Background: The epithelial Na+ channel (ENaC) functions as a pathway for Na+ absorption across epithelia.
Results: Seven acidic residues in the extracellular domain of γENaC and one in βENaC are required for regulation by acidic pH.
Conclusion: The ENaC extracellular domains function as sensors to detect changes in extracellular pH.
Significance: These findings provide new insights into mechanisms that regulate Na+ homeostasis and blood pressure.
Keywords: Biophysics, Electrophysiology, ENaC, Hypertension, Ion Channels, Kidney, Site-directed Mutagenesis
Abstract
A growing body of evidence suggests that the extracellular domain of the epithelial Na+ channel (ENaC) functions as a sensor that fine tunes channel activity in response to changes in the extracellular environment. We previously found that acidic pH increases the activity of human ENaC, which results from a decrease in Na+ self-inhibition. In the current work, we identified extracellular domain residues responsible for this regulation. We found that rat ENaC is less sensitive to pH than human ENaC, an effect mediated in part by the γ subunit. We identified a group of seven residues in the extracellular domain of γENaC (Asp-164, Gln-165, Asp-166, Glu-292, Asp-335, His-439, and Glu-455) that, when individually mutated to Ala, decreased proton activation of ENaC. γE455 is conserved in βENaC (Glu-446); mutation of this residue to neutral amino acids (Ala, Cys) reduced ENaC stimulation by acidic pH, whereas reintroduction of a negative charge (by MTSES modification of Cys) restored pH regulation. Combination of the seven γENaC mutations with βE446A generated a channel that was not activated by acidic pH, but inhibition by alkaline pH was intact. Moreover, these mutations reduced the effect of pH on Na+ self-inhibition. Together, the data identify eight extracellular domain residues in human β- and γENaC that are required for regulation by acidic pH.
Introduction
The epithelial Na+ channel (ENaC)4 is composed of three homologous subunits (α-, β-, and γENaC). Each subunit has relatively short cytoplasmic N and C termini, leaving the bulk of the protein exposed to the extracellular environment. ENaC functions as a pathway for Na+ reabsorption across epithelia in the kidney collecting duct, lung, distal colon, and sweat duct (1, 2). The channel is critical for the maintenance of Na+ homeostasis and control of the composition and quantity of fluid on the apical membrane of these epithelia. ENaC mutations, and defects in its regulation, cause inherited forms of hypertension and hypotension (1, 3) and cause lung disease with features similar to cystic fibrosis (4–6).
The large extracellular domain of ENaC is exposed to highly varied environments. A growing body of evidence suggests that this domain detects concentration changes in a number of molecules, producing compensatory changes in ENaC gating. As a complement to long term regulation by changes in channel abundance, this may provide a more rapid, fine-tuning of ENaC activity in response to a relentless assault of diverse extracellular challenges. For example, the concentrations of Na+ and Cl− in the urine change dynamically with shifts in volume state. Under conditions of volume depletion, Na+ and Cl− concentrations are very low (<10 mm) as a result of decreased delivery to the distal nephron (7). Conversely, Na+ and Cl− concentrations increase in response to volume excess (∼150 mm). Under these conditions, Na+ (via “Na+ self-inhibition” (8–10)) and Cl− (11, 12) inhibit ENaC gating, providing a negative feedback pathway to limit Na+ absorption.
In previous work, we found that ENaC activity is also regulated by changes in extracellular pH. In the kidney collecting duct, pH varies between 4.5 and 8 in response to metabolic acidosis and alkalosis as well as with changes in diet and volume status (7). In the lung, acidic airway surface liquid may contribute to the pathogenesis of cystic fibrosis (13). We found that pH modulates the activity of human ENaC. Acidic pH increased activity by reducing Na+ self-inhibition, whereas alkaline pH reduced ENaC activity (14). Acidic pH also has a second opposing effect on ENaC activity; by titrating a Cl− binding site, acidic pH enhances ENaC inhibition by extracellular Cl− (11).
In the current work, we explored the molecular mechanism by which acidic pH stimulates ENaC. We took advantage of species differences to identify extracellular domain residues that contribute to this regulation.
EXPERIMENTAL PROCEDURES
DNA Constructs
cDNAs for human α-, β-, and γENaC in pMT3 were cloned as previously described (15, 16). Mutations were generated by site-directed mutagenesis (QuikChange II XL; Agilent Technologies) and sequenced in the University of Iowa DNA Core.
Expression and Whole Cell Electrophysiology in Xenopus Oocytes
Oocytes were harvested from albino Xenopus laevis females and manually defolliculated following a 1-h treatment with 640 units/ml type IV collagenase (Worthington Biochemical Corporation) in Ca2+-free ND-96 (96 mm NaCl, 2 mm KCl, 1 mm MgCl2, 5 mm HEPES, pH adjusted to 7.4 with NaOH). Following nuclear injection of cDNAs encoding α-, β-, and γENaC (0.02 μg/μl each), cells were incubated at 18 °C in modified Barth's saline (88 mm NaCl, 1 mm KCl, 0.33 mm Ca(NO3)2, 0.41 mm CaCl2, 0.82 mm MgSO4, 2.4 mm NaHCO3, 10 mm HEPES, 50 μg/ml gentamycin sulfate, 10 μg/ml sodium penicillin, 10 μg/ml streptomycin sulfate, pH adjusted to 7.4 with NaOH) for 20–24 h prior to study.
Oocytes were voltage clamped (two-electrode voltage clamp), and currents were amplified with an Oocyte Clamp OC-725C (Warner Instruments), digitized with a MacLab/200 interface (ADInstruments), and recorded and analyzed with Chart software (ADInstruments). As noted, recordings were performed at −60 mV in a 58 mm Na2SO4 solution (58 mm Na2SO4, 58 mm d-mannitol, 2 mm KCl, 0.4 mm CaCl2, 1 mm MgCl2, 5 mm HEPES, pH adjusted to 7.4 with NaOH). High Na+ (116 mm NaCl, 2 mm KCl, 0.4 mm CaCl2, 1 mm MgCl2, 5 mm HEPES, pH adjusted to 7.4 with NaOH) and low Na+ solutions (1 mm NaCl, 115 mm N-methyl-d-glucamine, 2 mm KCl, 0.4 mm CaCl2, 1 mm MgCl2, 5 mm HEPES, pH adjusted with HCl) were used as indicated in the figure legends to measure Na+ self-inhibition. Amiloride-sensitive current was determined by adding 10 μm amiloride to the bathing solution.
pH-induced changes in amiloride-sensitive current were calculated as the -fold increase/decrease relative to the base-line current in pH 7.4 Na2SO4 Ringer's just prior to each test solution application. This was done to reduce the effect of time-dependent current run-down.
