Abstract
Background
The importance of clinical presentation and pre-transplantation course on outcome in children with dilated cardiomyopathy (DCM) listed for heart transplantation is not well defined.
Methods and Results
The impact of age, duration of illness, gender, race, ventricular geometry and the diagnosis of myocarditis on outcome in 261 DCM children enrolled in the Pediatric Cardiomyopathy Registry and Pediatric Heart Transplant Study was studied. Endpoints included: 1) listing as UNOS Status 1, 2) death while waiting and 3) death post-transplantation. The median age at the time of diagnosis was 3.4 years, and mean time from diagnosis to listing was 0.62±1.3 years. Risk factors associated with death while waiting were ventilator use and older age at listing in patients not mechanically ventilated (p=0.0006 and p=0.03, respectively). Shorter duration of illness (p=0.04) was associated with listing as UNOS Status 1. Death post-transplantation was associated with myocarditis at presentation (p=0.009), non-white race (p<0.0001) and a lower left ventricular end-diastolic dimension z-score at presentation (p=0.04). In the myocarditis group, 17% (4/23) died of acute rejection post-transplantation.
Conclusions
Mechanical ventilator use and older age at listing predicted death while waiting, while non-white race, smaller left ventricular dimension and myocarditis were associated with death post-transplantation. Although 97% of children with clinically or biopsy diagnosed myocarditis at presentation survived to transplantation, they had significantly higher mortality post-transplantation compared with children without myocarditis, raising the possibility that pre-existing viral infection or inflammation adversely affects graft survival.
Keywords: dilated cardiomyopathy, heart transplantation, myocarditis, pediatrics
Introduction and Background
In children, dilated cardiomyopathy (DCM) is the most common form of cardiomyopathy and the most common indication for heart transplantation.1, 2 The annual incidence of DCM is reported to be 0.37/100,000 in the United States and 0.57 to 1.13/100,000 in Australia.3-5 In children with end-stage heart failure secondary to DCM, heart transplantation is the only therapeutic option currently available. In prior studies from the Pediatric Cardiomyopathy Registry (PCMR) freedom from death or heart transplantation in children with DCM was 61% and 47% at 1 and 5 years, respectively.6,7 Refinements in the management of end-stage DCM in children over the last decade have significantly reduced early mortality, but transplantation remains the definitive treatment.8-11 Death after listing or after heart transplantation remains a significant problem in this group of children; even in the era of mechanical support, and as many as 38% of patients with DCM listed for heart transplantation have significant complications and morbidity while waiting for heart transplantation.12 Outcomes after heart transplantation are favorable in adults13, 14 as well as in children with DCM.2, 11, 15, 16 Children with DCM have a 68-72% 10-year survival rate after transplantation, higher than children transplanted for end-stage congenital heart disease.17,18 Improved surgical and peri-operative care has led to era improvements in outcomes post-transplantation.19
Studies of children with DCM requiring heart transplantation have focused on cohorts identified at listing.18,17 The impact of pre-listing factors on outcomes both post-listing and post-transplantation have not been studied. Better outcomes in children with DCM may be possible with better identification and management of pre-listing and pre-transplantation risk factors. We hypothesized that important risk factors for worse outcomes after transplantation listing and transplantation might be identified in children with DCM from their pre-listing status. In order to evaluate this hypothesis, we pooled longitudinal data regarding patients with DCM from 2 large pediatric data bases: the PCMR and the Pediatric Heart Transplant Study (PHTS) database20 and attempted to define risk factors at presentation and at listing that were associated with worse outcomes after listing in this cohort.
Methods
Patient Population
Patients were identified from a merged dataset that included all children enrolled in the PCMR who were also enrolled in the PHTS.20 The PCMR is a NIH/NHLBI-funded registry that enrolled children (less than 18 years at the time of presentation) who were diagnosed with all forms of primary cardiomyopathy from January 1, 1990 (Clinical Trial Registration- URL:http://www.clinicaltrials.gov unique identifier: NCT00005391). Data collected include echocardiographic measurements, and clinical and laboratory information at the time of presentation and at yearly follow-up visits. Patient follow-up in the PCMR is censored at heart transplantation or death.
The PHTS is an event-driven registry that has enrolled patients less than 18 years of age listed for heart transplantation at participating institutions since 1993. The PHTS dataset includes outcome after listing, peri-transplantation course and post-transplantation events such as rejection, infection, graft atherosclerosis, death, malignancy and the need for re-transplantation. The registries were merged to allow analysis of data in children with cardiomyopathy from the time of presentation through their post-transplantation course. The merged dataset included children transplanted in the US and Canada. The PHTS registry is currently active. The PCMR is no longer enrolling new patients, but analysis of previously enrolled patients continues. Follow-up for the merged dataset was complete through December 31, 2005.
Only children who met the PCMR definition of DCM were included in this analysis. In the PCMR dataset, DCM was defined by echocardiographic criteria that included a left ventricular (LV) end-diastolic dimension (EDD) ≥ 2 standard deviations (SD) for body surface area (BSA) and a LV percent fractional shortening (FS) ≤ 2SD for age.20 The diagnosis of myocarditis was defined in this study as it has been previously, 21 as either the histologic evidence of myocarditis (the presence of Dallas criteria) obtained at endomyocardial biopsy or at explantation,22 or clinically, by the investigator at the submitting institution if histology was not available.
Risk Factors Studied
We examined several potential diagnoses, and pre-listing, listing and transplantation risk factors for the following endpoints: 1) severity of illness as defined by the need to be listed as United Network for Organ Sharing (UNOS) Status 1 (highest priority UNOS status to receive an organ, a reflection of severity of illness at listing), 2) death after listing while waiting for a heart, and 3) death post-heart transplantation. The merged dataset included 10 children transplanted in Canada, which does not use UNOS listing criteria. For the variable UNOS Status 1 at listing, information was available for 3 children to be coded in the appropriate UNOS status and the other children were excluded from this portion of the analysis. The potential risk factors for death after listing while waiting for a heart included: age at listing, gender, race (white vs. non-white), diagnosis of myocarditis, time from diagnosis to listing (years), use of mechanical ventilation at the time of listing, and multiple echocardiographic measures of cardiac performance and size including LVFS z-score as well as LVEDD z-score at presentation. Echocardiographic measurements were expressed as the z-score for body surface area, in order to adjust for body size. In this analysis, older age was defined as >10 years old and younger age was defined as <1 year old, however in the regression analysis, age was analyzed as a continuous variable. UNOS Status 1 versus Status 2 was also included in the analyses of death while waiting and post-transplantation. Age at transplantation, use of mechanical ventilation at the time of transplant, and time spent waiting for a heart were included in the risk factor analysis for death after transplantation. Transplanted patients with and without the diagnosis of myocarditis were also analyzed.
