Background: The biochemical activities and tissue distribution of GADL1 have remained unknown.
Results: GADL1 is expressed in muscles and kidneys; it catalyzes decarboxylation of aspartate, cysteine sulfinic acid, and cysteic acid to produce β-alanine, hypotaurine, and taurine.
Conclusion: GADL1 has aspartate 1-decarboxylase and cysteine sulfinic acid decarboxylase activities.
Significance: GADL1 could potentially participate in mammalian taurine biosynthesis.
Keywords: Amino Acid, Aspartate, Decarboxylase, Mammal, Muscle, Beta-Alanine, GADL1, Hypotaurine, Taurine
Abstract
This manuscript concerns the tissue-specific transcription of mouse and cattle glutamate decarboxylase-like protein 1 (GADL1) and the biochemical activities of human GADL1 recombinant protein. Bioinformatic analysis suggested that GADL1 appears late in evolution, only being found in reptiles, birds, and mammals. RT-PCR determined that GADL1 mRNA is transcribed at high levels in mouse and cattle skeletal muscles and also in mouse kidneys. Substrate screening determined that GADL1, unlike its name implies, has no detectable GAD activity, but it is able to efficiently catalyze decarboxylation of aspartate, cysteine sulfinic acid, and cysteic acid to β-alanine, hypotaurine, and taurine, respectively. Western blot analysis verified the presence of GADL1 in mouse muscles, kidneys, C2C12 myoblasts, and C2C12 myotubes. Incubation of the supernatant of fresh muscle or kidney extracts with cysteine sulfinic acid resulted in the detection of hypotaurine or taurine in the reaction mixtures, suggesting the possible involvement of GADL1 in taurine biosynthesis. However, when the tissue samples were incubated with aspartate, no β-alanine production was observed. We proposed several possibilities that might explain the inactivation of ADC activity of GADL1 in tissue protein extracts. Although β-alanine-producing activity was not detected in the supernatant of tissue protein extracts, its potential role in β-alanine synthesis cannot be excluded. There are several inhibitors of the ADC activity of GADL1 identified. The discovery of GADL1 biochemical activities, in conjunction with its expression and activities in muscles and kidneys, provides some tangible insight toward establishing its physiological function(s).
Introduction
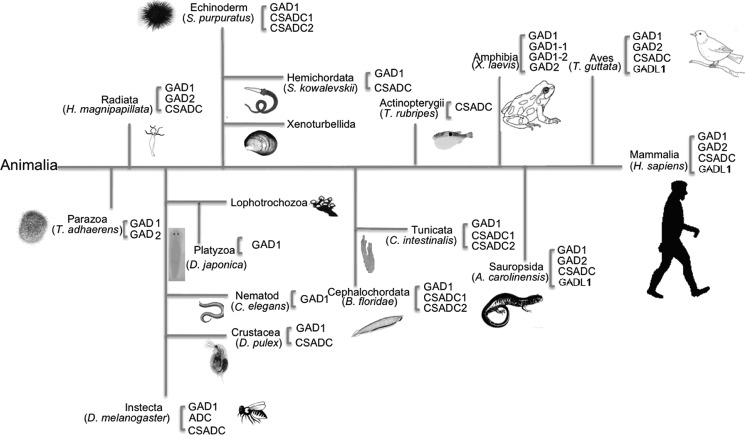
γ-Aminobutyric acid (GABA)2 is found in every class of living organisms (1). In higher organisms, GABA functions as an inhibitory neurotransmitter; for those without nervous systems, such as plants and bacteria, GABA is considered an important signaling molecule (2, 3). GAD catalyzes the synthesis of GABA from glutamate. Even though GAD is a pyridoxal-5′-phosphate (PLP)-containing enzyme, bacterial/plant and animal GADs are quite different (4, 5). Animal GAD is present in Parazoa and all later groups of animals with ∼40% sequence similarity throughout evolution (Fig. 1). In most animal species, there are two isoforms of GAD, which could be required for tissue-specific or developmental regulation and in turn reflect the important roles of GABA in living species (6–8). Other similar PLP-containing acidic amino acid decarboxylases, such as aspartate 1-decarboxylase (ADC), cysteine sulfinic acid decarboxylase (CSADC), and GADL1, seem to have evolved later than GAD in species evolution (Fig. 1).
FIGURE 1.
A cartoon evolutionary diagram showing the appearance of GADs, CSADC, ADC, and GADL1 during species evolution. The assignment of proteins in different classes was based on the overall sequence similarity with typical human GAD1 (GAD67), GAD2 (GAD65), CSADC, and GADL1, respectively. The classification of proteins used in the phylogenetic tree was based on the similarity with human GAD1, GAD2, CSADC, and GADL1.
Animal ADC is only found in insects (Fig. 1). Sequence analysis indicates that insect ADC shares high sequence homology with mammalian CSADC. Recently, we determined that insect ADC also is able to catalyze the decarboxylation of cysteine sulfinic acid to hypotaurine, the typical CSADC activity, but human CSADC has no activity to aspartate (9). HuGADL1 equivalent sequences are present in other available mammalian genomes, but there have been no publications addressing its activity or function. Despite its name, HuGADL1 actually shares higher sequence identity to human CSADC (59% identity) than to human GADs (50 and 51% identity). HuGADL1 also shares sequence similarity to insect ADC (51% identity). Therefore, it is possible that HuGADL1 could have activity for aspartate and/or cysteine sulfinic acid. Compared with the three-step reductive uracil degradation processes for β-alanine synthesis in mammals and some other species (10), insects seem to use a much more straightforward enzymatic pathway to produce β-alanine through aspartate decarboxylation. Mammals need β-alanine for the synthesis of a number of β-alanine-containing dipeptides (particularly carnosine), leading to the question of why a seemingly direct and simple aspartate to β-alanine pathway has not evolved in mammals. It has been established that skeletal muscles contain high concentrations of carnosine whose synthesis occurs primarily in muscles (based on the presence and activity of muscle carnosine synthase) (11, 12). β-Alanine is indispensible for carnosine synthesis, and oral consumption of β-alanine could significantly improve the muscular carnosine concentrations, although a considerable portion of the β-alanine would be digested before reaching to muscles (13, 14). This suggests that in situ synthesis of β-alanine would be advantageous for carnosine synthesis. Muscles have high levels of taurine concentration, whereas the muscular taurine is mostly synthesized in livers and transported to muscles via the taurine transporter (15). It has generally been considered that taurine and β-alanine are not synthesized in muscles, but the similarity of GADL1 to mammalian CSADC and insect ADC, together with its high expression levels in muscles, provides a basis to speculate that GADL1 could use cysteine sulfinic acid or/and aspartate as a substrate, therefore likely involving in taurine or/and β-alanine biosynthesis.