Na+ self-inhibition was measured by rapidly changing the bathing solution from low sodium (1 mm NaCl) to high sodium (116 mm NaCl) and quantitated as ((peak current − steady-state current)/peak current). Cl− inhibition was measured by rapidly changing the bathing solution from high chloride (116 mm NaCl) to low chloride (58 mm Na2SO4) and quantitated as -fold change in amiloride-sensitive current.
In all experiments, to account for day-to-day variation, values were normalized to values recorded from cells expressing wild-type ENaC on the same day. All values are reported as averages ± S.E. Statistical significance was calculated using a two-tailed Student's t test (p < 0.05).
RESULTS
Human γENaC Is Necessary for pH Regulation of Channel Activity
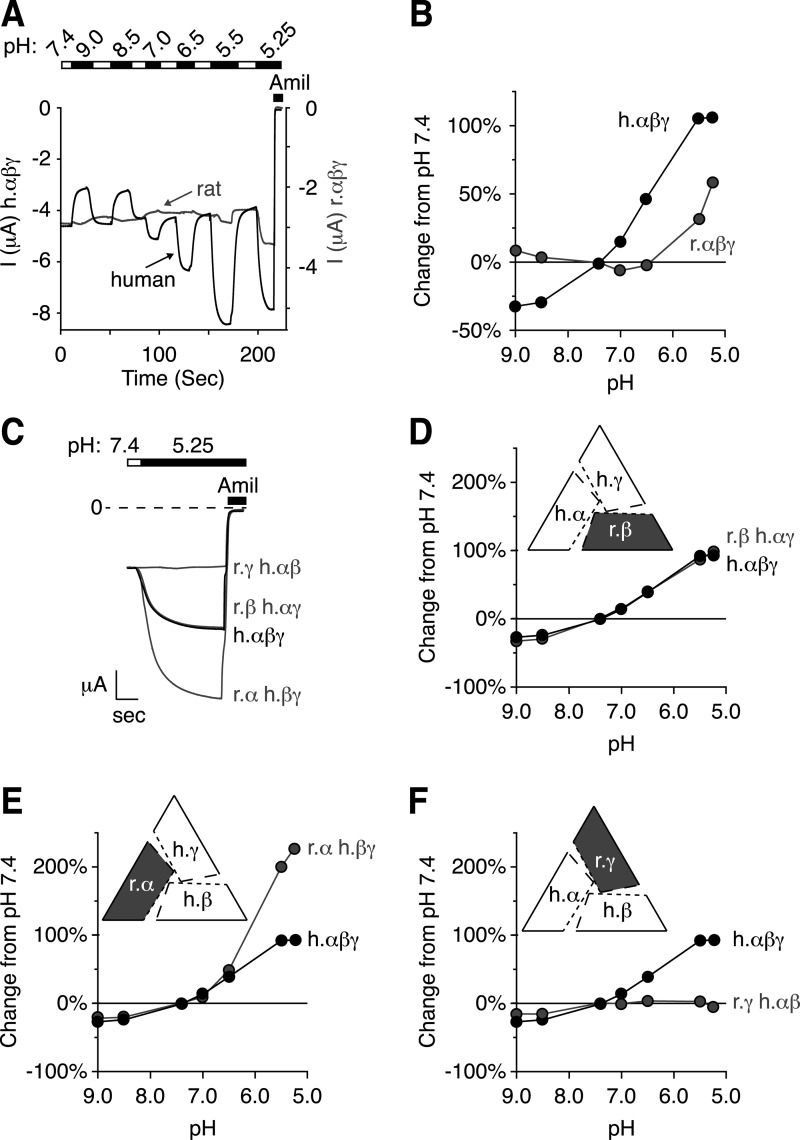
To identify residues in the ENaC extracellular domains that are required for regulation by acidic pH, we took advantage of the species dependent differences in pH sensitivity (14). In Xenopus oocytes, we measured the change in activity of human and rat ENaC in response to changes in extracellular pH. To focus on the stimulatory effect of acidic pH, we carried out our experiments in low Cl− solutions (11). We measured changes in ENaC activity over a pH range from 9.0 to 5.25. Fig. 1A shows representative current traces, and Fig. 1B plots the pH-induced change in current (relative to pH 7.4). Consistent with previous results, alkaline pH decreased human ENaC activity (black trace), and acidic pH increased human ENaC activity. However, rat ENaC current was relatively insensitive to pH over a range of 9.0 to 6.5, and pH 5.5 and 5.25 produced small increases in current (gray trace).
FIGURE 1.
Human γENaC is necessary for pH regulation of channel activity. A, representative traces of current versus time recorded at −60 mV from Xenopus oocytes expressing human αβγENaC (black) or rat αβγENaC (gray). To facilitate direct comparison of the data, we have scaled the y axes so that currents at pH 7.4 for rat ENaC and human ENaC are superimposed (left, current scale for human αβγENaC; right, current scale for rat αβγENaC). Currents were recorded under conditions of high Na+ (116 mm) and low extracellular Cl− (5 mm) over a range of pH from 9.0 to 5.25 (white bar, pH 7.4; black bar, pH exposures as indicated). After each test application, the cell was washed with pH 7.4 for comparison. ENaC-dependent inward Na+ current was determined by application of amiloride at the end of the experiment. B, D, E, and F, dose-response relationships showing the change in ENaC activity versus extracellular pH relative to pH 7.4 for cells expressing combinations of human and rat ENaC subunits (mean ± S.E., error bars are hidden by data symbols, human αβγENaC, black, n = 3–9; B, rat αβγENaC, gray, n = 6; D, human αγ- rat βENaC, gray, n = 6; E, human βγ- rat αENaC, gray, n = 3; F, human αβ- rat γENaC, gray, n = 9). C, representative trace showing the change in ENaC activity in response to pH 5.25 for cells expressing two human and one rat subunit, as indicated (scale bar: r.γ h.αβ = 0.47 μA, 6.5 s; h.αβγ = 1 μA, 5 s; r.β h.αγ = 1.7 μA, 4.25 s; r.αβγ = 2.4 μA, 5 s).