Data Collection
Institutional Review Board (IRB) approval was obtained for each registry (PCMR and PHTS) and the merged dataset at all study sites. A data sharing agreement was established between the data coordinating centers (New England Research Institutes, Watertown, MA and University of Alabama, Birmingham, AL) prior to the exchange of data.
Statistical Methods
Standard statistical methods for summarizing and displaying data were used. Data are reported as mean, median, with standard deviations (SD). Logistic regression was used to determine which variables were associated with listing as UNOS status 1. Kaplan-Meier analysis was used to estimate the time-related probability of death. Time zero for survival analysis for death while waiting was defined as the date of listing, while time zero for the analysis of death after transplant was the date of transplant. The log-rank test was used to compare the survival of designated subgroups. Cox proportional hazard regression was used to identify risk factors for death while waiting and death after transplantation; the results are reported as hazard ratios (HR). Alpha was set at 0.05, and all tests were two-tailed. Forward selection was used in the multivariable analysis. Comparison of the myocarditis and no myocarditis groups was performed using the unpaired t-test for normally distributed data and the Kruskal-Wallis test for skewed data. The SAS statistical analysis software (SAS Institute, Cary, NC) was used for all analyses.
Results
PCMR and PHTS Registry Merger
The merged dataset included 332 children from 16 participating centers, of whom 261 had a diagnosis of DCM and comprised the study cohort (Table 1). The remaining 71 children from the original merged dataset (n=332) were not included in this study because their cardiomyopathy phenotype was not DCM. Of the 261 children, 11% (n=29) died waiting for a heart. Eighty percent (209 of the 261) went on to heart transplantation, and the remaining 23 children were alive but still waiting at time of analysis. Of these 209 children transplanted, 40 children (19%) died after heart transplantation.
Table 1. Demographic and Clinical Characteristics of 261 Children with Dilated Cardiomyopathy Followed from Diagnosis through Heart Transplantation.
| Characteristic | At Listing for Transplant [n] | At Transplant [n] |
|---|---|---|
| Age, mean (SD), y median | 6.5 (6.2) 3.6 [261] |
7.0 (6.3) 4.4 [209] |
| Male, % | 51 [261] |
51 [209] |
| White, % | 66 [260] |
67 [209] |
| Ventilator use, % | 30 [261] |
22 [209] |
| UNOS Status 1, % | 79 [254] |
86 [209] |
| Time, Diagnosis to Listing, mean (SD), ymedian | 0.62 (1.3) 0.16 [261] |
0.69 (1.3) 0.22 [209] |
| Time, Listing to Transplant, mean (SD), y median |
…
[261] |
0.23 (0.7) 0.067 [209] |
| Myocarditis, % | 11 [261] |
11 [209] |
| LVEF, mean (SD), % | 26 (9) [38] |
25 (10) [34] |
| LVFS, mean (SD), z-score | -9.7 (2.4) [204] |
-9.6 (2.6) [161] |
| LVEDD, mean (SD), z-score | 5.2 (2.3) [185] |
5.2 (2.2) [144] |
Characteristics of Children with Dilated Cardiomyopathy Listed for Transplantation
Demographics of the 261 patients who were listed and the 209 who were transplanted are shown in Table 1. For the children who were listed, the median age at the time of diagnosis was 3.4 years (range; 0-17.9 years), and 43% were diagnosed at less than one year of age (Table 2). A third of the patients listed for transplantation presented after 10 years of age. Thirty percent of all patients listed were ventilator-dependent at the time of listing. Data on inotropic support were not available. Of the initial 261 children with DCM, 11% (29) were diagnosed with myocarditis either clinically or by biopsy at presentation.
Table 2. Age Distributions of 261 Children with Dilated Cardiomyopathy.
| Age Group years | At Diagnosis n (%) | At Listing n (%) | At Transplant n (%) |
|---|---|---|---|
| <0.5 | 86 (33) | 48 (18) | 23 (11) |
| 0.5-1 | 26 (10) | 32 (12) | 32 (15) |
| 1-10 | 62 (24) | 89 (34) | 75 (36) |
| >10 | 87 (33) | 92 (35) | 79 (38) |
|
| |||
| Total | 261 (100) | 261 (100) | 209 (100) |
Risk Factors Associated with Status 1 at Listing
Shorter duration of illness from diagnosis to listing was associated with listing as status 1 (p=0.04, odds ratio .789, 95% confidence interval .631-.988). No other factors were found to be associated with listing as Status 1.