In this study, we expressed recombinant human GADL1 and examined its activity to different amino acids, which resulted in the detection of decarboxylation activity of both aspartate and cysteine sulfinic acid. GADL1 does not work on glutamate as its name suggests. Subsequently, we analyzed the transcript and protein levels of GADL1 in mice and cattle, determining that its mRNA and protein were present primarily in skeletal muscles of both species. The transcription and expression of GADL1 in muscles and the ability of its recombinant protein to produce β-alanine and hypotaurine through decarboxylation of aspartate and cysteine sulfinic acid, respectively, suggest that the decarboxylation of aspartate and cysteine sulfinic acid could be a route of β-alanine and hypotaurine synthesis in skeletal muscles. Then, we were able to detect the hypotaurine-producing activities in the supernatant of protein extracts from muscle and kidney tissues. A number of endogenous compounds were shown to inhibit the ADC activity of GADL1.
EXPERIMENTAL PROCEDURES
Chemicals
All of the chemicals used in this report were from Sigma-Aldrich unless specified otherwise.
Tissue Collection
Mouse tissues were collected from two male mice ∼8 weeks old. Bovine tissues were collected from two Holstein bulls ∼5 years old at slaughter. The tissue samples were immediately frozen in liquid nitrogen and stored at −80 °C until RNA isolation.
Cell Culture
C2C12 myoblasts were cultured in growth medium (DMEM with 10% FBS and 1% antibiotic antimyotic). C2C12 myotubes were cultured in differentiation medium (DMEM with 2% horse serum and 1% antibiotic antimyotic) for 72 h before experiments.
Expression and Purification of GADL1
To express HuGADL1, a forward primer (5′-AAACATATGATTCCAAGTAAGAAGAATGCT-3′) containing a NdeI site (underlined nucleotides) and a reverse primer (5′-AAAGAATTCACATGTCTTTACCCAGTAAGTCTA-3′) containing an EcoRI site (underlined nucleotides) were designed and used to amplify HuGADL1 from human liver cDNA. The amplified HuGADL1 cDNA was cloned into an Impact-CN plasmid (New England Biolabs) for expression of its recombinant protein. The frame of the HuGADL1 was verified by DNA sequencing. Escherichia coli BL 21 cells with the expression vector were induced at 0.15 mm of isopropyl β-d-1-thiogalactopyranoside when optical density reached 1.0 and grew for 24 h at 15 °C before breaking the cells in a suggested lysis buffer. The recombinant enzyme was obtained from E. coli BL 21 cells. The concentrated protein sample was further purified by ion exchange and gel filtration chromatographies (Mono-Q column and Sepharose 12; GE Healthcare) with 20 mm phosphate buffer (pH 7.0). Protein concentrations were determined by a Bio-Rad protein assay using bovine serum albumin as a standard. The spectrum of HuGADL1 was recorded using a Hitachi U2800 UV-visible spectrophotometer.
Substrate Screening
The purified recombinant HuGADL1 was used for substrate screening. Each reaction mixture of 200 μl containing 10 μg of purified recombinant protein and 40 μl of 50 mm of a substrate (aspartate, cysteine sulfinic acid, cysteic acid, or glutamate) was prepared in 150 mm phosphate buffer (pH 7.0) with 0.40 μm of PLP. The reaction mixtures were incubated for 10 min at 25 °C and stopped by adding two volumes of 100% ethanol. The specific activities and standard deviations were calculated based on the averages of triplicates. The mixtures were derivatized by two volumes of o-phthaldialdehyde (OPT) agent as described in a previous method (9). Determination of the products was based on the detection of OPT derivatives by reverse phase liquid chromatography with electrochemical detection. The mobile phase consisted of 50 mm of phosphate buffer (pH 3.5) containing 25% acetonitrile at a flow rate of 0.5 ml per min. The oxidation potential of the working electrode was set to +0.75 V. The activities of HuGADL1 toward the substrates were calculated based on standard curves generated with authentic standards at the identical conditions.
After the substrate specificity of GADLI was determined, protein extracts from either mouse muscles or mouse kidneys were obtained and assayed for decarboxylation of aspartate and cysteine sulfinic acid. The extracts were obtained from raw tissues by homogenization in 100 mm HEPES buffer (pH 7.5). 1 mm of MgCl2, 10 μm of PLP, 2 mm of β-mercaptoethanol, and 20 μm of phenylmethanesulfonyl fluoride (protease inhibitor) were added into the homogenization buffer. The components of homogenization was the same to the previous lysis buffer except that the buffer compound is HEPES instead of Tris as the amino group of tris(hydroxymethyl)aminomethane will interfere with the following OPT assay. The protein extract was directly used for activity assay.
Kinetic Assays
The catalytic efficiencies of HuGADL1 toward aspartate and cysteine sulfinic acid were determined by incubating 20 μg of enzyme in the presence of varying concentrations (0.1, 0.5, 1, 2, 5, 10, and 20 mm) of aspartate and cysteine sulfinic acid in 500 μl of 150 mm of phosphate buffer (pH 7.0) containing 0.40 μm of PLP. Fifty millimolars of aspartate and cysteine sulfinic acid were prepared as stock and adjusted to pH 7.0 using 1 m of phosphate buffer (pH 7.0). Each reaction was incubated for 10 min at 25 °C before analysis. The kinetic parameters and standard deviations were calculated based on the averages of triplicates. Lineweaver-Burk double reciprocal plots (1/V versus 1/S) were used to determine the Michaelis-Menten constant Km and the maximum velocity Vmax.
RNA Isolation
Total RNA was isolated using TRI Reagent® according to the manufacturer's instruction (MRC, Cincinnati, OH). The extracted RNA was dissolved in diethypyrocarbonate-treated water. Concentrations of total RNA were determined using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE).
RT-PCR
Total RNA (0.1 μg) was reverse transcribed into cDNA in a total volume of 20 μl using the ImProm-II reverse transcriptase (Promega) according to the manufacturer's instruction. Ribosomal 18 S RNA was used as internal standard. To amplify the target gene, 5 ng of cDNA was mixed with 12.5 μl of 2 × PCR Master Mix (Promega) and 10 pmol of each corresponding primer in a total volume of 25 μl. The conditions for PCR were 32 cycles at 94 °C for 30 s, 55 °C for 1 min, and 72 °C for 30 s. The primers used for RT-PCR are shown in supplemental Table S1.