The difference in pH sensitivity between human and rat ENaC provided a strategy to identify the residues that contribute to changes in channel activity in response to changes in extracellular pH. ENaC functions as a heterotrimer of α-, β-, and γENaC subunits. To narrow our search for the residues that contribute to human ENaC pH sensitivity, we measured the change in ENaC activity in response to pH in cells expressing combinations of human and rat ENaC subunits. Given the decreased pH sensitivity of rat ENaC, we predicted that co-expression of rat ENaC subunits with human ENaC subunits would decrease pH sensitivity relative to a channel composed of only human ENaC subunits. Fig. 1C shows representative current traces, and Fig. 1, D–F, shows the pH dose-response relationships. Replacing human βENaC with rat βENaC (expressed with human α- and γENaC) had no effect on the pH dose response relative to cells expressing human αβγENaC (Fig. 1D, traces overlay). When human αENaC was replaced with rat αENaC, acidic pH produced a larger increase in current than cells expressing only human ENaC subunits (Fig. 1, C and E). However, when human γENaC was replaced with rat γENaC, ENaC activation by acidic pH was eliminated (Fig. 1, C and F). These data demonstrate that human γENaC is necessary for human ENaC to respond to acidic changes in pH.
Human γENaC Is Not Sufficient for pH Regulation of Channel Activity
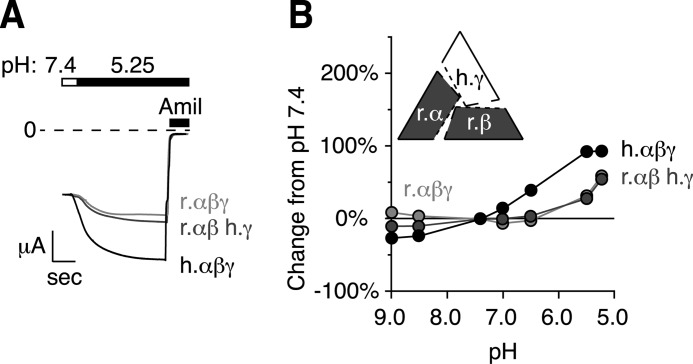
To test whether human γENaC is sufficient for the pH response, we expressed rat α- and βENaC with human γENaC. The pH dose-response relationship for channels expressing this combination of two rat and one human subunit was very similar to channels expressing only rat αβγENaC (Fig. 2). This demonstrates that although human γENaC was required, it was not sufficient to restore pH sensitivity to rat ENaC. Therefore, the data suggest that more than one subunit is involved in the pH response seen in human ENaC.
FIGURE 2.
Human γENaC is not sufficient for pH regulation of channel activity. A, representative traces of current versus time recorded at −60 mV from Xenopus oocytes expressing human αβγENaC (black, scale bar = 1 μA, 5 s), rat αβγENaC (light gray, scale bar = 1.3 μA, 5 s), or rat αβ- human γENaC (gray, scale bar = 1.3 μA, 6.25 s). Traces are scaled to pH 7.4 amiloride-sensitive current. Currents were recorded under conditions of high Na+ (116 mm) and low extracellular Cl− (5 mm) over a range of pH from 9.0 to 5.25 (only pH 5.25 is shown). ENaC-dependent inward Na+ current was determined by application of amiloride at the end of the experiment. B, dose-response relationship showing the change in ENaC activity versus extracellular pH relative to pH 7.4 for cells expressing combinations of human and rat ENaC subunits (mean ± S.E., error bars are hidden by data symbols, human αβγENaC, black, n = 6; human γ- rat αβENaC, dark gray, n = 6, rat αβγENaC, light gray, n = 6).
