Background: The plant photoreceptor “phototropin” is a light-regulated kinase containing the LOV photosensory domains.
Results: Photosensitivity of the kinase activation involves the lifetime of an intermediate in the photocycle.
Conclusion: Lifetime of the adduct state of LOV2 is a determinant in the activation of kinase.
Significance: This work examines and explains the different photosensitivities observed for phototropin 1 and 2.
Keywords: Photobiology, Photoreceptors, Phototransduction, Plant, Serine-Threonine Protein Phosphatase, LOV Domain, Photoregulation, Phototropin
Abstract
Phototropin (phot) is a light-regulated protein kinase that mediates a variety of photoresponses in plants, such as phototropism, chloroplast positioning, and stomata opening. Arabidopsis has two homologues, phot1 and phot2, that share physiological functions depending on light intensity. A phot molecule has two photoreceptive light oxygen voltage-sensing domains, LOV1 and LOV2, and a Ser/Thr kinase domain. The LOV domains undergo a photocycle upon blue light (BL) stimulation, including transient adduct formation between the chromophore and a conserved cysteine (S390 intermediate) that leads to activation of the kinase. To uncover the mechanism underlying the photoactivation of the kinase, we have introduced a kinase assay system composed of a phot1 LOV2-linker-kinase polypeptide as a light-regulated kinase and its N-terminal polypeptide as an artificial substrate (Okajima, K., Matsuoka, D., and Tokutomi, S. (2011) LOV2-linker-kinase phosphorylates LOV1-containing N-terminal polypeptide substrate via photoreaction of LOV2 in Arabidopsis phototropin1. FEBS Lett. 585, 3391–3395). In the present study, we extended the assay system to phot2 and compared the photochemistry and kinase activation by BL between phot1 and phot2 to gain insight into the molecular basis for the different photosensitivities of phot1 and phot2. Photosensitivity of kinase activation by BL and the lifetime of S390 of phot1 were 10 times higher and longer, respectively, than those of phot2. This correlation was confirmed by an amino acid substitution experiment with phot1 to shorten the lifetime of S390. The present results demonstrated that the photosensitivity of kinase activation in phot involves the lifetime of S390 in LOV2, suggesting that the lifetime is one of the key factors for the different photosensitivities observed for phot1 and phot2.
Introduction
Light is one of the most essential environmental signals for plants. To sense and respond to the variation in environmental light conditions, plants have developed two major light sensing systems: phytochrome (1) and blue light (BL)2 receptors, such as cryptochrome (2) or phototropin (phot) (3). phot was first identified as a receptor for phototropic response (4) and is now known to act as a receptor for chloroplast relocation (5), stomata opening (6), leaf flattening (7), and leaf photomorphogenesis (8). All of these regulatory responses maximize the efficiency of photosynthetic activity.
Most plants, including Arabidopsis thaliana (At), have two homologues of phot named phot1 and phot2, which share physiological responses depending on light intensity. In Arabidopsis, phot1 is responsible for the phototropic curvature in etiolated seedlings from low to high light conditions, whereas phot2 plays a role only under high light (9). For chloroplast relocation, the accumulation response is mediated by both phot1 and phot2 in light intensity regions similar to those of the tropic responses (9). However, avoidance response to high light is solely mediated by phot2 (5). Thus, phot1 and phot2 serve as blue light sensors working in a broad range of light intensities and at high light intensities, respectively. In contrast, stomata opening is mediated by both phot1 and phot2 (6).
phot molecules consist of residues of ∼1000 amino acids and have two Light oxygen voltage-sensing domains (LOV1 and LOV2) (10) in the N-terminal region and a Ser/Thr kinase domain at the C terminus (Fig. 1). LOV2 is connected to the kinase domain with a linker, and phot is thought to be a light-regulated Ser/Thr protein kinase (3). LOV domains bind a flavin mononucleotide (FMN) named D450 noncovalently in the dark. When FMN is excited by BL, it forms an adduct (a long-lived intermediate designated S390) with a conserved Cys residue (11) via intersystem crossing to a triplet excited state (a short-lived intermediate designated L660) (12). The adduct dissociates and reverts to D450 with different rate constants among the LOV domains in a time range from seconds to minutes (13). In oat phot1, an α-helix named Jα exists downstream of the C-terminal end of LOV2 in the linker region (Fig. 1) that interacts with the surface of a five-stranded anti-parallel β-sheet, making a scaffold for the FMN (14). The Jα helix unfolds and possibly dissociates from the LOV2 core upon adduct formation (15).
FIGURE 1.
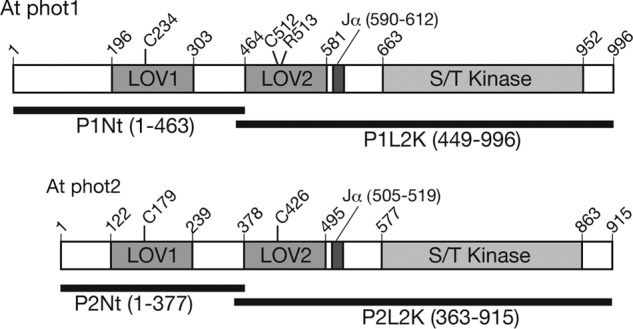
Schematic of the domain structures of At phot1 and phot2. Horizontal bars indicate the regions for the N-terminal substrate (P1Nt and P2Nt) and the LOV2-linker-kinase domain (P1L2K and P2L2K) of phot1 and phot2 used in the study.