Western Blot Analysis
Frozen tissues (∼0.5 g) and C2C12 cells were lysed in ice-cold radioimmunoprecipitation assay buffer (50 mm Tris-HCl, pH 8, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS) supplemented with protease inhibitors (Roche Applied Science) and phosphatase inhibitors (0.5 mm Na3VO4, 5 mm sodium pyrophosphate, 50 mm NaF, 10 mm sodium β-glycerophosphate). The lysates were centrifuged at 12,000 × g for 15 min at 4 °C. The final supernatants were collected and stored at −80 °C. Protein concentrations of the supernatants were determined with a BCA protein assay kit (Thermo Scientific, Rockford, IL). For Western blot analyses, 40 μg of isolated total protein or 10 μg of purified CSADC protein was resolved by 10% SDS-PAGE and then transferred to a nitrocellulose membrane (Bio-Rad). The membrane was blocked with 5% nonfat dried milk in TBST buffer (20 mm Tris-HCl, 500 mm NaCl, and 0.05% Tween 20) and incubated with GADL1 antibody (Thermo Scientific) at 1:100 dilution in TBST with 5% BSA overnight at 4 °C. This antibody was detected using a horseradish peroxidase-conjugated goat IgG antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and SuperSignal West Pico chemiluminescence substrate (Thermo Scientific). Following detection of GADL1 protein, the membrane was stripped with Restore Western blot Stripping Buffer (Thermo Scientific) and re-probed with the β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
RESULTS
Presence of GADL1 Gene in Species
Although not specified in the databases, human GADL1 is likely the first one named as GADL1, and the other GADL1 sequences likely have been based on the human sequence. To date, there have been several GADL1 genes in the databases. The deduced sequences from these genes are highly conserved. For example, GADL1 sequences from human, cattle, and mice share more than 90% sequence identity (supplemental Fig. S1). In human, GADL1 also shares considerable similarity with human GAD65 and GAD67, which likely explains why it has been named GAD-like protein. However, GADL1 shares noticeable better similarity (59% identity) with CSADC than with mammalian GAD sequences (50 and 51% identity) (supplemental Fig. S2).
UV-visible Spectrum of Recombinant HuGADL1
The concentrated protein solution showed a visible light yellow color. Spectral analysis of purified HuGADL1 revealed the presence of typical visible absorbance peaks with their λmax at 340 and 430 nm, respectively (Fig. 2). These visible absorption peaks were due to the presence of PLP cofactor. The ratio of the 340-nm peak versus the 430-nm peak reflected the relative contents of PLP tautomers. Overall, the visible spectrum of HuGADL1 was quite similar to that of HuCSADC (9), except that HuGADL1 had a higher 340-nm/430-nm peak ratio (Fig. 2).
FIGURE 2.
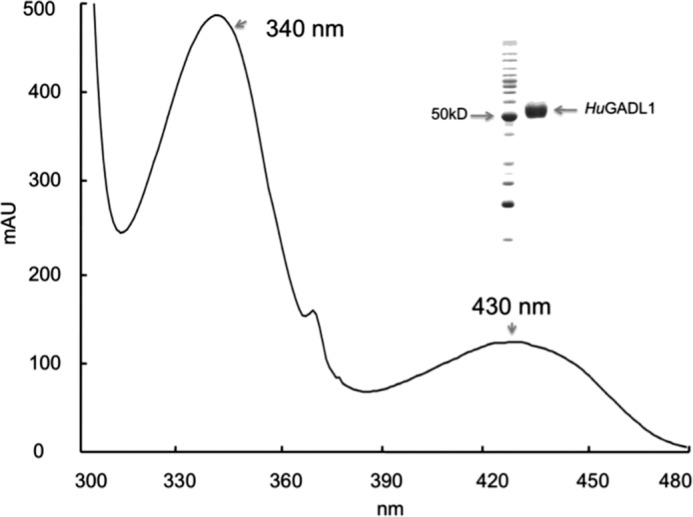
Spectral characteristics of recombinant HuGADL1. Extensively purified HuGADL1 was prepared in 20 mm phosphate buffer (pH 7.0), and its absorbance from 300 to 480 nm was determined using a Hitachi U-2800A spectrophotometer. The inset illustrates purified protein and reference molecular mass marker.
Substrate Specificity of HuGADL1
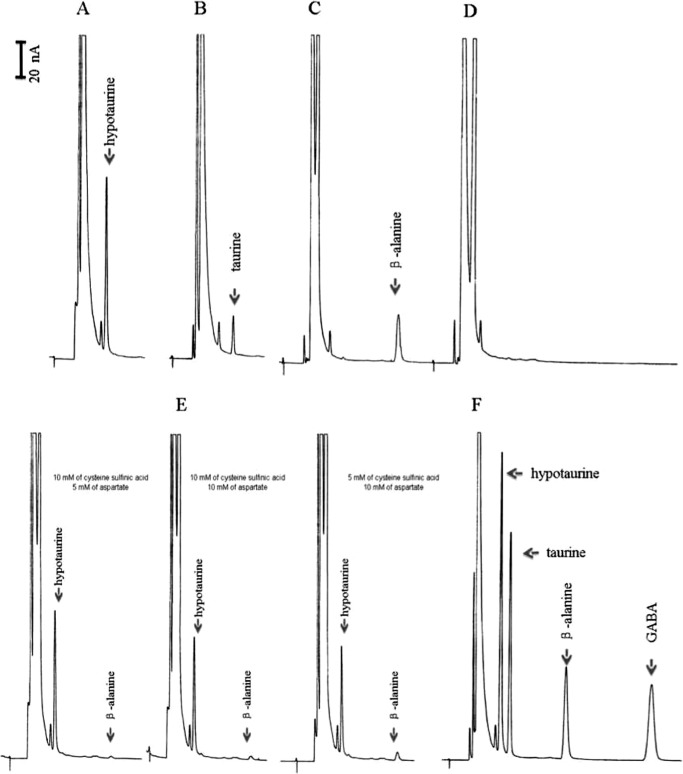
HuGADL1 was screened against all 20 proteogenic amino acids and also cysteine sulfinic acid and cysteic acid for decarboxylation activity. Among them, HuGADL1 showed decarboxylation activities with aspartate, cysteine sulfinic acid, and cysteic acid (Fig. 3, A–C) but displayed no activity with glutamate (Fig. 3D) and other amino acids. Fig. 3 (A–C) demonstrates that HuGADL1 has both ADC activity and CSADC activity. Under the applied assay conditions, the specific activity of HuGADL1 was 1.3 ± 0.2 μmol min−1 mg−1 to aspartate, 2.08 ± 0.3 μmol min−1 mg−1 to cysteine sulfinic acid, and 0.46 μmol min−1 mg−1 to cysteic acid, respectively. Based on its substrate specificity, HuGADL1 could be called a HuCSADC isozyme or named HuADC. When the enzyme was incubated with aspartate and cysteine sulfinic acid at the same time, its CSADC activity was not affected to any significant degree, but its ADC activity was considerably reduced (Fig. 3E), indicating that cysteine sulfinic acid competes much more effectively than aspartate in HuGADL1 binding. For example, in the presence of 10 mm of cysteine sulfinic acid and 5 mm of aspartate, the calculated CSADC activity and ADC activity were ∼1.67 and 0.06 μmol min−1 mg−1 (Fig. 3E, left panel), respectively. When GADL1 was incubated with cysteine sulfinic acid and aspartate with each at 10 mm final concentration, its CSADC activity and ADC activity were ∼1.41 and 0.12 μmol min−1 mg−1 (Fig. 3E, middle panel), respectively. When the enzyme was incubated with 5 mm of cysteine sulfinic acid and 10 mm of aspartate, the CSADC activity and ADC activity were ∼1.29 and 0.22 μmol min−1 mg−1 (Fig. 3E, right panel), respectively. Fig. 3F shows the elution profile of hypotaurine, taurine, β-alanine, and GABA in our assay conditions.