Identification of Extracellular Residues in γENaC That Contribute to Regulation by Acidic pH
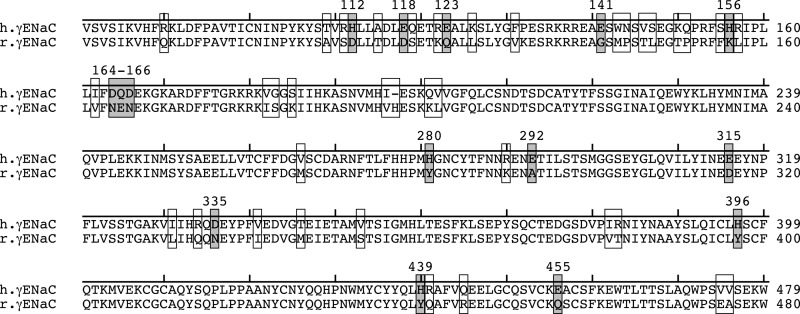
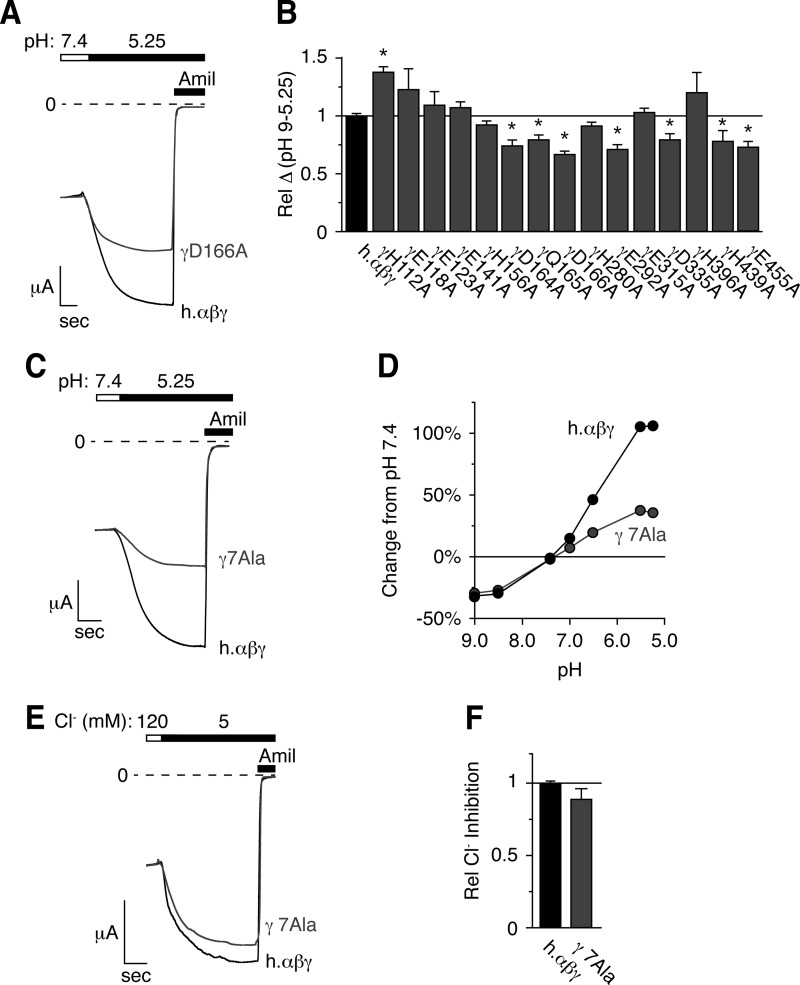
We hypothesized that sequence differences between the extracellular domains of human and rat γENaC contributed to differences in pH regulation. Fig. 3 shows a partial sequence alignment of the extracellular domain of human and rat γENaC. In total, 50 residues differ between the two species in the extracellular domains of γENaC (indicated by boxes). Of these, we focused on 15 residues that H+ might titrate within the physiologically relevant acidic pH range (acidic residues and histidines, Fig. 3, gray boxes). We mutated each of these residues to Ala and tested the effect of pH changes on ENaC current. Fig. 4A shows representative current traces for wild type ENaC and one of the mutants, γD166A. This mutation reduced ENaC stimulation by acidic pH. In Fig. 4B, we plotted the total change in ENaC current between pH 9.0 and pH 5.25 for each of the 15 mutants (relative to wild type ENaC). We found that seven of the mutations (γD164A, γQ165A, γD166A, γE292A, γD335A, γH439A, and γE455A) decreased activation of ENaC current by acidic pH (Fig. 4B). In contrast, the other mutations had no effect or slightly increased the pH response (Fig. 4B).
FIGURE 3.
15 titratable residues present in the extracellular domain of human but not rat γENaC. Partial sequence alignment of the extracellular domains of human and rat γENaC is shown. Residues that differ between the two subunits are indicated with an open box. Acidic residues and histidines (Asp, Glu, His) that differ between the two subunits are indicated with a gray box.
FIGURE 4.
Mutation of titratable residues in the extracellular domain of γENaC decreases the response to acidic pH. A, C, and E, representative traces of current versus time recorded at −60 mV from Xenopus oocytes expressing human αβγENaC (A and C, black, scale bar = 1 μA, 5 s), human αβ- and γD166AENaC (A, gray, scale bar = 2 μA, 6.5 s), or human αβ- and γD164A,Q165A,D166A,E292A,D335A,H439A,E455AENaC (γ7Ala, C, gray, scale bar = 2 μA, 3.6 s). Currents were recorded under conditions of high Na+ (116 mm) and low extracellular Cl− (5 mm) over a range of pH from 9.0 to 5.25 (only pH 5.25 is shown). ENaC-dependent inward Na+ current was determined by application of amiloride at the end of the experiment. B, total pH response for each acidic residue identified in Fig. 3 (calculated as the difference in ENaC activity between pH 9.0 and 5.25) relative to wild type human αβγENaC (mean ± S.E. (error bars), n = 3–8, *, p < .05). D, dose-response relationship showing the change in ENaC activity versus extracellular pH relative to pH 7.4 for cells expressing wild type (black) and human αβγ7AlaENaC (gray, mean ± S.E., error bars are hidden by data symbols, n = 11). E, representative trace of Cl− inhibition recorded at −60 mV from oocytes expressing wild type (black) or αβγ7AlaENaC (gray). F, Cl− inhibition relative to wild type ENaC (mean ± S.E., n = 11).
As no single mutation abolished the response to pH, the data indicate that multiple residues in the extracellular domain of human γENaC are required. To test this idea further, we generated a human γENaC construct in which the seven residues that reduced the response to acidic pH were mutated simultaneously (γD164A,Q165A,D166A,E292A,D335A,H439A,E455A, labeled γ7Ala). Cells expressing human αβγ7AlaENaC had a greater reduction in their response to acidic pH than any single mutation alone (Fig. 4, C and D), consistent with a role for multiple residues. However, the response to acidic pH was not abolished, indicating that additional ENaC residues also contribute. Interestingly, ENaC inhibition by alkaline pH (pH 7.4–9.0) was identical to wild type ENaC (Fig. 4D). Thus, different residues are responsible for ENaC regulation by acidic and alkaline pH.
In previous work, we found that extracellular Cl− inhibits ENaC. To test the specificity of these mutations for pH, we measured the inhibition of ENaC activity by extracellular Cl−. A reduction in extracellular Cl− increased the γ7Ala mutant current to an extent similar to wild type ENaC (Fig. 4, E and F). This demonstrates that the decrease in the response to acidic pH seen with the γ7Ala mutations did not result from a nonspecific change in channel activity.