Death While Waiting for Transplantation
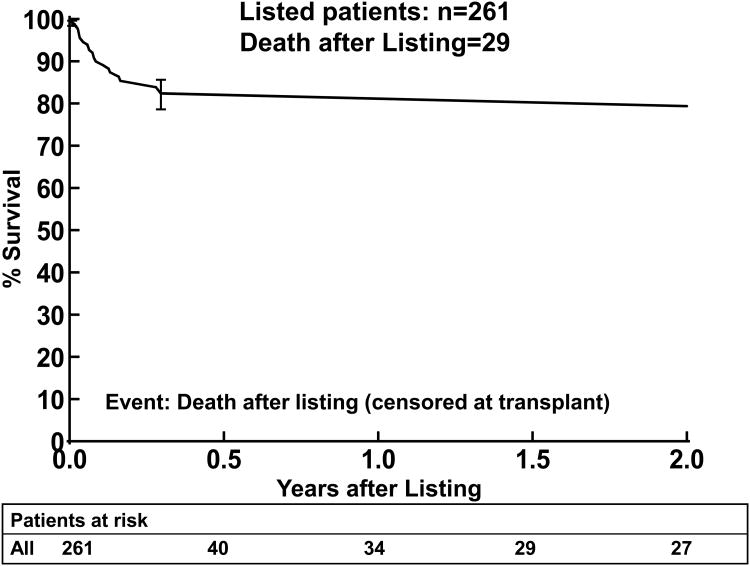
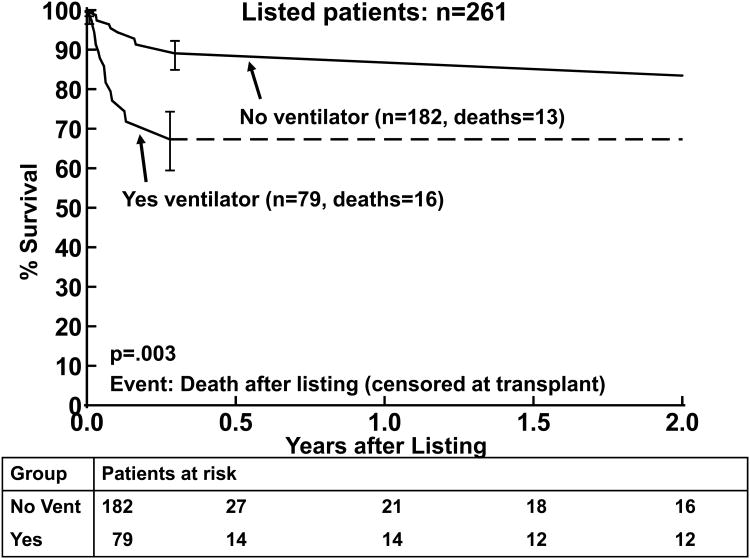
There were 29 deaths prior to transplant, three occurring after 2 years from listing. By 2 years after listing, 11% (n=26) of the patients had died while waiting, 11% (n=26) were still alive waiting, and 79% (n=206) had been transplanted (Figure 1). The primary cause of death was cardiac in nature (primary cardiac failure, sudden death, or multisystem failure) in 16 of the 29 children (55%; Table 3). Neurologic events were a common cause of death, occurring in 6/29 (21%). Most of the risk factors analyzed did not correlate with death while waiting, Tables 4 and 5. The time on the wait-list for children who died while waiting was not significantly shorter than the time from listing to transplantation in those who were transplanted. Mechanical ventilation at listing (p=0.003) was associated with death while waiting by Kaplan-Meier analysis (Figure 2). Mechanical ventilation at listing had a hazard ratio of 4.06 and was strongly associated with death while waiting in the multivariable analysis (p=0.0006). Age was not a risk factor for death while waiting in the univariate analysis, Table 4a. A higher proportion of younger patients were ventilated at listing compared to older patients, thus when mechanical ventilation was included in the analysis, multivariable analysis demonstrated that older age at listing was a risk factor (Table 5a). One child with myocarditis died while waiting for heart transplantation. A subgroup analysis in children with DCM without myocarditis (n=232) also showed ventilator use and older age at listing as risk factors for death (p=0.0001, and p=0.01, respectively).
Figure 1.
Kaplan-Meier survival curve for the first two years after listing (censored at transplantation) for children with DCM (n=261, 26 deaths by two years after listing). The error bars represent 70% confidence limits.
Table 3. Primary Causes of Death among 29 Children with Dilated Cardiomyopathy who Died after Listing for Cardiac Transplantation.
| Primary Cause of Death | n | % |
|---|---|---|
| Cardiac Failure | 12 | 41 |
| Neurologic or Cerebrovascular Accident | 6 | 21 |
| Multi-organ or System Failure | 2 | 7 |
| Sudden Cardiac Death | 2 | 7 |
| Respiratory Failure | 1 | 4 |
| Pulmonary Hemorrhage | 1 | 4 |
| Other | 5 | 17 |
|
| ||
| Total | 29 | 100 |
Table 4. Univariate Risk Factors for Death among Children with Dilated Cardiomyopathy.
| A. Death while waiting for transplantation (29 deaths of 261 children) | |
|---|---|
|
| |
| Potential Risk Factor | Univariate P Value |
| White race | 0.12 |
| Male gender | 0.73 |
| On ventilator at listing | 0.003 |
| Older age at listing* | 0.30 |
| UNOS Status1 at listing | 0.10 |
| Time from diagnosis to listing | 0.45 |
| LV FS z-score at presentation | 0.52 |
| LVEDD z-score at presentation | 0.89 |
|
| |
| *mechanical ventilation removed from analysis | |
| B. Death after transplantation (40 deaths of 209 children) | |
|---|---|
|
| |
| Potential Risk Factor | Univariate P Value |
| Non-white race | <.0001 |
| Male gender | 0.94 |
| On ventilator at transplant | 0.97 |
| Older age at transplant | 0.05 |
| UNOS Status 1 at transplant | 0.48 |
| Myocarditis at diagnosis | 0.02 |
| Time from diagnosis to listing | 0.83 |
| Time from listing to transplant | 0.47 |
| LV FS z-score at presentation | 0.07 |
| LVEDD z-score at presentation (lower) | 0.10 |
UNOS: United Organ Sharing network
LVEDD: Left Ventricular End Diastolic Dimension
Table 5. Multivariable Risk Factors for Death among Children with Dilated Cardiomyopathy.
| A. Death while waiting for transplantation (29 deaths of 261 children) | |||
|---|---|---|---|
|
| |||
| Potential Risk Factor | Multivariable P Value | Hazard Ratio | 95% Conf Limits |
| On ventilator at listing | 0.0006 | 4.06 | 1.79 – 9.19 |
| Older age at listing | 0.03 | 1.07 | 1.01 – 1.14 |
| B. Death after transplantation (40 deaths of 209 children) | |||
|---|---|---|---|
|
| |||
| Potential Risk Factor | Multivariable P Value | Hazard Ratio | 95% Conf Limits |
| Non-white race | <.0001 | 4.55 | 2.35 – 8.83 |
| Myocarditis at diagnosis | 0.009 | 2.71 | 1.27 – 5.82 |
| LVEDD z-score at presentation (lower) | 0.04 | 1.19 | 1.01 – 1.42 |
LVEDD: Left Ventricular End Diastolic Dimension
Figure 2.
Kaplan-Meier survival curves after listing (censored at transplantation) for patients who were (Yes vent) and were not (No vent) mechanically ventilated at listing. The error bars represent 70% confidence limits.
Characteristics of Children with Dilated Cardiomyopathy who Survived to Transplantation
Of the 261 patients listed for transplantation 209 (80%) received heart transplants. The median age at transplantation was 4.4 years. UNOS Status 1 at listing did not correlate with death while waiting. The transplanted group had a higher proportion of children who were UNOS Status1 at the time of transplantation compared to the time of listing (86% versus 79%). In the myocarditis group 23 of 29 were transplanted (1 died waiting, 5 still waiting at analysis). The proportion of children who were Status 1 at the time of transplantation was the same in the myocarditis and non-myocarditis groups. At the time of heart transplantation 22% of children were ventilator dependent.