FIGURE 3.
Substrate specificities of recombinant HuGADL1. Chromatograms A, B, and C show the accumulation of hypotaurine in a HuGADL1 and cysteine sulfinic acid reaction mixture, the accumulation of taurine in a HuGADL1 and cysteic acid reaction mixture, and the accumulation of β-alanine in a HuGADL1 and aspartate reaction mixture, respectively. Chromatogram D shows the absence of GABA in the HuGADL1 and glutamate reaction mixture. Chromatogram E shows a comparative study of the substrate preference of HuGADL1 under different concentrations of substrates. Chromatogram F illustrates the retention time of hypotaurine, taurine, β-alanine, and GABA standards under the same assay conditions. The reaction was initiated by the addition of purified HuGADL1 into each substrate preparation. An equal volume of 100% ethanol was added to each reaction mixture at 10 min after incubation at 25 °C, followed by the addition of two volumes of OPT agent. The mixture was incubated for 3 min. Determination of the products was based on the detection of OPT derivatives by reverse phase liquid chromatography with electrochemical detection. A C18 reverse phase column (4.6 mm × 100 mm, 5-μm spherical particle) was used for substrate and product separation. The mobile phase consisted of 50 mm phosphate buffer (pH 3.5) containing 25% acetonitrile with a flow rate of 0.5 ml/min. The working electrode was maintained at +0.75 V versus an Ag/AgCl reference electrode.
Kinetic Parameters of HuGADL1 to Aspartate and Cysteine Sulfinic Acid
HuGADL1 showed moderate substrate affinity to aspartate and cysteine sulfinic acid. Although the enzyme showed overall better affinity and catalytic efficiency to cysteine sulfinic acid than to aspartate (Table 1), the concentration of aspartate is generally much higher than that of cysteine sulfinic acid in many tissues. Therefore, the enzyme could be functional primarily as ADC or CSADC, depending on its locations.
TABLE 1.
Kinetic parameters of HuGADL1
| Enzyme | Substrate | Km | Vmax | kcat | kcat/Km |
|---|---|---|---|---|---|
| mm | μmol/min/mg | s−1 | mm − 1 s − 1 | ||
| HuGADL1 | Aspartate | 2.72 ± 0.22 | 1.56 ± 0.12 | 1.3 | 0.48 |
| Cysteine sulfinic acid | 1.14 ± 0.14 | 2.31 ± 0.27 | 1.9 | 1.67 |
Tissue Distribution of GADL1 mRNA
Tissue distributions of GADL1 mRNA of mice and cattle were determined by RT-PCR using ribosomal 18 S RNA as an internal standard for cDNA normalization. In mice, GADL1 mRNA expression was detected at high levels only in skeletal muscles and kidneys (Fig. 4A). In cattle, GADL1 mRNA was detected in skeletal muscles and hearts, but transcript abundance was much greater in muscles than in hearts (Fig. 4B). GADL1 mRNA was not found in mouse brains (supplemental Fig. S3). The GADL1 mRNA was detected from C2C12 myoblasts but not from C2C12 myotubes.
FIGURE 4.
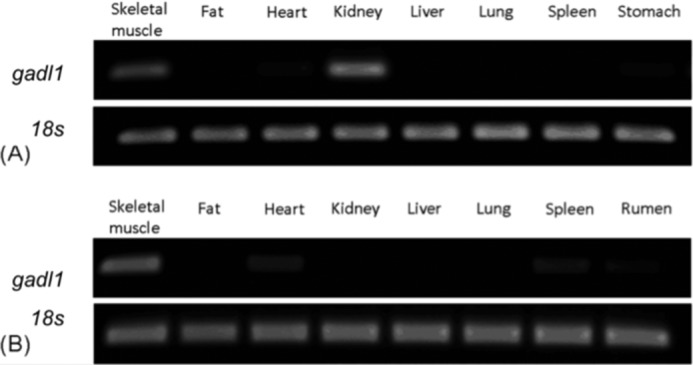
RT-PCR analysis of GADL1 in mice and cattle tissues. The tissue distribution of GADL1 mRNA was studied in mice (A) and cattle (B). Ribosomal 18 S RNA was used as an internal standard for both species.
Western Blot Analysis
The presence of GADL1 was investigated in mouse muscles, kidneys, C2C12 myoblasts, and C2C12 myotubes. Antibody against β-actin was used as an internal control. Purified recombinant mouse CSADC was used to test the specificity of the GADL1 antibody. The result showed that GADL1 is present in muscles, kidneys, C2C12 myoblasts, and C2C12 myotubes (Fig. 5, A and B). These results are comparable with the RT-PCR data, except that GADL1 transcript was not detected from C2C12 myotubes (supplemental Fig. S3). It is possible that GADL1 in C2C12 myotubes might be carried over from the myoblast stage of the muscle cells. It was also noted that the GADL1 antibody cross-reacted with mouse CSADC (Fig. 5C). Trace amounts of CSADC were detected from kidney samples but not from muscle samples (Fig. 5A).
FIGURE 5.
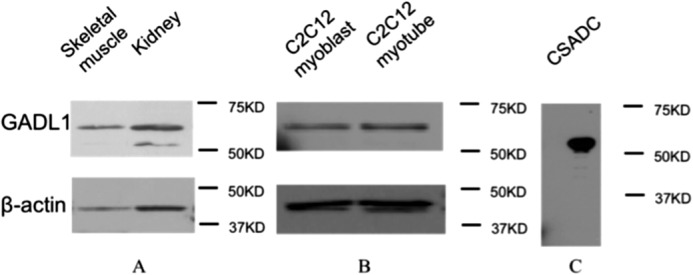
Western blot analysis of GADL1 in tissues and cell culture. A, the presence of GADL1 in mouse kidneys and muscles. Noticed that a lower band was detected in kidneys; this band corresponded to CSADC. B, the presence of GADL1 in mouse C2C12 myoblasts and myotubes. C, 10 μg of purified mouse CSADC was used to detect whether the GADL1 antibody had cross-reactivity. Antibody against β-actin was used as a control.