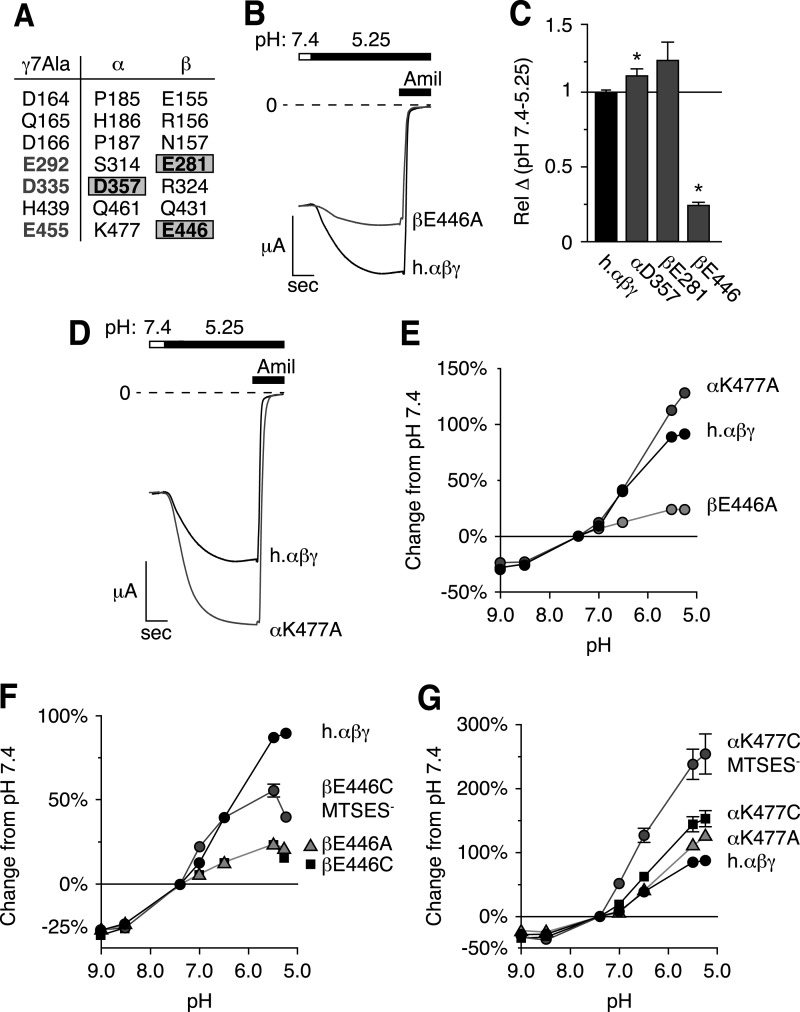
Residues Equivalent to γE455 in α- and βENaC Also Modulate the pH Response
Because the γ7Ala mutations did not abolish ENaC regulation by acidic pH, we searched for additional contributing residues. We turned our attention to α- and βENaC; our finding that human γENaC alone was not sufficient to explain the species difference in pH regulation supported a role for one or more additional subunit(s). Of the seven human γENaC residues that contributed to pH regulation, one was conserved in αENaC (αD357,γD335), and two were conserved in βENaC (βE281,γE292 and βE446,γE455, Fig. 5A). To test their role in pH regulation, we mutated each residue individually to Ala. Two of the mutations (αD357A and βE281A) did not reduce the effect of pH on ENaC current (Fig. 5C). In contrast, βE446A had a large effect, decreasing the acidic pH response by 75% (Fig. 5, B and C). Fig. 5E shows the pH dose-response relationship for βE446A; this mutation selectively reduced ENaC stimulation by acidic pH but had no effect on inhibition by alkaline pH. In αENaC, the corresponding residue carries a positive charge (αK477). In contrast to results with β- and γENaC, mutation of αK477 to Ala increased ENaC stimulation by acidic pH (Fig. 5, D and E).
FIGURE 5.
Residues equivalent to γE455 in α- and βENaC also modulate the pH response. A, seven residues in γENaC that decrease the response to acidic pH and the equivalent residues in α- and βENaC. B and D, representative traces of current versus time recorded at −60 mV from Xenopus oocytes expressing human αβγENaC (B and D, black, scale bar = 1 μA, 5 s), αβE446AγENaC (B, gray, scale bar = 2 μA, 5 s), or αK477Aβγ (D, gray, scale bar = 2 μA, 5 s). Currents were recorded under conditions of high Na+ (116 mm) and low extracellular Cl− (5 mm) over a range of pH from 9.0 to 5.25 (only pH 5.25 is shown). ENaC-dependent inward Na+ current was determined by application of amiloride at the end of the experiment. C, total change in ENaC activity between pH 7.4 and 5.25 relative to wild type (mean ± S.E. (error bars), n = 7–10, *, p < .05). E–G, dose-response relationships showing the change in ENaC activity versus extracellular pH (relative to pH 7.4) for cells expressing wild type ENaC (E–G, black), αK477A (E, dark gray) or βE446A mutations (E, light gray, mean ± S.E., n = 6–9, error bars hidden by data symbols); βE446C after modification with MTSES (F, dark gray), βE446A (F, gray triangles), and βE446C (F, black squares, mean ± S.E., n = 3–9, some error bars hidden by data symbols); αK477A (G, gray triangles), αK477C (G, black squares), and αK477C after modification with MTSES (G, dark gray, mean ± S.E., n = 3–9, some error bars hidden by data symbols).
Together, the data suggest that the negative charge of βE446 and equivalent positions in α- and γENaC are important for protons to modulate ENaC activity. To investigate this possibility further, we replaced βE446 with Cys. This mutation reduced ENaC activation by acidic pH to the same degree as the Ala mutation at this position (Fig. 5F). Modification of the Cys with MTSES, to restore the negative charge, partially rescued pH regulation of ENaC (Fig. 5F). Mutating βE446 to Lys elicited a pH dose response nearly identical to βE446A or βE446C (data not shown). These findings indicate that the negative charge at this position is important for ENaC activation by acidic pH.