Causes of Death Post-Transplantation
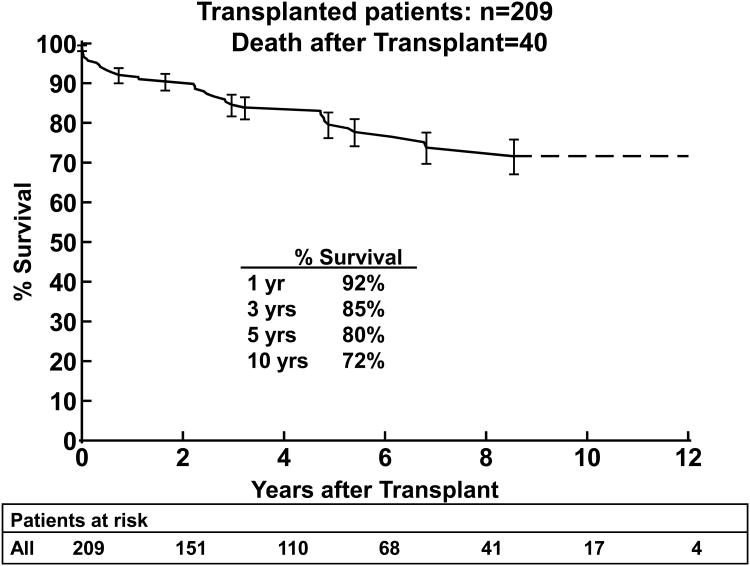
Among the 209 children who underwent heart transplantation, 40 died; 1-year survival was 92%, 5-year survival was 80%, and 10-year survival was 72%, (Figure 3a). The most common cause of death post-transplantation was the development of graft vasculopathy and/or myocardial infarction in 11 patients (28% of deaths). Sudden cardiac death occurred in 4 children (10% of deaths), likely related to either vasculopathy or acute rejection.17 Rejection was the cause of death in 10 patients (9 acute, 1 hyperacute rejection; 25% of deaths). There were 2 deaths from early graft failure, and 1 from hyperacute rejection.
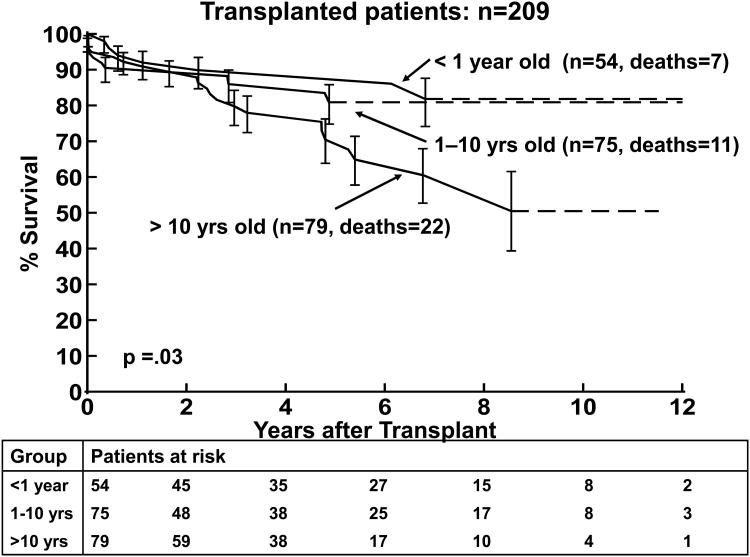
Figure 3.
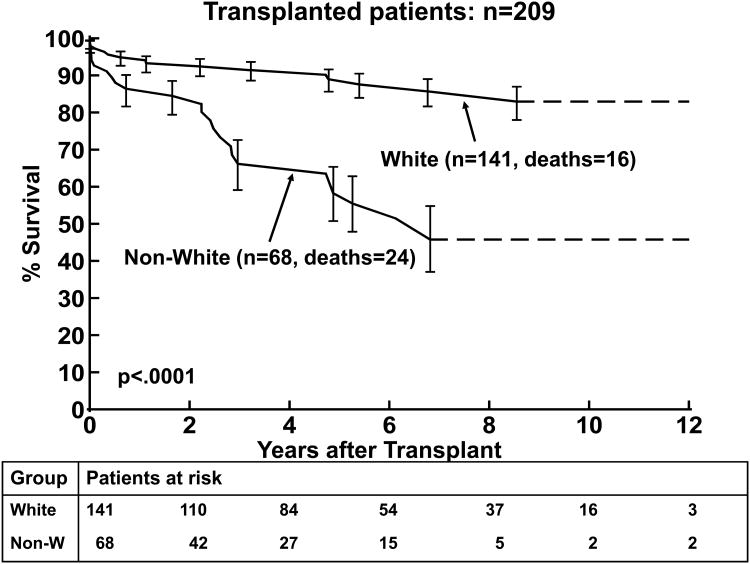
3(a). Kaplan-Meier post transplantation survival curve for children with DCM (n=209). 3(b). Kaplan Meier post-transplantation curve for children < 1 year, 1-10 years, and >10 years of age at transplantation. 3(c). Kaplan-Meier post–transplantation survival curves for children with non-white versus white race. The error bars represent 70% confidence limits.
Risk Factors for Death Post-Transplantation
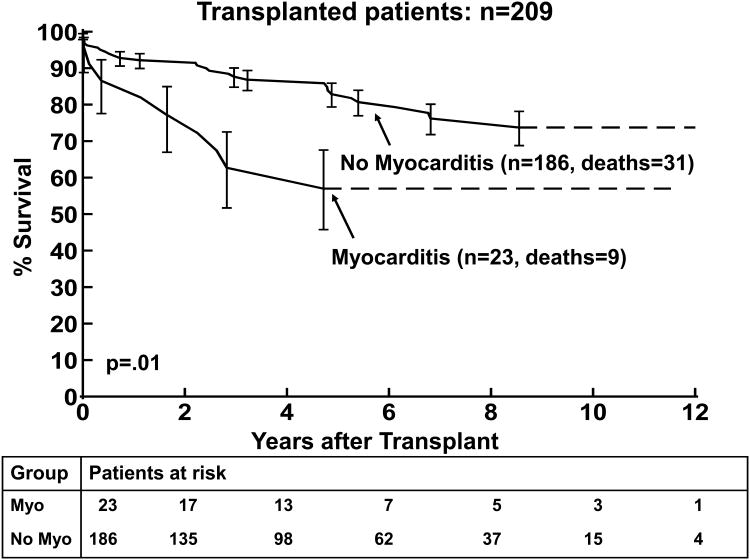
The results of the univariate and multivariable analysis of death post-transplantation are shown in Tables 4 and 5, respectively. Older age at transplantation was associated with death post transplantation (p=0.05) by univariate analysis but not on multivariable analysis, Figure 3b. Non-white race was significantly associated with worse survival in both the univariate and multivariable analyses (p<0.0001, Figure 3c). A diagnosis of myocarditis was associated with worse survival in the univariate and multivariable analyses (p=0.02 and p=0.009, respectively; Figure 4a). A lower LVEDD z-score was also associated with worse post-transplantation survival by multivariable analysis (p=0.04).