Activity Assay Using Protein Extracts
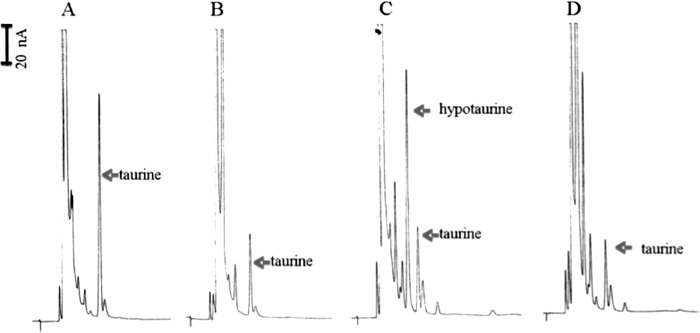
The ADC and CSADC activities were investigated with fresh protein extracts from kidneys and muscles, respectively. When the supernatant of freshly prepared muscle protein extracts was mixed with cysteine sulfinic acid and incubated for 10 min, no apparent accumulation of hypotaurine was observed, but the relative amount of taurine (Fig. 6A) was much greater than that of the endogenous taurine present in the supernatant of muscle protein extracts (see Fig. 6B as a reference). When the freshly prepared muscle sample was mixed with aspartate for 10 min, no apparent accumulation of β-alanine was observed in the reaction mixture (Fig. 6B). Because only aspartate was incubated with the supernatant, the taurine peak corresponded to the relative base level of taurine in the supernatant of muscle protein extracts (Fig. 6B). Based on the apparent increase of taurine concentration in the reaction mixture with cysteine sulfinic acid as a substrate, it seemed that no hypotaurine was produced in the reaction mixture, but hypotaurine, once formed, was converted to taurine. When hypotaurine was directly mixed with muscle extract sample, hypotaurine was also converted to taurine (supplemental Fig. S4), suggesting that crude muscle extract contains factor(s) capable of oxidation of hypotaurine to taurine. Compared with the high concentration of endogenous taurine, no endogenous β-alanine peak was observed in the supernatant of muscle protein extracts. Also, no apparent decrease of β-alanine was observed when the compound was incubated with muscle extract sample (supplemental Fig. S5).
FIGURE 6.
ADC and CSADC activity assays of the supernatant of mouse muscle or kidney protein extracts. Five microliters of protein extract supernatant (containing ∼90 mg/ml total protein) were added into 45 μl of reaction mixture containing 10 mm of cysteine sulfinic acid or 10 mm of aspartate. The reaction mixtures were incubated at 25 °C for 10 min. Chromatograms illustrate substrate, product, and endogenous taurine in the supernatant of muscle protein extracts in the presence of 10 mm of cysteine sulfinic acid (A), in the supernatant of muscle protein extracts in the presence of 10 mm of aspartate (B), in the supernatant of kidney protein extracts in the presence of 10 mm of cysteine sulfinic acid (C), and in the supernatant of kidney protein extracts in the presence of 10 mm of aspartate (D).
When the supernatant of freshly prepared kidney protein extracts was incubated with cysteine sulfinic acid as a substrate, accumulation of hypotaurine was observed in the reaction mixture (Fig. 6C). When the kidney sample was incubated with aspartate, no apparent accumulation of β-alanine was observed in the reaction mixture (Fig. 6D). It is worth noting that, when substrate was incubated with the supernatant of muscle protein extracts, the majority of product was found in the form of taurine, not hypotaurine (Fig. 6A). The CSADC activity in muscle and kidney protein extracts was calculated ∼11 and 13 nmol min−1 mg−1, respectively. GADL1 was not stable in protein extracts because attempts to get rid of the endogenous taurine in protein extracts, such as dialysis and ammonia sulfate precipitation/resuspension, led to the disappearance of CSADC activity in both muscle and kidney tissue samples (data not shown).
DISCUSSION
In this manuscript, we reported for the first time the biochemical activities of recombinant HuGADL1 and the distribution of GADL1 mRNA in mice and cattle. The recombinant HuGADL1, expressed in E. coli, has a UV-visible spectrum similar to that of other identified glutamate/cysteine sulfinic acid/aspartate 1-decarboxylases. HuGADL1 has different substrate usage than the name would imply. The enzyme catalyzes decarboxylation of α-carboxyl group of cysteine sulfinic acid and aspartate but not glutamate. GADL1 mRNA showed tissue-specific expression in cattle and mice, with relatively high levels in the muscle of both species. High levels of GADL1 transcript were also detected in mouse kidneys. The presence of GADL1 in muscles and kidneys has been confirmed by Western blot analysis and activity assays with tissue samples. It is likely that GADL1 may contribute to in vivo taurine or/and β-alanine biosynthesis (supplemental Fig. S6). Consistent with our results in terms of GADL1 expression, online human microarray data indicated that GADL1 expression went up significantly under conditions of neuromuscular pain (ArrayExpress: E-GEOD-7307, Gene Expression Atlas). These online data have been derived from a number of independent DNA array studies. The linking of GADL1 with muscular pain conditions provides some basis to suggest that GADL1 plays a physiological role in muscles.
There are only a limited number of GADL1 genes in the Gene database with most of them from mammals. It was interesting to observe that although GADL1 from cattle and mice is highly similar in primary sequence and both are transcribed primarily in skeletal muscles, the mouse gene is also transcribed a lot in kidney, and the cattle gene is transcribed a little in heart. In addition, GADL1 mRNA was detected in C2C12 myoblasts, a cell line corresponding to earlier stages of muscle ontogeny, but it disappeared in the next C2C12 myotube stage, indicating that GADL1 might be tightly regulated during myogenesis. The Western blot data indicated the presence of GADL1 in mouse muscles, kidneys, C2C12 myoblasts, and C2C12 myotubes, which is comparable with our RT-PCR data except for the C2C12 myotube result. Hypotaurine is the indispensable intermediate along the cysteine sulfinic acid to taurine pathway. Accumulation of taurine in the supernatant of muscle extracts and cysteine sulfinic acid reaction mixture determined the CSADC activity in muscles. Based on the Western blot analysis, however, typical CSADC was absent in muscles. Accordingly, the CSADC activity in muscles likely is primarily due to the presence of GADL1, whereas in kidney tissues, activity might well be a contribution by both typical CSADC and GADL1 as seen in Fig. 5A. It has been suggested that the hypotaurine-to-taurine reaction was enzyme-mediated, but there have been no reports or evidence showing the presence of such an enzyme. Conversion of hypotaurine to taurine in muscle samples suggested that a molecule/enzyme, capable of catalyzing the production of hypotaurine to taurine, is present in muscles (supplemental Fig. S4). No such activity was detected from brains and kidneys (data not shown). It is therefore worthwhile to work on the exact enzyme that was able to catalyze the conversion of hypotaurine to taurine.