We performed a similar series of experiments at the αK477 position. We mutated αK477 to Cys. This mutation increased the response to acidic pH to a degree similar to the Ala mutation (Fig. 5G). When we introduced a negative charge by modifying the Cys mutant with MTSES, the response to acidic pH increased even further (Fig. 5G). These data indicate that a negative charge at this position in α-, β-, or γENaC allows ENaC to be activated by protons.
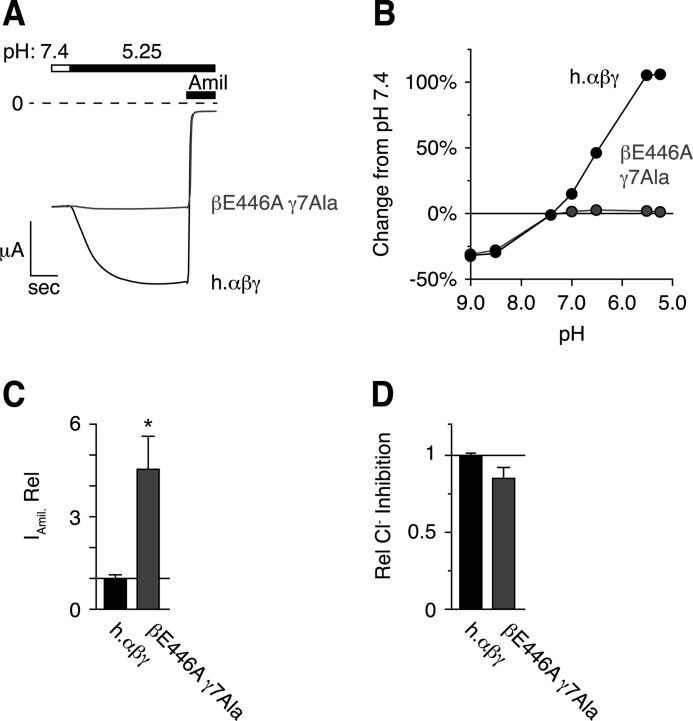
Mutation of Titratable Residues in β- and γENaC Specifically Eliminates the Response to Acidic pH
In Fig. 6, A and B, we co-expressed βE446A with γ7Ala (and wild type α). Acidic pH failed to stimulate this mutant channel, whereas inhibition by alkaline pH was intact. Cells expressing αβE446Aγ7AlaENaC also showed increased amiloride-sensitive current at pH 7.4 (in 116 mm NaCl Ringer's), suggesting that Ala mutations mimic ENaC stimulation by the protonated state of the wild type acidic residues (Fig. 6C). However, ENaC inhibition by extracellular Cl− was not significantly reduced (Fig. 6D), demonstrating that the mutations are specific to activation by extracellular pH. These results identify eight residues in the extracellular domains of β- and γENaC that are required for regulation by acidic pH but are not involved in regulation by alkaline pH or Cl−.
FIGURE 6.
Mutation of titratable residues in β- and γENaC specifically eliminate the response to acidic pH. A, representative traces of current versus time recorded at −60 mV from a Xenopus oocyte expressing human αβγENaC (black, scale bar = 1 μA, 5 s) or human αβE446AγD164A, Q165A, D166A, E292A, D335A, H439A, E455A ENaC (βE446A γ7Ala, gray, scale bar = 4 μA, 4 s). Currents were recorded under conditions of high Na+ (116 mm) and low extracellular Cl− (5 mm) over a range of pH from 9.0 to 5.25. Only pH 5.25 is shown. ENaC-dependent inward Na+ current was determined by application of amiloride at the end of the experiment. B, dose-response relationship showing the change in ENaC activity versus extracellular pH relative to pH 7.4 for cells expressing wild type or βE446Aγ7Ala ENaC mutations (gray, mean ± S.E., n = 6, error bars hidden by data symbols). C, amiloride-sensitive current relative to wild type ENaC (mean ± S.E., n = 6, * p < .05). D, Cl− inhibition relative to wild type ENaC (mean ± S.E., n = 6).
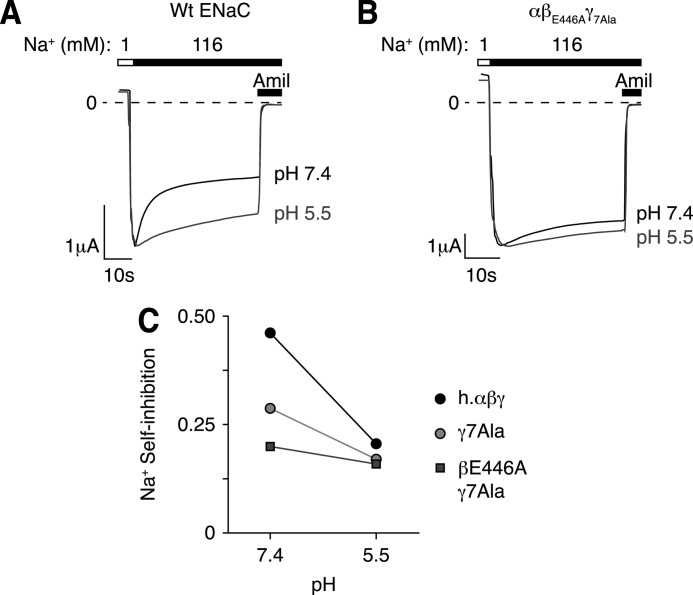
Ala Mutations Disrupt Na+ Self-inhibition
In previous work, we found that protons stimulate ENaC in part by reducing Na+ self-inhibition, a process by which extracellular Na+ decreases ENaC activity. Here we asked whether protons modulate Na+ self-inhibition through the eight titratable residues we identified in β- and γENaC. We varied the extracellular pH (7.4 or 5.5) and measured Na+ self-inhibition by shifting the extracellular Na+ concentration from 1 to 116 mm. This produced a large peak inward current (Ip) that decreased to a lower steady-state current (Iss, Fig. 7A); the fraction of current inhibited by Na+ (Na+ self-inhibition) is plotted in Fig. 7C. For wild type ENaC, Na+ inhibited channel activity to a greater extent at pH 7.4 than at pH 5.5 (Fig. 7, A and C), consistent with our previous work (14). Mutation of the eight residues (βE446A and γ7Ala) dramatically reduced Na+ self-inhibition at pH 7.4 and nearly abolished the effect of acidic pH on Na+ self-inhibition (Fig. 7, B and C). Mutation of the γENaC subunit alone (γ7Ala) had an intermediate effect (Fig. 7C). Intriguingly, Na+ self-inhibition for wild type ENaC and the mutants converged at pH 5.5 (Fig. 7C), suggesting that neutralization of the acidic residues by protonation or mutagenesis have equivalent effects on Na+ self-inhibition.
