. Indeed, dendritic cells have been shown to be capable of inducing Th2 differentiation via expression of cell surface molecules such as Jagged and OX-40L15,16. However, IL-4 production from a non-T cell source has been shown to be necessary in the generation of functional Th2 mediated immunity in many different model systems of Type-2 immunity17,18. If dendritic cells were incapable of providing that IL-4, what was the necessary source?
Upon activation, basophils are capable of rapid IL-4 production. This ability has long prompted others to postulate that basophils may be that elusive, necessary supplier of IL-4 involved in Th2 skewing. Several clues pointing to a role for basophils in initiating Th2 differentiation existed. IRF2−/− mice and Lyn−/− mice both have increased numbers of basophils under steady state conditions19,20. Interestingly, both exhibit spontaneous Th2 differentiation, but was this was due to increased systemic IL-4 levels or direct effects of basophils19,20? Using a model of Type-2 immunity in which subcutaneous immunization with the protease allergen papain leads to antigen-specific Th2 differentiation and IgE production, we showed that basophils played an essential role in initiating this response. Three days after subcutaneous papain immunization, basophils enter and transiently reside in the T cell zone of the draining lymph node13. For the 24 hours preceding the appearance of Th2 cells these activated basophils provide the cytokines IL-4 and TSLP, which instruct Th2 differentiation13. After immunization with protease allergens as well as the helminth T. muris, they are necessary for Th2 differentiation13,21. In both models of Type-2 immunity the depletion of basophils, via pre-treatment with the MAR-1 antibody to FcεRI, leads to the loss of Th2 differentiation13,21. Interestingly, the production of soluble factors by basophils has recently also been implicated in CD8+ T cell differentiation. Basophil production of IL-4 and IL-6 in in vitro systems promotes generation of IL-10 producing CD8+ T cells22. In varied models, basophils play important roles as accessory cells to T cell differentiation, but could their role also include that of antigen presenting cell?
Although basophils were only recently described to express MHC Class II on their surface, their possible role as antigen presenting cells was provided by earlier in vitro experiments. In a three cell culture system including basophils, dendritic cells and T cells, direct cell-cell interactions between basophils and T cells was shown to be an important factor in Th2 skewing in vitro23. Evidence that these interactions could be in the form of antigen presentation was provided by three groups using three different models of Type-2 immunity: protease allergen mediated, IgE mediated and the response to infection with a helminth parasite14,21,24. All three groups showed that basophils constitutively express MHC Class II and costimulatory molecules such as CD40, CD80 and CD86. Just like LPS activated dendritic cells upregulate expression of these molecules, papain stimulation led to upregulation of MHC Class II and costimulatory molecules14. Basophil expression of these molecules had functional consequences; naïve T cells mixed in culture with basophils and OVA peptide underwent Th2 differentiation in the absence of any other cells or stimuli14,21,24. Basophils were capable of forming immune synapses with T cells within 60 minutes of co-culture14. In the papain immunization model of Type-2 immunity, this Th2 differentiation capacity was singular to basophils; dendritic cells sorted from the draining lymph nodes of papain immunized mice were unable to induce Th2 differentiation in the absence of basophils in vitro14.
Basophils have an estimated in vivo life span of only 60 hours and they are very fragile cells with poor survival after standard sorting procedures25. Thus, it is technically difficult to perform transfer experiments with basophils. In order to get around these difficulties, two groups used transgenic mice in which MHC Class II expression was restricted to dendritic cells, and showed that while basophil migration to the draining lymph node remained intact, Th2 differentiation was lost14,21. This experiment is interesting for two reasons. First, it shows that dendritic cells are not sufficient for Th2 differentiation after immunization with helminth parasites or protease allergens. But perhaps more importantly, it illustrates that the role of basophils in Th2 differentiation is more than that of a cytokine source or accessory cell. However, the direct demonstration that basophils could act as functional antigen presenting cells in vivo required basophil transfer. To get around the technical difficulties of basophil transfer the two groups used two separate methods, either boosting the in vivo response after basophil transfer or transferring basophils from Bcl2 transgenic mice14,24. In both cases the transferred basophils were capable of inducing Th2 differentiation. Finally, to directly implicate basophils and rule out actions by any other antigen presenting cells, antigen loaded basophils were transferred into MHC Class II deficient mice14. As before, this led to Th2 differentiation illustrating that at least in these models basophils can be necessary and sufficient antigen presenting cells in the Type-2 immune response.