Figure 4.
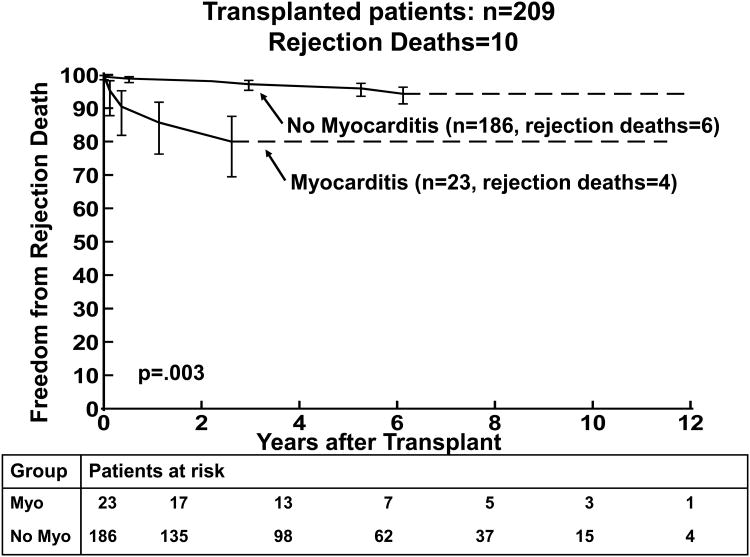
4(a) Kaplan-Meier post–transplantation survival curve for children with the diagnosis of myocarditis versus no myocarditis (at presentation). 4(b) Kaplan-Meier curves comparing freedom from rejection death post-transplantation for children with the diagnosis of myocarditis versus no myocarditis. The error bars represent 70% confidence limits.
Myocarditis and Outcomes
Survival at 1 and 3 years post-transplantation was 83% and 65% in children with myocarditis compared to 93% and 88% in the group without myocarditis (p=0.01, Figure 4a). The median age at transplantation was 11.4 years in the children with myocarditis at presentation and 3.6 years in the children who did not have myocarditis at transplantation (p=0.03). (Table 6) LV FS z-score was not significantly worse in the myocarditis group. The LVEDD z-score was similar between the two groups. Smaller LVEDD z-score was a risk factor for death in the non-myocarditis subgroup (p=0.02). Because of small numbers LVEDD z-score could not be analyzed in the myocarditis subgroup. Death from acute rejection was more common in the myocarditis group compared to the non-myocarditis group (17% versus 3%, respectively, p=0.003, Figure 4b).
Table 6. Characteristics of 209 Children with and without Myocarditis at the Time of Cardiac Transplantation.
| Characteristic | Children with Myocarditis (n=23) [n] | Children without Myocarditis (n=186) [n] |
|---|---|---|
| Age at Transplant, mean (SD), y median | 9.4 (6.2) 11.4 [23] |
6.7 (6.3) 3.6 [186] |
| Male, % | 57 [23] |
51 [186] |
| White, % | 74 [23] |
67 [186] |
| LV FS z-score at presentation | -9.5 (3.4) [17] |
-9.6 (2.5) [144] |
| LVEDD z-score at presentation | 5.5 (1.1) [14] |
5.2 (2.6) [130] |
| Ventilator-Dependent, % | 30 [23] |
22 [186] |
| UNOS Status 1, % | 83 [23] |
87 [180] |
| Time from Diagnosis to Listing, mean(SD) median, y | 1.1 (1.8) 0.44 [23] |
0.63 (1.3) 0.21 [186] |
| Time from Listing to Transplant, mean(SD) median, y | 0.12 (0.1) 0.05 [23] |
0.24 (0.8) 0.07 [186] |
LV FS: Left ventricular fractional shortening
LVEDD: Left ventricular end diastolic dimension
UNOS: United Organ Sharing network
Discussion
Identification of risk factors for worse outcome after listing is an important step toward optimizing the management of children with DCM. The merged dataset available from the PCMR and PHTS offers a unique opportunity to follow patients from the time of diagnosis through the post-transplantation period. In our study, mortality on the heart transplantation wait-list was 11%. None of the variables measured at the time of diagnosis of DCM were associated with death while waiting for heart transplantation except mechanical ventilation and older age, which have previously been described as risk factors.23, 24 Death post-transplantation was associated with three factors: non-white race, a small left ventricular dimension and a diagnosis of myocarditis at the time of presentation.
Other studies have identified older age and black race as risk factors for worse survival after transplantation.2, 17, 18, 25, 26 In our study, older age was a predictor for death post-transplantation in univariate analysis, but not in the multivariable analysis. Older age, in particular, adolescence, is a known predictor for death post-transplantation in children.2, 17, 18 This finding has been attributed to adolescent defiance, non-adherence to treatment and risk-taking behavior. Worse outcome in this age group is not limited to heart transplantation patients, it is also seen in other solid organ transplantation patients,27 and with other chronic diseases such as diabetes.28 Black race is a common finding in many transplantation outcome studies. Higher mortality and morbidity in black race, compared to other races, have largely been attributed to lower socioeconomic status, lower level of education, and limited access to health care, but may also be attributed to genetic differences in drug metabolism and immunity.25 Although the black population may have genetic causes, as opposed to demographic, or social characteristics that predispose them to a worse outcome, this study was not powered to address these possibilities.