HuGADL1 is active to cysteine sulfinic acid. Accordingly, it is reasonable to name it CSADC or CSADC isozyme. Although CSADC has generally been considered the primary enzyme for taurine synthesis, only a few mammalian CSADC enzymes have been experimentally characterized (16–18). Typical CSADC catalyzes cysteine sulfinic acid to hypotaurine that is the precursor of taurine and can be oxidized in vivo to taurine, but the enzyme has no activity to aspartate (9). Although past studies are not very consistent regarding the tissue distribution of mammalian CSADC, it is generally agreed it is expressed in both liver and brain (16, 19, 20). Although hearts and skeletal muscles have high concentrations of taurine, CSADC was not considered to be present in skeletal muscles and kidneys (16), and it was suggested that taurine was produced elsewhere and transported to these tissues (21, 22). In this study, the expression and biochemical activity of GADL1 in muscles suggested that muscle taurine might be produced in situ.
The other product of recombinant GADL1-catalyzed reactions is β-alanine, a naturally occurring β-amino acid that is commonly found in many living species. β-Alanine is a central component of pantothenate (vitamin B5), the essential precursor of coenzyme A (23). β-Alanine is also an important component of several dipeptides in animals, such as carnosine, carcinine, and N-β-alanyldopamine. In humans (perhaps all other mammalian species as well), β-alanine is necessary for carnosine synthesis. Carnosine, the dipeptide between l-histidine and β-alanine, has long been considered the dominant buffering component in muscles (24). Increasing the intracellular carnosine levels has been exploited for improving the buffering capacity of skeletal muscles, thus enhancing exercise performance (14, 25). Carnosine is present at high concentrations in vertebrate skeletal muscles (24). In addition to muscles, carnosine is also found in brains and hearts and is therefore suspected to be more than a buffering reagent in physiology (26–30). Studies of carnosine have suggested that it has neuroprotective (31, 32), antiglycative (33), antioxidative (34), and anti-aging activities (35, 36).
Living species seem to have evolved different biochemical processes to generate β-alanine, and some have more than one β-alanine producing pathway. It has been a general consensus that β-alanine is mainly derived from the degradation of uracil in plants, fungi, and vertebrates (37). In contrast, β-alanine is produced primarily through one-step decarboxylation of α-carboxyl group of aspartate by ADC in bacteria and insects, although the insect ADC and bacterial ADC share no sequence homology and are recruited independently by convergent evolution (38, 39). The bacterial ADC is a homotetramer with a covalently bound pyruvoyl cofactor (40), whereas the insect ADC is a dimer with PLP as a cofactor (9).
The discovery of ADC activity of recombinant HuGADL1 is interesting and potentially quite important. Currently, except for insect ADC, no animal enzyme is known that is capable of catalyzing the decarboxylation of the α-carboxyl group of aspartate to β-alanine. Endogenous carnosine is synthesized by carnosine synthetase using histidine and β-alanine as substrates in muscles (41, 42). β-Alanine is the limiting compound in carnosine synthesis because its plasma concentration is much lower than that of histidine (24), and the enzyme has a lower affinity to β-alanine than to histidine (43, 44). All the currently known β-alanine-synthesizing enzymes have low expression levels in muscles, where the demand for β-alanine is great; accordingly, it is generally considered that the intramuscular β-alanine is transported from elsewhere to make carnosine (13). A study with chicken embryonic muscle cell culture indicated that a β-amino acid transporter is present in the cells (45); hence the sarcoplasmic β-alanine delivery is the rate-limiting factor for muscle carnosine synthesis. β-Alanine supplementation has been exploited to boost the blood β-alanine concentration, drive the carnosine synthesis in muscles, and improve muscle performance (46). Long term β-alanine intake has been shown to augment muscle carnosine concentrations (25, 47, 48), but such delivery is inefficient and somewhat problematic. Even with multiple high doses of 800 mg of β-alanine/day over 4 weeks, the mean increase of carnosine was only ∼60% (24). Moreover, acute oral β-alanine intake (>800 mg) can cause paraesthia, generally known as the feelings of “pins and needles” (49). Compared with the enzymatic β-alanine production and transportation pathways mentioned above, GADL1-mediated β-alanine production could be the simple and energy-efficient pathway.
ADC activity in muscle extracts remains to be established. Our recent data showed that cysteine could inactivate the insect ADC, and the two activities of the enzyme responded differently to cysteine inactivation, with its ADC activity being more potently inactivated (50). Cysteine sulfinic acid or/and hypotaurine can inhibit the ADC activity of GADL1 (Fig. 3E). Further analysis indicated that cysteine and taurine could severely inhibit the ADC activity of GADL1 as well (supplemental Fig. S7). It is possible that homogenization disrupted most organelles, which might expose GADL1 to endogenous inhibitors, which may explain in part our inability to detect ADC activity in the extracted protein samples.
From an evolutionary perspective, GAD and CSADC appeared early in evolution, but so far GADL1 has only been found only in birds, reptiles, and mammals (Fig. 1). Although insect ADC possesses a similar substrate usage to GADL1 (9, 50), insect ADC-type enzymes have not been found in non-insect species (Fig. 1). GADL1 appeared late in species evolution. One might argue that GADL1 is still an evolving protein, and its appearance may help those animals better adapt the much more complex terrestrial environment and whose better survival may somewhat depend on their muscle performance.
In summary, although the in vivo ADC activity of GADL1 is still in question, the distribution and biochemical activities of the recombinant HuGADL1 provide a basis to more intelligently explore its physiological function(s). The hypotaurine-generating activity of GADL1 was shown in muscles and kidneys; the up-regulation of GADL1 in muscles under disease conditions (reported by independent mRNA array analyses) indicates that the protein plays a physiological function(s) in muscles. Consequently, results of this study provide 1) useful information toward a more appropriate classification/annotation of GADL1 proteins, 2) a tangible basis in terms of directions for revealing its true physiological function(s), and 3) a stimulating momentum for studying these intriguing proteins.
This work was supported, in whole or in part, by National Institutes of Health Grant AI19769.