FIGURE 7.
Mutation of titratable residues in β- and γENaC reduce pH sensitivity of Na+ self-inhibition. A and B, representative current traces recorded at −60 mV from Xenopus oocytes expressing human αβγENaC (A) or human αβE446AγD164A, Q165A, D166A, E292A, D335A, H439A, E455A ENaC (B, βE446A γ7Ala). Traces show representative examples of Na+ self-inhibition induced by rapidly changing the extracellular bath from low to high Na+ conditions under high Cl− conditions (116 mm) at pH 7.4 (black) and 5.5 (gray). C, Na+ self-inhibition at pH 7.4 and pH 5.5 for wild type (black), γ7Ala (light gray) and βE446A γ7Ala (dark gray) mutations (mean ± S.E., n = 6, error bars hidden by data symbols).
DISCUSSION
In this work, we identified eight residues that participate in the regulation of ENaC activity by extracellular acidification. Mutation of seven residues in γENaC (Asp-164, Gln-165, Asp-166, Glu-292, Asp-335, His-439, and Glu-455) and one residue in βENaC (Glu-446) each reduced ENaC stimulation by acidic pH, whereas simultaneous mutation of all eight abolished stimulation. Although ENaC is a constitutively active channel, a wide variety of extracellular stimuli can modulate its activity, including H+, Cl−, Na+, Cu2+, Zn2+, Ni2+, shear stress, and proteases (8, 11, 14, 17–20). In this regard, it is clear that the extracellular domain functions as a sensor to detect changes in the composition and quantity of the highly varied extracellular milieu in which ENaC resides.
The residues we identified could function as proton sensors through titration of their side chains. Alternatively, they could function downstream of proton binding by transducing conformational changes that underlie channel gating. Several lines of evidence support the first model in which these residues are directly titrated by protons. First, all of the residues have side chains that are titrated in the acidic pH range (Glu, Asp, His), with the exception of Gln-165. Although the role of Gln-165 in the acidic pH response may seem contrary to this idea, its location may alter the pKa or accessibility of two surrounding acidic residues (Asp-164 and Asp-166). Second, the charge of the residues was critical. Neutralization of the charge, by mutation to Ala, mimicked the effect of acidic pH by increasing ENaC current. Conversely, restoration of the negative charge (by MTSES modification of βE446C) restored ENaC regulation by acidic pH. Likewise, introduction of a negative charge at the equivalent position in αENaC (via MTSES modification of αK477C) enhanced ENaC activation by acidic pH. Finally, mutation of these eight residues abolished ENaC regulation by acidic pH, but did not alter its regulation by alkaline pH or Cl−. Therefore, the mutations did not cause general changes in ENaC gating. This finding also indicates that different residues mediate ENaC regulation by acidic pH and by alkaline pH. Thus we favor a model in which the eight identified residues are titrated by protons but we cannot exclude other potential mechanisms.
Although ENaC subunits share a high degree of sequence similarity, there is growing evidence to suggest that the functional roles of each subunit are strikingly different. Several examples exist. In earlier work, we demonstrated that the three ENaC subunits play differing roles in regulation by extracellular Cl− (12). Mutation of equivalent histidine residues in α- and γENaC have opposite effects on Na+ self-inhibition (10, 14). ENaC is activated by proteolytic cleavage of α- and γ-, but not βENaC (21–23). Moreover, mutations at the DEG position also have varying effects when introduced into each ENaC subunit (24, 25). Here we identified conserved negatively charged residues in βENaC (Glu-446) and γENaC (Glu-455) that contribute to regulation by acidic pH. However, there is a charge reversal at the equivalent position in αENaC (Lys-477). Mutation of this basic residue increased the effect of acidic pH on ENaC current, opposite to the effects of mutating the acidic residues in β- and γENaC. This finding indicates that there is functional asymmetry among the three ENaC subunits in regard to regulation by acidic pH.
We took advantage of species differences in ENaC regulation to identify residues required for regulation by pH; rat (and mouse) ENaC is much less sensitive to pH than human ENaC. We do not yet know why pH regulation of ENaC varies among species. In humans, differences in diet can produce wide variations in urine pH. For example, consumption of meat produces an acidic urine, whereas vegetarians have an alkaline urine. Perhaps rats and mice lacked selective pressure to develop or retain the pH response because of a less varied diet.
ENaC is exposed to extreme changes in pH. Our data suggest that the ENaC extracellular domain functions as a sensor to detect these pH changes and to respond with appropriate changes in ENaC activity, and hence, in Na+ absorption. It is intriguing that acidic pH has dual effects on ENaC activity. In addition to stimulatory effect investigated here, acidic pH also reduces ENaC activity by enhancing Cl− inhibition (11). This inhibitory effect occurs through αH418 and γH396, which contribute to Cl− binding sites in the extracellular domain of ENaC. Protonation of these residues is thought to enhance Cl− binding through electrostatic effects. These opposing effects of pH may function to fine-tune renal Na+ absorption. With hypovolemia, delivery of NaCl to the collecting duct is low and the urine is acidic. Under these low Cl− conditions, the stimulatory effect of protons predominates, maximizing Na+ absorption. Conversely, with hypervolemia, the inhibitory effect of protons is enhanced by rising Cl− concentrations. This serves to reduce Na+ absorption. By modulating renal sodium absorption, changes in extracellular pH and ion concentrations may collaborate to maintain volume homeostasis and to regulate blood pressure.
Acknowledgments
We thank Diane Olson and Abigail Hamilton for assistance and acknowledge the University of Iowa DNA Core Facility for reagents and DNA sequencing.