Although these observations are compelling, it should be stressed that they do not negate important roles for other accessory or antigen presenting cells in Type-2 immunity. It simply reveals the existence of another antigen presenting cell, and an important addition to the known roles of basophils. Dendritic cells can induce Th2 differentiation in vitro and in vivo via Notch ligand and OX40 signaling15,16. Indeed, despite similar results in three different models of Type-2 immunity, it was recently shown that basophils may not be essential for Th2 differentiation after infection with the helminth N. brasiliensis26. In this model basophils are recruited to the draining lymph nodes with similar kinetics as those seen in other models, but their depletion did not impact the Th2 differentiation in these experiments26. This observation again underlines the complexity of innate control of Type-2 immunity, indicating that there may be multiple pathways to its activation. However, the common thread of basophil migration to the draining lymph node underscores the crucial role played by basophils in orchestrating Type-2 immunity.
Via antigen presentation and cytokine production, the role of basophils in the Type-2 immune response mimics that of dendritic cells in the Type-1 immune response. However, despite their functional similarities, they appear to target different types of antigens during the primary immune response. While immature dendritic cells avidly and efficiently phagocytose particulate antigens, basophils are unable to take up particulate antigens in the absence of antigen specific IgE14. Instead, the source of antigen for basophils in the naïve state appears to consist of soluble antigens. In vivo, basophils can endocytose soluble, fluorescently labeled ovalbumin with an efficiency that equals if not surpasses that of dendritic cells14. This may allow basophils in naïve mice to target the soluble antigens shed from the surface of large multicellular helminth parasites. In turn, this could unintentionally shuttle small, soluble, environmental antigens to cells central to the allergic response.
Regulation of the Antibody Response by Basophils
Just as B cell IgE production ultimately impacts basophil function, basophils have also been described to impact antibody production by B cells. In vitro studies using KU812 cells, a human basophil-like cell line, have shown that activated basophils can lead to IgE production in B cells27,28. Basophils express low levels of CD40L under steady state conditions and this expression increases upon basophil activation27–30. Along with CD40L, basophils also express BAFF and APRIL, which together can lead to T cell independent Ig production30. Although this can be induced in basophils by IgE crosslinking, IgD crosslinking more efficiently leads to basophil activation and expression of these B cell activating factors30. This may explain the mechanism by which injection of anti-IgD antibody leads to a Type-2 immune response as well as mechanistically explaining the chronic inflammation that accompanies hyper-IgD states. More importantly, it reveals that basophils may promote the Type-2 immune response not only through Th2 activation and differentiation, but also by directly activating and promoting IgE production by B cells.
Recent studies have shown an important role for basophils in initiating the Th2 mediated immune response and in direct activation of B cells. However, they may also play a role in maintaining Type-2 immune memory. Despite an estimated lifespan of only 60 hours in the mouse, basophils can extend the half-life of IgE by binding circulating IgE to FcεRI. This was illustrated in experiments in which APC specific IgE remained bound to basophils six weeks after immunization of naïve mice with APC and heat killed Bordetella pertussis31. However, it is unclear whether this indicates continuous production of antigen-specific IgE or whether this indicates retention of APC-specific IgE from the original immune response. This is specifically in question since long-lived IgE producing plasma cells have yet to be discovered. Despite these questions, these experiments illustrated that basophils can boost the memory immune response. IgE crosslinking on basophils leads to production of IL-4 and IL-6, cytokines known to be important in antibody production. Basophil production of these cytokines appears to be important in boosting production of antibody during the memory response; depletion of basophils leads to decreased production of IgG1 and IgG2a31. Whether this is due to direct effects on antibody production or to general effects on B cell activation remains to be seen.
Conclusion
Despite their long history of relative obscurity, basophils are beginning to be appreciated as an important cell type in instructing the Type-2 immune response from initiation to the memory response. Basophils are capable of directly activating and instructing naïve T cells to become antigen specific Th2 cells. They also seem to be capable of promoting antigen specific IgE production via their effects on Th2 activation and via direct activation of B cells. In these ways they appear to be central regulators of the Type-2 immune response. However, despite the current enthusiasm for basophils, many questions and controversies remain. One controversy involves whether murine and human basophils are actually analogous cells given their distinct histologic appearance32. Additionally, it should be noted that given the wide variety of Type-2 inducing stimuli it is very likely that there are many different pathways to Type-2 immunity, some of which are mediated by dendritic cells. Additionally, a potential role of alternatively activated macrophages in Th2 induction is of particular interest33. Thus multiple pathways of Th2 induction appear to exist and the future challenge is to understand their differential functions and activation requirements.
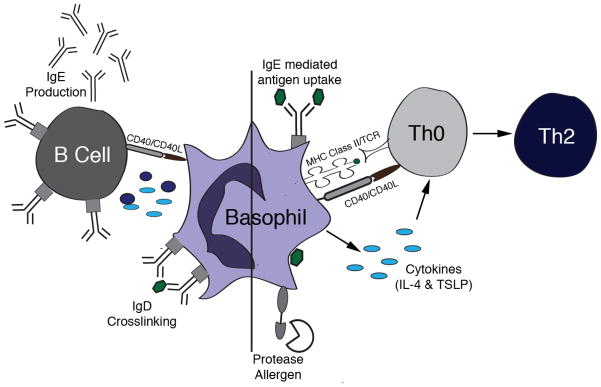
Figure.