In this study, the diagnosis of myocarditis was not associated with death while waiting for heart transplantation, but, strikingly, myocarditis was associated with a worse outcome after transplantation compared with children without the diagnosis of myocarditis. In fact, 97% of the children listed who had myocarditis at presentation, survived to heart transplantation. Efforts to be certain that these results could not be explained by more severe cardiac dysfunction in the myocarditis group before transplantation were limited by the lack of complete echocardiographic and hemodynamic data at the time of transplantation. However, the data available suggests that children with DCM, with or without myocarditis, were similar in their severity of illness at the time of transplantation. The finding that a smaller LVEDD z-score was associated with death post-transplantation may represent a group of children with more acute cardiomyopathy in whom the LV has not yet dilated. However, this finding was only significant in multivariable analysis including the diagnosis of myocarditis, so other factors may be involved. One consideration as to the validity of a combined histologic and clinical definition of myocarditis is that this approach is supported by a PCMR-sponsored analysis that compared children with biopsy confirmed myocarditis to those with clinically diagnosed myocarditis and demonstrated similar survival outcomes between the two groups.21 Sub-group analysis of each of these two methods of diagnosis for myocarditis separately was not feasible because of small numbers and the retrospective nature of the study design. In a PHTS analysis of children with DCM, myocarditis identified at transplantation was not a risk factor for death post-transplantation.18 Differences between that study and the present study may be best addressed with a prospective study of children with biopsy-proven myocarditis to resolve this discrepancy.
Our finding that the diagnosis of myocarditis was associated with worse survival post transplantation raises the possibility that infectious or immune mechanisms persist that may affect outcome. The finding that acute rejection was a common cause of death in the myocarditis group supports this hypothesis. Possible explanations include ongoing sub-clinical viral infection, a change in immune or “heterologous” memory, viral reactivation after transplantation, or ongoing autoimmunity. In animal studies persistent viral infection at the time of heart transplantation increases the tempo and likelihood of acute rejection, and precludes the induction of allograft tolerance.29,30 Heterologous immunity and acquired immune memory shape immune responses based on infections encountered. Because heterologous immunity has been shown to be associated with increased acute rejection it has potential to increase the need for immunosuppression to avoid allograft rejection. Viral myocarditis has been shown to occasionally relapse after transplantation.31 Parvovirus B19 has been associated with death in children after heart transplantation and may contribute to cardiac transplantation rejection.32,33 In this respect, myocardial damage may be directly mediated by the virus itself, as in the case of Coxsackie B3 viral myocarditis.34,35 In heart transplantation patients, viral genome detection by PCR in endomyocardial biopsies from children following transplantation has been associated with acute rejection, transplant vasculopathy and graft loss.36,37 On the other hand, the immune response and subsequent autoimmunity may be important in the pathogenesis of myocardial dysfunction. Nonspecific markers of inflammation, such as C-reactive protein (CRP) have been associated with worse outcome in myocarditis.38 Persistent viral immunity may also have a role in the pathogenesis of myocarditis. In support of this hypothesis, several reports describe favorable outcomes in children with biopsy-proven myocarditis in DCM who improved with either immunomodulation (immune globulin)39, 40 or immunosuppression.41-44 Unfortunately, in our study no information was available on the presence of active virus at transplantation, nor viral PCR testing of endomyocardial biopsies.
The clinical implications of the finding that DCM caused by myocarditis is a risk factor for worse survival after transplantation suggest the need for improved pre- and post-transplantation interventions in children. The need to standardize the assessment of chronic viral infection, and inflammation of the myocardium has long been a goal of the pediatric cardiology community, and appears to be pertinent in the assessment for transplantation eligibility as well.45 Treatment also needs to be standardized since as many as 5% of patients with DCM receive immunomodulatory therapy without objective evidence of myocarditis.9 Post-transplant immunosuppression protocols varied among the centers in our study. Therefore, it was not possible to determine the impact of the type and intensity of immunosuppression, or use of induction regimens (including the routine use of intravenous immune globulin) on the outcome of patients with myocarditis.
Limitations of the Study Design
Pediatric DCM is a diverse collection of diseases, with both acquired and genetic causes. As a result, registry-based studies like ours have limitations in that heterogeneity may obscure causal relationships. This study was limited by its retrospective nature, which despite the merger of two registries, had a relatively modest number of patients. Subgroup analysis, such as myocarditis, results in even smaller numbers in each group, which make robust statistical statements challenging. Data were limited to what was available and included in the databases prior to the merger in 2005. Data were also limited to the number of variables collected in the registries, without the possibility of additional collection. Despite these limitations, the information is valuable in predicting outcome and counseling families. These limitations remain a reality of research into rare diseases, such as pediatric cardiomyopathy, and further emphasize the need for larger and more detailed continued data collection efforts by registries. In addition, although diagnostic evaluations and treatment strategies were similar at the data collection sites, standardized protocols were not used within the study group. Multiple physicians initiated, manipulated and terminated therapy for individual patients in different ways. This limitation can only be overcome with a prospective randomized study design, which would be challenging, due to the low incidence of children presenting with severe heart failure and myocarditis.
Conclusions
We conclude that mechanical ventilation and older age at listing in patients not mechanically ventilated were risk factors for death while waiting for heart transplantation, factors that allow risk-stratification of patients at the time of listing. Outcomes of children with DCM post-transplantation are affected by non-white race and older age. Myocarditis is associated with a higher mortality post-transplantation and suggests a persistent infectious or immune mechanism.
Factors that affect outcome in pediatric heart diseases, such as dilated cardiomyopathy (DCM), are often difficult to define because of the rarity of disease. In order to study the potential importance of factors at presentation and at listing for heart transplantation in children with DCM on outcome, we analyzed data from two large pediatric registries the Pediatric Cardiomyopathy Registry and Pediatric Heart Transplant Study. In the merged data set there were 261 children with DCM. Among the factors studied were age, duration of illness, gender, race, ventricular geometry and the clinical or histologic diagnosis of myocarditis at presentation. We found that death while waiting was associated with ventilator use and older age at listing. A shorter duration of illness was associated with a more urgent listing status (UNOS Status 1). Death post-transplantation was associated black race, and lower left ventricular end-diastolic dimension z-score at presentation. We also found that death post-transplantation was associated with the diagnosis myocarditis at presentation (p=<0.009). Death while waiting was not associated with the diagnosis of myocarditis and 97% of children with myocarditis survived to transplantation. Further, the most common cause of death post-transplantation in the myocarditis group was acute rejection (17%). This is the first study to show that children with DCM and myocarditis have significantly higher mortality post-transplantation compared with children without myocarditis. This finding suggests that pre-existing viral infection, or inflammation, could adversely affect heart allograft survival and has implications the management of these children pre and post heart transplantation in the future.
