Abstract
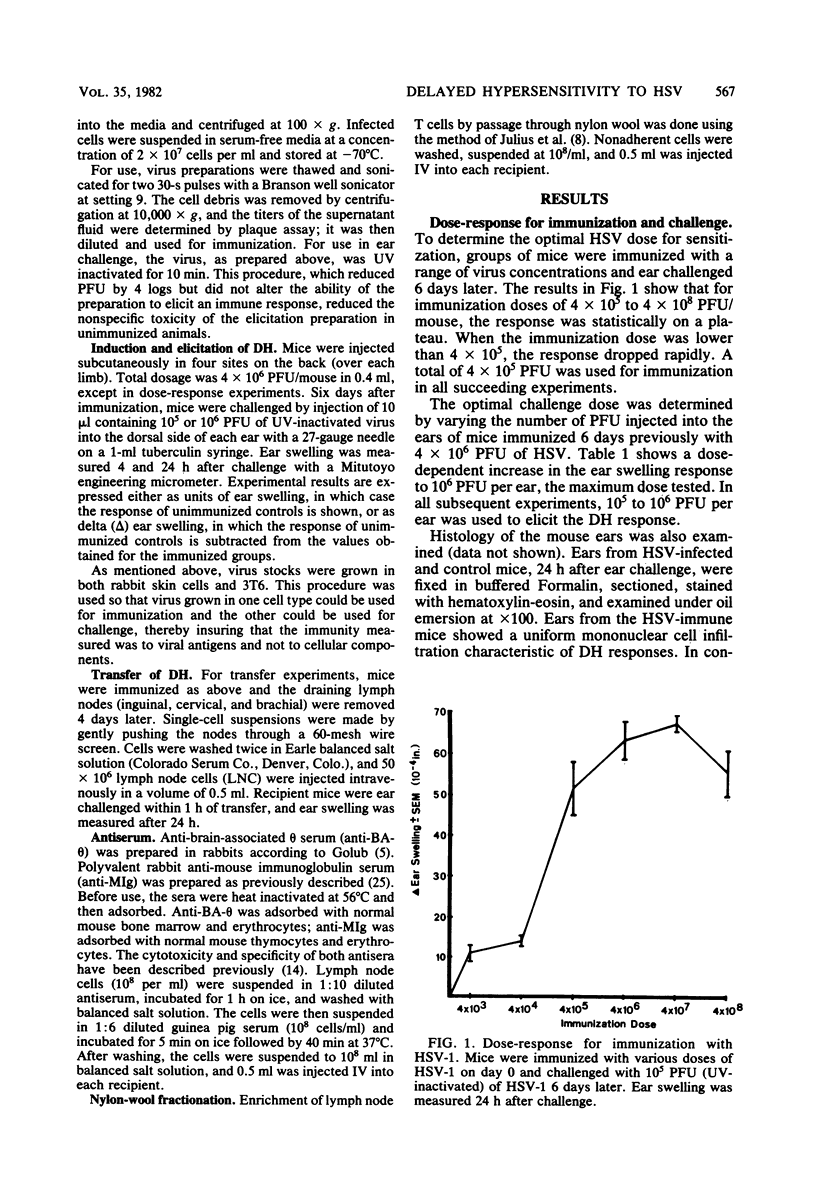
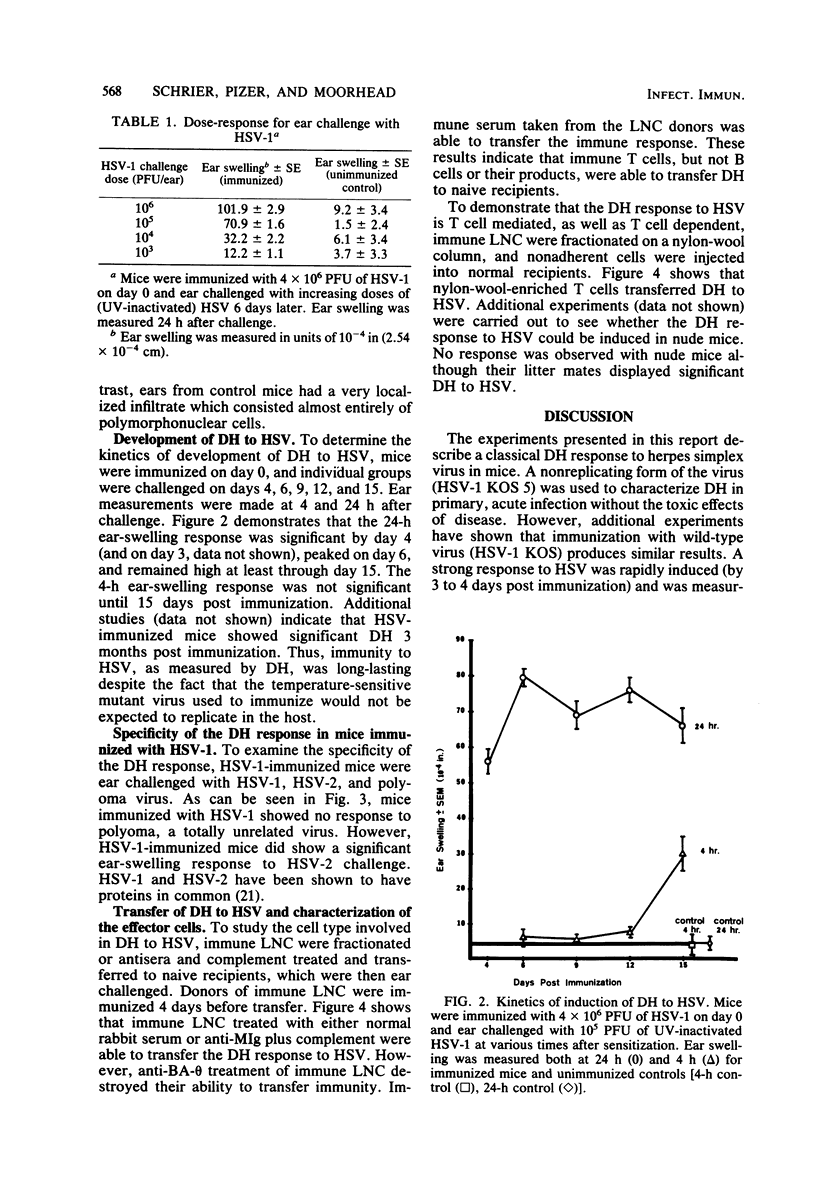
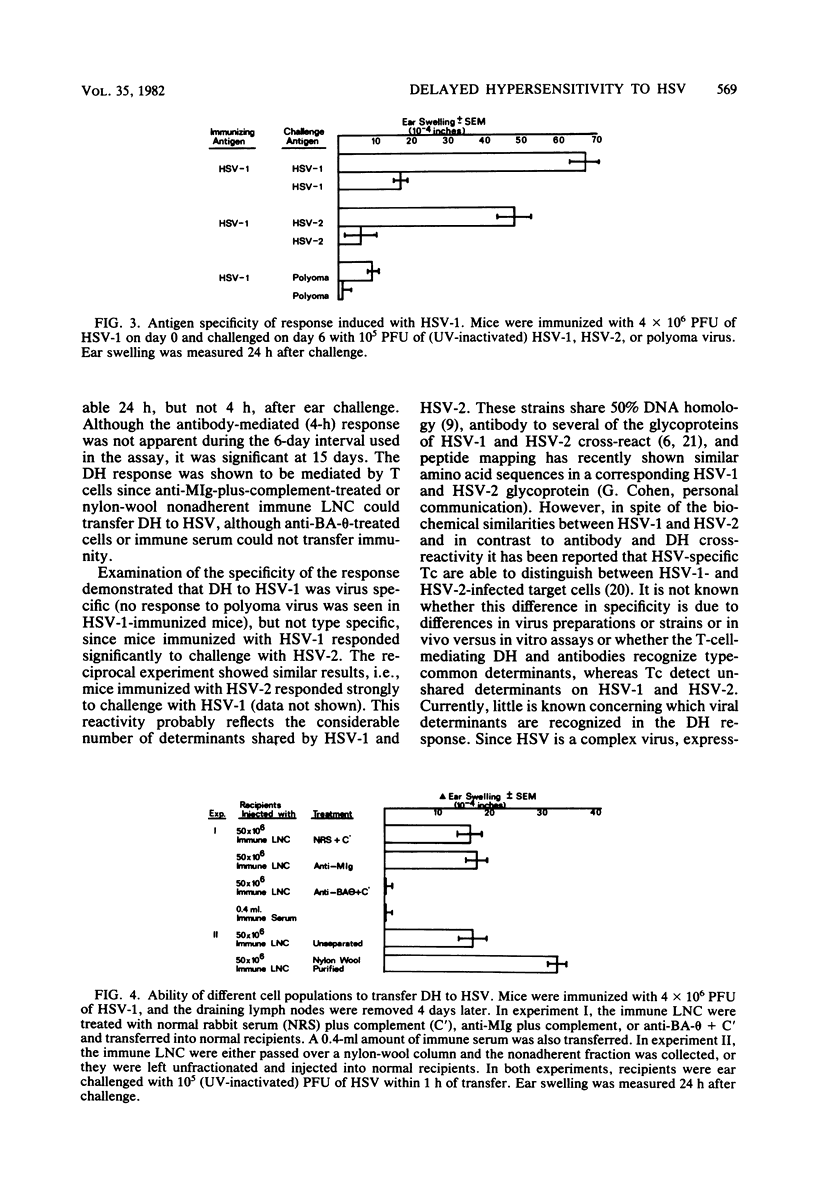
Cell-mediated immunity has been shown to be clinically important in recovery from herpes simplex virus (HSV) infections. To investigate the role of delayed hypersensitivity (DH) in immunity and protection against HSV, we developed a murine model using the ear-swelling assay. Mice were infected subcutaneously with HSV-1 and ear-challenged, and the swelling was quantified. Significant ear swelling was detected by 3 to 4 days postinfection and peaked at 6 days. The kinetics of development of ear swelling were typical of DH: maximal swelling occurred 24 h post challenge and was diminished by 48 h, and the cellular infiltrate was predominantly mononuclear. Four-hour swelling, indicative of antibody-mediated, immediate-type hypersensitivity, was not detected until 15 days post immunization. The DH response was virus specific and could be transferred to normal recipients with lymph node T cells, but not with B cells or immune serum. This system will provide a useful model for evaluating the protective role of DH in HSV infection and for studying the specificity and interaction of T cells which mediate the response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D., Young L. S., Meyer R. D., Blevins A. H. Infectious complications of neoplastic disease. Med Clin North Am. 1971 May;55(3):729–745. doi: 10.1016/s0025-7125(16)32514-7. [DOI] [PubMed] [Google Scholar]