This article contains supplemental Table S1 and Figs. S1–S7.
- GABA
- γ-aminobutyric acid
- GADL1
- glutamate decarboxylase-like protein 1
- GAD
- glutamate decarboxylase
- PLP
- pyridoxal-5′-phosphate
- ADC
- aspartate 1-decarboxylase
- CSADC
- cysteine sulfinic acid decarboxylase
- OPT
- o-phthaldialdehyde.
REFERENCES
- 1. Bouché N., Lacombe B., Fromm H. (2003) GABA signaling. A conserved and ubiquitous mechanism. Trends Cell Biol. 13, 607–610 [DOI] [PubMed] [Google Scholar]
- 2. Bouché N., Fait A., Bouchez D., Møller S. G., Fromm H. (2003) Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. U.S.A. 100, 6843–6848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chevrot R., Rosen R., Haudecoeur E., Cirou A., Shelp B. J., Ron E., Faure D. (2006) GABA controls the level of quorum-sensing signal in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. U.S.A. 103, 7460–7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fenalti G., Law R. H., Buckle A. M., Langendorf C., Tuck K., Rosado C. J., Faux N. G., Mahmood K., Hampe C. S., Banga J. P., Wilce M., Schmidberger J., Rossjohn J., El-Kabbani O., Pike R. N., Smith A. I., Mackay I. R., Rowley M. J., Whisstock J. C. (2007) GABA production by glutamic acid decarboxylase is regulated by a dynamic catalytic loop. Nat. Struct. Mol. Biol. 14, 280–286 [DOI] [PubMed] [Google Scholar]
- 5. Capitani G., De Biase D., Aurizi C., Gut H., Bossa F., Grütter M. G. (2003) Crystal structure and functional analysis of Escherichia coli glutamate decarboxylase. EMBO J. 22, 4027–4037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Battaglioli G., Liu H., Martin D. L. (2003) Kinetic differences between the isoforms of glutamate decarboxylase. Implications for the regulation of GABA synthesis. J. Neurochem. 86, 879–887 [DOI] [PubMed] [Google Scholar]
- 7. Asada H., Kawamura Y., Maruyama K., Kume H., Ding R. G., Kanbara N., Kuzume H., Sanbo M., Yagi T., Obata K. (1997) Cleft palate and decreased brain γ-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc. Natl. Acad. Sci. U.S.A. 94, 6496–6499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asada H., Kawamura Y., Maruyama K., Kume H., Ding R., Ji F. Y., Kanbara N., Kuzume H., Sanbo M., Yagi T., Obata K. (1996) Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem. Biophys. Res. Commun. 229, 891–895 [DOI] [PubMed] [Google Scholar]
- 9. Liu P., Ding H., Christensen B. M., Li J. (2012) Cysteine sulfinic acid decarboxylase activity of Aedes aegypti aspartate 1-decarboxylase. The structural basis of its substrate selectivity. Insect. Biochem. Mol. Biol. 42, 396–403 [DOI] [PubMed] [Google Scholar]
- 10. Lohkamp B., Voevodskaya N., Lindqvist Y., Dobritzsch D. (2010) Insights into the mechanism of dihydropyrimidine dehydrogenase from site-directed mutagenesis targeting the active site loop and redox cofactor coordination. Biochim. Biophys. Acta 1804, 2198–2206 [DOI] [PubMed] [Google Scholar]
- 11. Hipkiss A. R. (2009) Carnosine and its possible roles in nutrition and health. Adv. Food Nutr. Res. 57, 87–154 [DOI] [PubMed] [Google Scholar]
- 12. Derave W., Everaert I., Beeckman S., Baguet A. (2010) Muscle carnosine metabolism and β-alanine supplementation in relation to exercise and training. Sports Med. 40, 247–263 [DOI] [PubMed] [Google Scholar]
- 13. Artioli G. G., Gualano B., Smith A., Stout J., Lancha A. H., Jr. (2010) Role of β-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exerc. 42, 1162–1173 [DOI] [PubMed] [Google Scholar]
- 14. Hill C. A., Harris R. C., Kim H. J., Harris B. D., Sale C., Boobis L. H., Kim C. K., Wise J. A. (2007) Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32, 225–233 [DOI] [PubMed] [Google Scholar]
- 15. Warskulat U., Heller-Stilb B., Oermann E., Zilles K., Haas H., Lang F., Häussinger D. (2007) Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 428, 439–458 [DOI] [PubMed] [Google Scholar]
- 16. Park E., Park S. Y., Wang C., Xu J., LaFauci G., Schuller-Levis G. (2002) Cloning of murine cysteine sulfinic acid decarboxylase and its mRNA expression in murine tissues. Biochim. Biophys. Acta 1574, 403–406 [DOI] [PubMed] [Google Scholar]
- 17. Wu J. Y. (1982) Purification and characterization of cysteic acid and cysteine sulfinic acid decarboxylase and l-glutamate decarboxylase from bovine brain. Proc. Natl. Acad. Sci. U.S.A. 79, 4270–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sköldberg F., Rorsman F., Perheentupa J., Landin-Olsson M., Husebye E. S., Gustafsson J., Kämpe O. (2004) Analysis of antibody reactivity against cysteine sulfinic acid decarboxylase, a pyridoxal phosphate-dependent enzyme, in endocrine autoimmune disease. J. Clin. Endocrinol. Metab. 89, 1636–1640 [DOI] [PubMed] [Google Scholar]
- 19. Ubuka T., Okada A., Nakamura H. (2008) Production of hypotaurine from l-cysteinesulfinate by rat liver mitochondria. Amino Acids 35, 53–58 [DOI] [PubMed] [Google Scholar]
- 20. Wu J. Y., Tang X. W., Schloss J. V., Faiman M. D. (1998) Regulation of taurine biosynthesis and its physiological significance in the brain. Adv. Exp. Med. Biol. 442, 339–345 [DOI] [PubMed] [Google Scholar]
- 21. Heller-Stilb B., van Roeyen C., Rascher K., Hartwig H. G., Huth A., Seeliger M. W., Warskulat U., Häussinger D. (2002) Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J. 16, 231–233 [DOI] [PubMed] [Google Scholar]
- 22. Ito T., Oishi S., Takai M., Kimura Y., Uozumi Y., Fujio Y., Schaffer S. W., Azuma J. (2010) Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. J. Biomed. Sci. 17, (Suppl. 1) S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Webb M. E., Smith A. G., Abell C. (2004) Biosynthesis of pantothenate. Nat. Prod. Rep. 21, 695–721 [DOI] [PubMed] [Google Scholar]