Acknowledgments
We thank the participating PCMR and PHTS clinical centers for patient recruitment, Susan Myers, BA for statistical analysis assistance and Tom Lang, MA for editorial assistance. Dr. Anne Dipchand for assistance in the review and coding of data from Canadian children. Mr. Lang was compensated for his presubmittal editorial review of the manuscript.
Funding Sources: RO1 HL 53392 National Institutes of Health, National Heart Blood and Lung Institute.
Footnotes
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez JA, Wilkinson JD, Lipshultz SE, Group PCRS. Outcome predictors for pediatric dilated cardiomyopathy: a systematic review. Progress in Pediatric Cardiology. 2007;23:25–32. doi: 10.1016/j.ppedcard.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucek MM, Aurora P, Edwards LB, Taylor DO, Trulock EP, Christie J, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: tenth official pediatric heart transplantation report--2007. J Heart Lung Transplant. 2007;26:796–807. doi: 10.1016/j.healun.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, Lurie PR, McCoy KL, McDonald MA, Messere JE, Colan SD. The incidence of pediatric cardiomyopathy in two regions of the United States. N Engl J Med. 2003;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 4.Daubeney PE, Nugent AW, Chondros P, Carlin JB, Colan SD, Cheung M, Davis AM, Chow CW, Weintraub RG. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation. 2006;114:2671–2678. doi: 10.1161/CIRCULATIONAHA.106.635128. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson JD, Sleeper LA, Alvarez JA, Bublik N, Lipshultz SE. The Pediatric Cardiomyopathy Registry: 1995-2007. Prog Pediatr Cardiol. 2008;25:31–36. doi: 10.1016/j.ppedcard.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 7.Towbin JA. Cardiomyopathy and heart transplantation in children. Curr Opin Cardiol. 2002;17:274–279. doi: 10.1097/00001573-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:1377–1384. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Harmon WG, Sleeper LA, Cuniberti L, Messere J, Colan SD, Orav EJ, Towbin JA, Wilkinson JD, Lipshultz SE. Treating children with idiopathic dilated cardiomyopathy (from the Pediatric Cardiomyopathy Registry) Am J Cardiol. 2009;104:281–286. doi: 10.1016/j.amjcard.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon AM, van Doorn C, Burch M, Whitmore P, Neligan S, Rees P, Radley-Smith R, Goldman A, Brown K, Cohen G, Tsang V, Elliott M, de Leval MR. Improved early outcome for end-stage dilated cardiomyopathy in children. J Thorac Cardiovasc Surg. 2003;126:1781–1787. doi: 10.1016/j.jtcvs.2003.07.029. [DOI] [PubMed] [Google Scholar]
- 11.Hsu DT, Canter CE. Dilated cardiomyopathy and heart failure in children. Heart Fail Clin. 2010;6:415–432. vii. doi: 10.1016/j.hfc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal DN, Dubin AM, Chin C, Falco D, Gamberg P, Bernstein D. Outcome while awaiting heart transplantation in children: a comparison of congenital heart disease and cardiomyopathy. J Heart Lung Transplant. 2000;19:751–755. doi: 10.1016/s1053-2498(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 13.Valantine HA, Hunt SA, Fowler MB, Billingham ME, Schroeder JS. Frequency of familial nature of dilated cardiomyopathy and usefulness of cardiac transplantation in this subset. Am J Cardiol. 1989;63:959–963. doi: 10.1016/0002-9149(89)90148-3. [DOI] [PubMed] [Google Scholar]
- 14.Taylor DO, Edwards LB, Boucek MM, Trulock EP, Aurora P, Christie J, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report--2007. J Heart Lung Transplant. 2007;26:769–781. doi: 10.1016/j.healun.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Adwani SS, Whitehead BF, Rees PG, Whitmore P, Fabre JW, Elliott MJ, de Leval MR. Heart transplantation for dilated cardiomyopathy. Arch Dis Child. 1995;73:447–452. doi: 10.1136/adc.73.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaddy RE, Naftel DC, Kirklin JK, Boyle G, McGiffin DC, Towbin JA, Ring WS, Pearce B, Addonizio L, Morrow WR. Outcome of cardiac transplantation in children. Survival in a contemporary multi-institutional experience. Pediatric Heart Transplant Study. Circulation. 1996(94):II69–73. [PubMed] [Google Scholar]
- 17.Dipchand AI, Naftel DC, Feingold B, Spicer R, Yung D, Kaufman B, Kirklin JK, Allain-Rooney T, Hsu D. Outcomes of children with cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28:1312–1321. doi: 10.1016/j.healun.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Kirk R, Naftel D, Hoffman TM, Almond C, Boyle G, Caldwell RL, Kirklin JK, White K, Dipchand AI. Outcome of pediatric patients with dilated cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28:1322–1328. doi: 10.1016/j.healun.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirk R, Edwards LB, Aurora P, Taylor DO, Christie JD, Dobbels F, Kucheryavaya AY, Rahmel AO, Stehlik J, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twelfth Official Pediatric Heart Transplantation Report-2009. J Heart Lung Transplant. 2009;28:993–1006. doi: 10.1016/j.healun.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Grenier MA, Osganian SK, Cox GF, Towbin JA, Colan SD, Lurie PR, Sleeper LA, Orav EJ, Lipshultz SE. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139:S86–95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 21.Foerster SR, Canter CE, Cinar A, Sleeper LA, Webber SA, Pahl E, Kantor PF, Alvarez JA, Colan SD, Jefferies JL, Lamour JM, Margossian R, Messere JE, Rusconi PG, Shaddy RE, Towbin JA, Wilkinson JD, Lipshultz SE. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: an outcomes study from the Pediatric Cardiomyopathy Registry. Circ Heart Fail. 2010;3:689–697. doi: 10.1161/CIRCHEARTFAILURE.109.902833. [DOI] [PubMed] [Google Scholar]
- 22.Arola A, Tuominen J, Ruuskanen O, Jokinen E. Idiopathic dilated cardiomyopathy in children: prognostic indicators and outcome. Pediatrics. 1998;101:369–376. doi: 10.1542/peds.101.3.369. [DOI] [PubMed] [Google Scholar]