- Ching C., Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979 Oct;26(1):49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Wells M. Immune control of herpes simplex virus infections. Cancer Res. 1974 May;34(5):1140–1145. [PubMed] [Google Scholar]
- Glorioso J. C., Smith J. W. Immune interactions with cells infected with herpes simplex virus: antibodies to radioiodinated surface antigens. J Immunol. 1977 Jan;118(1):114–121. [PubMed] [Google Scholar]
- Golub E. S. Brain-associated theta antigen: reactivity of rabbit anti-mouse brain with mouse lymphoid cells. Cell Immunol. 1971 Aug;2(4):353–361. doi: 10.1016/0008-8749(71)90070-0. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Howes E. L., Taylor W., Mitchison N. A., Simpson E. MHC matching shows that at least two T-cell subsets determine resistance to HSV. Nature. 1979 Jan 4;277(5691):66–68. doi: 10.1038/277067a0. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kieff E., Hoyer B., Bachenheimer S., Roizman B. Genetic relatedness of type 1 and type 2 herpes simplex viruses. J Virol. 1972 May;9(5):738–745. doi: 10.1128/jvi.9.5.738-745.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Cells mediating delayed-type hypersensitivity in the lungs of mice infected with an influenza A virus. Scand J Immunol. 1980;12(5):393–400. doi: 10.1111/j.1365-3083.1980.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Russell S. M., Brand C. M. Induction and characterization of delayed-type hypersensitivity to influenza virus in mice. Eur J Immunol. 1979 Oct;9(10):783–790. doi: 10.1002/eji.1830091008. [DOI] [PubMed] [Google Scholar]