Basophils directly influence both T cell differentiation and antibody production by B cells. On the right, a basophil is depicted taking up antigen either via IgE mediated antigen uptake or by endocytosis of soluble antigen (hexagon). After activation stimulus, either activation by a protease allergen or IgE crosslinking, antigen is processed and presented on MHC Class II. Basophils also upregulated costimulatory molecules such as CD40 and secrete cytokines such as IL-4 and TSLP that together activate Th0 cells and induce skewing to the Th2 phenotype. On the left, IgD crosslinking leads to basophil activation, exhibited by upregulation of CD40L and production of IL-4 and BAFF. This results in T independent IgE production from B cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehrlich P. Beitrage zur kenntnis der granuliertan bindegewebszellen und der eosinophilen leukocyten. Arch Anat Physiol. 1879;3 [Google Scholar]
- 2.Dvorak AM, et al. Ultrastructural identification of the mouse basophil. Blood. 1982;59:1279–85. [PubMed] [Google Scholar]
- 3.Schroeder JT. Basophils beyond effector cells of allergic inflammation. Adv Immunol. 2009;101:123–61. doi: 10.1016/S0065-2776(08)01004-3. [DOI] [PubMed] [Google Scholar]
- 4.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–17. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–11. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 6.Hida S, et al. Fc receptor gamma-chain, a constitutive component of the IL-3 receptor, is required for IL-3-induced IL-4 production in basophils. Nat Immunol. 2009;10:214–22. doi: 10.1038/ni.1686. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Sasson SZ, Le Gros G, Conrad DH, Finkelman FD, Paul WE. Cross-linking Fc receptors stimulate splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. Proc Natl Acad Sci U S A. 1990;87:1421–5. doi: 10.1073/pnas.87.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsujimura Y, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–9. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Schramm G, et al. Molecular characterization of an interleukin-4-inducing factor from Schistosoma mansoni eggs. J Biol Chem. 2003;278:18384–92. doi: 10.1074/jbc.M300497200. [DOI] [PubMed] [Google Scholar]
- 10.Schramm G, et al. Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J Immunol. 2007;178:6023–7. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- 11.Finkelman FD, Urban JF., Jr Cytokines: making the right choice. Parasitol Today. 1992;8:311–4. doi: 10.1016/0169-4758(92)90105-b. [DOI] [PubMed] [Google Scholar]
- 12.Phillips C, Coward WR, Pritchard DI, Hewitt CR. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol. 2003;73:165–71. doi: 10.1189/jlb.0702356. [DOI] [PubMed] [Google Scholar]
- 13.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–20. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohshima Y, et al. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–45. [PubMed] [Google Scholar]
- 16.Amsen D, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 17.Shimoda K, et al. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–3. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 18.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–46. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hida S, Tadachi M, Saito T, Taki S. Negative control of basophil expansion by IRF-2 critical for the regulation of Th1/Th2 balance. Blood. 2005;106:2011–7. doi: 10.1182/blood-2005-04-1344. [DOI] [PubMed] [Google Scholar]
- 20.Charles N, et al. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity. 2009;30:533–43. doi: 10.1016/j.immuni.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Shen T, Min B. Basophils can directly present or cross-present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL-10-producing phenotypes. J Immunol. 2009;183:3033–9. doi: 10.4049/jimmunol.0900332. [DOI] [PubMed] [Google Scholar]
- 23.Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109:2921–7. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–12. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 25.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–25. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, et al. Cutting Edge: Basophils Are Transiently Recruited into the Draining Lymph Nodes during Helminth Infection via IL-3, but Infection-Induced Th2 Immunity Can Develop without Basophil Lymph Node Recruitment or IL-3. J Immunol. 2009 doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gauchat JF, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 28.Yanagihara Y, et al. Induction of human IgE synthesis in B cells by a basophilic cell line, KU812. Clin Exp Immunol. 1997;108:295–301. doi: 10.1046/j.1365-2249.1997.d01-1001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagihara Y, et al. Cultured basophils but not cultured mast cells induce human IgE synthesis in B cells after immunologic stimulation. Clin Exp Immunol. 1998;111:136–43. doi: 10.1046/j.1365-2249.1998.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–98. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denzel A, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 32.Lee JJ, McGarry MP. When is a mouse basophil not a basophil? Blood. 2007;109:859–61. doi: 10.1182/blood-2006-06-027490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–6. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]