Footnotes
- ENaC
- epithelial Na+ channel
- MTSES
- sodium (2-sulfonatoethyl)methanethiosulfonate.
REFERENCES
- 1. Schild L. (2004) The epithelial sodium channel: from molecule to disease. Rev. Physiol. Biochem. Pharmacol. 151, 93–107 [DOI] [PubMed] [Google Scholar]
- 2. Snyder P. M. (2005) Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology 146, 5079–5085 [DOI] [PubMed] [Google Scholar]
- 3. Lifton R. P. (1996) Molecular genetics of human blood pressure variation. Science 272, 676–680 [DOI] [PubMed] [Google Scholar]
- 4. Boucher R. C., Stutts M. J., Knowles M. R., Cantley L., Gatzy J. T. (1986) Na+ transport in cystic fibrosis respiratory epithelia: abnormal basal rate and response to adenylate cyclase activation. J. Clin. Invest. 78, 1245–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boase N. A., Rychkov G. Y., Townley S. L., Dinudom A., Candi E., Voss A. K., Tsoutsman T., Semsarian C., Melino G., Koentgen F., Cook D. I., Kumar S. (2011) Respiratory distress and perinatal lethality in Nedd4-2-deficient mice. Nat. Commun. 2, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimura T., Kawabe H., Jiang C., Zhang W., Xiang Y. Y., Lu C., Salter M. W., Brose N., Lu W. Y., Rotin D. (2011) Deletion of the ubiquitin ligase Nedd4L in lung epithelia causes cystic fibrosis-like disease. Proc. Natl. Acad. Sci. U.S.A. 108, 3216–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rose B. D. (1984) Clinical Physiology of Acid-Base and Electrolyte Disorders, pp. 274–275, 2nd Ed., McGraw-Hill, New York [Google Scholar]
- 8. Garty H., Palmer L. G. (1997) Epithelial sodium channels: function, structure, and regulation. Physiol. Rev. 77, 359–396 [DOI] [PubMed] [Google Scholar]
- 9. Chraïbi A., Horisberger J. D. (2002) Na self inhibition of human epithelial Na channel: temperature dependence and effect of extracellular proteases. J. Gen. Physiol. 120, 133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheng S., Bruns J. B., Kleyman T. R. (2004) Extracellular histidine residues crucial for Na+ self-inhibition of epithelial Na+ channels. J. Biol. Chem. 279, 9743–9749 [DOI] [PubMed] [Google Scholar]
- 11. Collier D. M., Snyder P. M. (2009) Extracellular chloride regulates the epithelial sodium channel. J. Biol. Chem. 284, 29320–29325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collier D. M., Snyder P. M. (2011) Identification of epithelial Na+ channel (ENaC) intersubunit Cl− inhibitory residues suggests a trimeric αγβ channel architecture. J. Biol. Chem. 286, 6027–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pezzulo A. A., Tang X. X., Hoegger M. J., Alaiwa M. H., Ramachandran S., Moninger T. O., Karp P. H., Wohlford-Lenane C. L., Haagsman H. P., van Eijk M., Bánfi B., Horswill A. R., Stoltz D. A., McCray P. B., Jr., Welsh M. J., Zabner J. (2012) Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collier D. M., Snyder P. M. (2009) Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J. Biol. Chem. 284, 792–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McDonald F. J., Price M. P., Snyder P. M., Welsh M. J. (1995) Cloning and expression of the β- and γ-subunits of the human epithelial sodium channel. Am. J. Physiol. 268, C1157–1163 [DOI] [PubMed] [Google Scholar]
- 16. McDonald F. J., Snyder P. M., McCray P. B., Jr., Welsh M. J. (1994) Cloning, expression, and tissue distribution of a human amiloride-sensitive Na+ channel. Am. J. Physiol. 266, L728–734 [DOI] [PubMed] [Google Scholar]
- 17. Sheng S., Perry C. J., Kleyman T. R. (2004) Extracellular Zn2+ activates epithelial Na+ channels by eliminating Na+ self-inhibition. J. Biol. Chem. 279, 31687–31696 [DOI] [PubMed] [Google Scholar]
- 18. Kusama N., Harding A. M., Benson C. J. (2010) Extracellular chloride modulates the desensitization kinetics of acid-sensing ion channel 1a (ASIC1a). J. Biol. Chem. 285, 17425–17431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carattino M. D., Sheng S., Kleyman T. R. (2004) Epithelial Na+ channels are activated by laminar shear stress. J. Biol. Chem. 279, 4120–4126 [DOI] [PubMed] [Google Scholar]
- 20. Kavanaugh J. S., Rogers P. H., Case D. A., Arnone A. (1992) High-resolution x-ray study of deoxyhemoglobin Rothschild 37 β Trp–Arg: a mutation that creates an intersubunit chloride-binding site. Biochemistry 31, 4111–4121 [DOI] [PubMed] [Google Scholar]
- 21. Vallet V., Chraibi A., Gaeggeler H. P., Horisberger J. D., Rossier B. C. (1997) An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389, 607–610 [DOI] [PubMed] [Google Scholar]
- 22. Carattino M. D., Hughey R. P., Kleyman T. R. (2008) Proteolytic processing of the epithelial sodium channel γ subunit has a dominant role in channel activation. J. Biol. Chem. 283, 25290–25295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hughey R. P., Bruns J. B., Kinlough C. L., Harkleroad K. L., Tong Q., Carattino M. D., Johnson J. P., Stockand J. D., Kleyman T. R. (2004) Epithelial sodium channels are activated by furin-dependent proteolysis. J. Biol. Chem. 279, 18111–18114 [DOI] [PubMed] [Google Scholar]
- 24. Snyder P. M., Olson D. R., Bucher D. B. (1999) A pore segment in DEG/ENaC Na+ channels. J. Biol. Chem. 274, 28484–28490 [DOI] [PubMed] [Google Scholar]
- 25. Snyder P. M., Bucher D. B., Olson D. R. (2000) Gating induces a conformational change in the outer vestibule of ENaC. J. Gen. Physiol. 116, 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]