- 23.McGiffin DC, Naftel DC, Kirklin JK, Morrow WR, Towbin J, Shaddy R, Alejos J, Rossi A. Predicting outcome after listing for heart transplantation in children: comparison of Kaplan-Meier and parametric competing risk analysis. Pediatric Heart Transplant Study Group. J Heart Lung Transplant. 1997;16:713–722. [PubMed] [Google Scholar]
- 24.Morrow WR, Frazier E, Naftel DC. Survival after listing for cardiac transplantation in children. Prog Pediatr Cardiol. 2000;11:99–105. doi: 10.1016/s1058-9813(00)00041-2. [DOI] [PubMed] [Google Scholar]
- 25.Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr. 2005;147:739–743. doi: 10.1016/j.jpeds.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Tsirka AE, Trinkaus K, Chen SC, Lipshultz SE, Towbin JA, Colan SD, Exil V, Strauss AW, Canter CE. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol. 2004;44:391–397. doi: 10.1016/j.jacc.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Pediatr Transplant. 2007;11:366–373. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 28.Bryden KS, Dunger DB, Mayou RA, Peveler RC, Neil HA. Poor prognosis of young adults with type 1 diabetes: a longitudinal study. Diabetes Care. 2003;26:1052–1057. doi: 10.2337/diacare.26.4.1052. [DOI] [PubMed] [Google Scholar]
- 29.Williams MA, Onami TM, Adams AB, Durham MM, Pearson TC, Ahmed R, Larsen CP. Cutting edge: persistent viral infection prevents tolerance induction and escapes immune control following CD28/CD40 blockade-based regimen. J Immunol. 2002;169:5387–5391. doi: 10.4049/jimmunol.169.10.5387. [DOI] [PubMed] [Google Scholar]
- 30.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunol Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 31.Calabrese F, Rigo E, Milanesi O, Boffa GM, Angelini A, Valente M, Thiene G. Molecular diagnosis of myocarditis and dilated cardiomyopathy in children: clinicopathologic features and prognostic implications. Diagn Mol Pathol. 2002;11:212–221. doi: 10.1097/00019606-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Francalanci P, Chance JL, Vatta M, Jimenez S, Li H, Towbin JA, Bowles NE. Cardiotropic viruses in the myocardium of children with end-stage heart disease. J Heart Lung Transplant. 2004;23:1046–1052. doi: 10.1016/j.healun.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Schowengerdt KO, Ni J, Denfield SW, Gajarski RJ, Bowles NE, Rosenthal G, Kearney DL, Price JK, Rogers BB, Schauer GM, Chinnock RE, Towbin JA. Association of parvovirus B19 genome in children with myocarditis and cardiac allograft rejection: diagnosis using the polymerase chain reaction. Circulation. 1997;96:3549–3554. doi: 10.1161/01.cir.96.10.3549. [DOI] [PubMed] [Google Scholar]
- 34.Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, Knowlton KU. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- 35.Towbin JA, Bowles KR, Bowles NE. Etiologies of cardiomyopathy and heart failure. Nat Med. 1999;5:266–267. doi: 10.1038/6474. [DOI] [PubMed] [Google Scholar]
- 36.Shirali GS, Ni J, Chinnock RE, Johnston JK, Rosenthal GL, Bowles NE, Towbin JA. Association of viral genome with graft loss in children after cardiac transplantation. N Engl J Med. 2001;344:1498–1503. doi: 10.1056/NEJM200105173442002. [DOI] [PubMed] [Google Scholar]
- 37.Moulik M, Breinholt JP, Dreyer WJ, Kearney DL, Price JF, Clunie SK, Moffett BS, Kim JJ, Rossano JW, Jefferies JL, Bowles KR, O'Brian Smith E, Bowles NE, Denfield SW, Towbin JA. Viral endomyocardial infection is an independent predictor and potentially treatable risk factor for graft loss and coronary vasculopathy in pediatric cardiac transplant recipients. J Am Coll Cardiol. 2010;56:582–592. doi: 10.1016/j.jacc.2010.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaneko K, Kanda T, Hasegawa A, Suzuki T, Kobayashi I, Nagai R. C-reactive protein as a prognostic marker in lymphocytic myocarditis. Jpn Heart J. 2000;41:41–47. doi: 10.1536/jhj.41.41. [DOI] [PubMed] [Google Scholar]
- 39.Maisch B, Herzum M, Hufnagel G, Bethge C, Schonian U. Immunosuppressive treatment for myocarditis and dilated cardiomyopathy. Eur Heart J. 1995;16(O):153–161. doi: 10.1093/eurheartj/16.suppl_o.153. [DOI] [PubMed] [Google Scholar]
- 40.McNamara DM, Rosenblum WD, Janosko KM, Trost MK, Villaneuva FS, Demetris AJ, Murali S, Feldman AM. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation. 1997;95:2476–2478. doi: 10.1161/01.cir.95.11.2476. [DOI] [PubMed] [Google Scholar]
- 41.Gagliardi MG, Bevilacqua M, Squitieri C, Boldrini R, Di Julio DP, Marcelletti C. Dilated cardiomyopathy caused by acute myocarditis in pediatric patients: evolution of myocardial damage in a group of potential heart transplant candidates. J Heart Lung Transplant. 1993;12:S224–229. [PubMed] [Google Scholar]
- 42.Kleinert S, Weintraub RG, Wilkinson JL, Chow CW. Myocarditis in children with dilated cardiomyopathy: incidence and outcome after dual therapy immunosuppression. J Heart Lung Transplant. 1997;16:1248–1254. [PubMed] [Google Scholar]
- 43.Gagliardi MG, Bevilacqua M, Bassano C, Leonardi B, Boldrini R, Camassei FD, Fierabracci A, Ugazio AG, Bottazzo GF. Long term follow up of children with myocarditis treated by immunosuppression and of children with dilated cardiomyopathy. Heart. 2004;90:1167–1171. doi: 10.1136/hrt.2003.026641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hia CP, Yip WC, Tai BC, Quek SC. Immunosuppressive therapy in acute myocarditis: an 18 year systematic review. Arch Dis Child. 2004;89:580–584. doi: 10.1136/adc.2003.034686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipshultz SE, Wilkinson JD. Epidemiological and Outcomes Research in Children with Pediatric Cardiomyopathy: Discussions from the International Workshop on Primary and Idiopathic Cardiomyopathies in Children. Prog Pediatr Cardiol. 2008;25:23–25. doi: 10.1016/j.ppedcard.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]