- Lopez C., O'Reilly R. J. Cell-mediated immune responses in recurrent herpesvirus infections. I. Lymphocyte proliferation assay. J Immunol. 1977 Mar;118(3):895–902. [PubMed] [Google Scholar]
- Moorhead J. W. Tolerance and contact sensitivity to DNFA in mice. VIII. Identification of distinct T cell subpopulations that mediate in vivo and in vitro manifestations of delayed hypersensitivity. J Immunol. 1978 Jan;120(1):137–144. [PubMed] [Google Scholar]
- Muller S. A., Herrmann E. C., Jr, Winkelmann R. K. Herpes simplex infections in hematologic malignancies. Am J Med. 1972 Jan;52(1):102–114. doi: 10.1016/0002-9343(72)90012-5. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Oda H., Mori R., Taniguchi T. Mechanism of acquired resistance to herpes simplex virus infection as studied in nude mice. J Gen Virol. 1979 Sep;44(3):715–723. doi: 10.1099/0022-1317-44-3-715. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Field H. J., Quartey-Papafio R. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J Gen Virol. 1980 Jun;48(Pt 2):351–357. doi: 10.1099/0022-1317-48-2-351. [DOI] [PubMed] [Google Scholar]
- Oakes J. E. Role for cell-mediated immunity in the resistance of mice to subcutaneous herpes simplex virus infection. Infect Immun. 1975 Jul;12(1):166–172. doi: 10.1128/iai.12.1.166-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw H., Asher L. V., Wohlenberg C., Sekizawa T., Notkins A. L. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol. 1979 Jul;44(1):205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Randall R. E., Killington R. A., Watson D. H. Glycoproteins with type common and type specific antigenic sites excreted from cells infected with herpes simplex virus types 1 and 2. J Gen Virol. 1980 Jun;48(Pt 2):297–310. doi: 10.1099/0022-1317-48-2-297. [DOI] [PubMed] [Google Scholar]