- 24. Harris R. C., Tallon M. J., Dunnett M., Boobis L., Coakley J., Kim H. J., Fallowfield J. L., Hill C. A., Sale C., Wise J. A. (2006) The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30, 279–289 [DOI] [PubMed] [Google Scholar]
- 25. Derave W., Ozdemir M. S., Harris R. C., Pottier A., Reyngoudt H., Koppo K., Wise J. A., Achten E. (2007) β-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J. Appl. Physiol. 103, 1736–1743 [DOI] [PubMed] [Google Scholar]
- 26. Zaloga G. P., Roberts P. R., Nelson T. E. (1996) Carnosine. A novel peptide regulator of intracellular calcium and contractility in cardiac muscle. New Horiz. 4, 26–35 [PubMed] [Google Scholar]
- 27. Boldyrev A. A. (1995) [Carnosine metabolism in excitable tissues. Biological significance]. Vestn. Ross Akad. Med. Nauk. 1995, 3–7 [PubMed] [Google Scholar]
- 28. Jackson M. C., Lenney J. F. (1996) The distribution of carnosine and related dipeptides in rat and human tissues. Inflamm. Res. 45, 132–135 [DOI] [PubMed] [Google Scholar]
- 29. Zaloga G. P., Roberts P. R., Black K. W., Lin M., Zapata-Sudo G., Sudo R. T., Nelson T. E. (1997) Carnosine is a novel peptide modulator of intracellular calcium and contractility in cardiac cells. Am. J. Physiol. 272, H462–H468 [DOI] [PubMed] [Google Scholar]
- 30. Aydin A. F., Küçükgergin C., Ozdemirler-Erata G., Koçak-Toker N., Uysal M. (2010) The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology 11, 103–109 [DOI] [PubMed] [Google Scholar]
- 31. Horning M. S., Blakemore L. J., Trombley P. Q. (2000) Endogenous mechanisms of neuroprotection. Role of zinc, copper, and carnosine. Brain Res. 852, 56–61 [DOI] [PubMed] [Google Scholar]
- 32. Trombley P. Q., Horning M. S., Blakemore L. J. (2000) Interactions between carnosine and zinc and copper. Implications for neuromodulation and neuroprotection. Biochemistry 65, 807–816 [PubMed] [Google Scholar]
- 33. Hipkiss A. R., Michaelis J., Syrris P. (1995) Non-enzymatic glycosylation of the dipeptide l-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 371, 81–85 [DOI] [PubMed] [Google Scholar]
- 34. Boldyrev A. A. (1993) Does carnosine possess direct antioxidant activity? Int. J. Biochem. 25, 1101–1107 [DOI] [PubMed] [Google Scholar]
- 35. Chez M. G., Buchanan C. P., Aimonovitch M. C., Becker M., Schaefer K., Black C., Komen J. (2002) Double-blind, placebo-controlled study of l-carnosine supplementation in children with autistic spectrum disorders. J. Child Neurol. 17, 833–837 [DOI] [PubMed] [Google Scholar]
- 36. Hipkiss A. R. (2009) On the enigma of carnosine's anti-ageing actions. Exp. Gerontol. 44, 237–242 [DOI] [PubMed] [Google Scholar]
- 37. Brown G. M., Williamson J. M. (1982) Biosynthesis of riboflavin, folic acid, thiamine, and pantothenic acid. Adv. Enzymol. Relat. Areas Mol. Biol. 53, 345–381 [DOI] [PubMed] [Google Scholar]
- 38. Cronan J. E., Jr. (1980) β-Alanine synthesis in Escherichia coli. J. Bacteriol. 141, 1291–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richardson G., Ding H., Rocheleau T., Mayhew G., Reddy E., Han Q., Christensen B. M., Li J. (2010) An examination of aspartate decarboxylase and glutamate decarboxylase activity in mosquitoes. Mol. Biol. Rep. 37, 3199–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmitzberger F., Kilkenny M. L., Lobley C. M., Webb M. E., Vinkovic M., Matak-Vinkovic D., Witty M., Chirgadze D. Y., Smith A. G., Abell C., Blundell T. L. (2003) Structural constraints on protein self-processing in l-aspartate-α-decarboxylase. EMBO J. 22, 6193–6204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drozak J., Veiga-da-Cunha M., Vertommen D., Stroobant V., Van Schaftingen E. (2010) Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J. Biol. Chem. 285, 9346–9356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bulygina E. R., Kramarenko G. G. (1995) [Isolation of carnosine synthetase from animal and human muscles]. Vopr. Med. Khim. 41, 27–30 [PubMed] [Google Scholar]
- 43. Horinishi H., Grillo M., Margolis F. L. (1978) Purification and characterization of carnosine synthetase from mouse olfactory bulbs. J. Neurochem. 31, 909–919 [DOI] [PubMed] [Google Scholar]
- 44. Ng R. H., Marshall F. D. (1978) Regional and subcellular distribution of homocarnosine-carnosine synthetase in the central nervous system of rats. J. Neurochem. 30, I87–90 [DOI] [PubMed] [Google Scholar]
- 45. Bakardjiev A., Bauer K. (1994) Transport of β-alanine and biosynthesis of carnosine by skeletal muscle cells in primary culture. Eur. J. Biochem. 225, 617–623 [DOI] [PubMed] [Google Scholar]
- 46. Stellingwerff T., Decombaz J., Harris R. C., Boesch C. (2012) Optimizing human in vivo dosing and delivery of β-alanine supplements for muscle carnosine synthesis. Amino Acids 43, 57–65 [DOI] [PubMed] [Google Scholar]
- 47. Kendrick I. P., Harris R. C., Kim H. J., Kim C. K., Dang V. H., Lam T. Q., Bui T. T., Smith M., Wise J. A. (2008) The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids 34, 547–554 [DOI] [PubMed] [Google Scholar]
- 48. Kendrick I. P., Kim H. J., Harris R. C., Kim C. K., Dang V. H., Lam T. Q., Bui T. T., Wise J. A. (2009) The effect of 4 weeks β-alanine supplementation and isokinetic training on carnosine concentrations in type I and II human skeletal muscle fibres. Eur. J. Appl. Physiol. 106, 131–138 [DOI] [PubMed] [Google Scholar]
- 49. Décombaz J., Beaumont M., Vuichoud J., Bouisset F., Stellingwerff T. (2012) Effect of slow-release β-alanine tablets on absorption kinetics and paresthesia. Amino Acids 43, 67–76 [DOI] [PubMed] [Google Scholar]
- 50. Liu P., Torrens-Spence M. P., Ding H., Christensen B. M., Li J. (2012) Mechanism of cysteine-dependent inactivation of aspartate/glutamate/cysteine sulfinic acid α-decarboxylases. Amino Acids DOI [DOI] [PubMed] [Google Scholar]