- Schlabach A. J., Martinez D., Field A. K., Tytell A. A. Resistance of C58 mice to primary systemic herpes simplex virus infection: macrophage dependence and T-cell independence. Infect Immun. 1979 Nov;26(2):615–620. doi: 10.1128/iai.26.2.615-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer E. S., Andersen H. K. Clinically evident, non-terminal infections with herpesviruses and the wart virus in immunosuppressed renal allograft recipients. Br Med J. 1970 Aug 1;3(5717):251–254. doi: 10.1136/bmj.3.5717.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A. L., Smithwick E. M., Seligman S. J., Kim D. S. Fatal disseminated herpesvirus hominis type 2 infection in an adult with associated thymic dysplasia. Am J Med. 1974 Apr;56(4):545–553. doi: 10.1016/0002-9343(74)90487-2. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Greene M. I., Fields B. N. Delayed hypersensitivity in mice infected with reovirus. I. Identification of host and viral gene products responsible for the immune response. J Immunol. 1980 Jul;125(1):278–282. [PubMed] [Google Scholar]
- Weiner H. L., Moorhead J. W., Yamaga K., Kubo R. T. Anti-immunoglobulin stimulation of murine lymphocytes. II. Identification of cell surface target molecules and requirements for cross-linkage. J Immunol. 1976 Nov;117(5 Pt 1):1527–1531. [PubMed] [Google Scholar]