Light-induced conformational changes in the linker region were detected in At phot1 with small angle x-ray scattering (SAXS) (16), FTIR spectroscopy (17, 18), and transient grating (19). Recently, light-induced movement of the LOV2 domain relative to the kinase domain in At phot2 was visualized by SAXS (20). These conformational changes in the linker region may cancel the inhibition of kinase activity by LOV2 and activate the phot kinase (21) to phosphorylate phot itself (22–24), as well as artificial substrates (25, 26). Nine phosphorylation sites have been identified for At phot1, among which those in the activation loop of the kinase domain are essential for the expression of physiological responses (27, 28). Recently, ATP-binding cassette B19 (ABCB19) protein, an auxin transporter, was proven to be a substrate of phot1 kinase and to mediate phototropic response (29).
To understand the molecular mechanism underlying the photoregulation of the phot kinase, suitable assay systems for kinase activity are required. Because LOV2 plays a major role in the photoregulation of the kinase (22, 25, 30, 31), we recently introduced a new in vitro assay system consisting of the At phot1 LOV2-linker-kinase polypeptide as a light-regulated kinase and its N-terminal polypeptide including six of nine autophosphorylation sites as a substrate (26). The LOV2-linker-kinase showed a photoreaction similar to that of the LOV2 domain and phosphorylated the N-terminal polypeptide in a light-dependent manner, indicating that the in vitro system could serve as a useful tool in elucidating the molecular basis for photoregulation of the kinase. Although LOV2, a main molecular switch of the kinase, showed the same photoreaction pathways in At phot1 and phot2, the lifetimes of S390 differed significantly between them (13, 22). phot2, a high light sensor, showed much shorter half-lives of LOV2 than phot1, which can sense low light. This raised an interesting question regarding whether the lifetime of S390 was involved in the light-induced activity of phot kinase because S390 may elicit physiological responses through activation of the kinase. Correlation between the degree of light-induced autophosphorylation and the lifetime of S390 was studied and discussed using LOV1/LOV2 chimeric phot1 (31). However, the involvement of the S390 lifetime in the photoactivation of kinases in phot1 and phot2 remains to be understood. To investigate the correlation between the S390 lifetime of LOV2 and the light-induced activity of phot kinase quantitatively, a previously reported in vitro assay system (26) served as a useful tool. In the present study, we extended the in vitro assay system to At phot2 and compared the photoactivation of the kinase between phot1 and phot2 to gain insight into the molecular origin of their different light sensitivities.
EXPERIMENTAL PROCEDURES
Construction of Expression Vectors
DNA of the LOV2 + linker + kinase (positions 362–915) region of At phot2 (P2L2K; Fig. 1) was synthesized with PCR and the oligonucleotide primers (5′-accatatgGACAGTTGGGACCTATCG-3′ and 5′-gtttctcgagTTAGAAGAGGTCAATGTCCAAGTCCGTAGAgTTCACAAGCAC-3′) using pGEX_phot2 of Arabidopsis as a template (25). The amplified DNA fragment was inserted into the NdeI/XhoI site of the pET28a bacterial expression vector (Amersham Biosciences) as a translational fusion to the N-terminal His6 tag. For P2L2K-C426A and P2L2K-D750A, whose Cys-426 and Asp-750 residues were substituted with Ala and Asn, respectively, and P1L2K-R513K, of which Arg-513 was substituted with Lys, the substitutions were inserted into the pET28a_P2L2K vector by PCR-based site-directed mutagenesis using oligonucleotide primers (25). For the N-terminal fragment of At phot2 consisting of the amino acid region 1–377 (P2Nt; Fig. 1), a stop codon was inserted into pGEX_phot2 by PCR-based site-directed mutagenesis using the oligonucleotide primers 5′-ATATACGCCAAGGGtaaGATCTAGCTA-3′ and 5′-TAGCTAGATCttaCCCTTGGCGTATAT-3′. For the LOV2 + linker + kinase DNA region of At phot1 (P1L2K) (449–996) and its N-terminal fragment (1–463) (P1Nt) (Fig. 1), see Ref. 26. DNA sequences of produced vectors were verified by DNA sequencing with a CEQ2000XL DNA analysis system (Beckman Coulter).
Expression and Purification of Recombinant Proteins
The Escherichia coli BL21 (DE3) strain transformed with the pET28a_P1L2K (26) or pET28a_P2L2K vectors was precultured at 37 °C in LB medium containing 30 μg ml−1 kanamycin overnight. The preculture was then transferred into 1 liter of LB medium and incubated for 8 h at 37 °C. It was next incubated further in the presence of 0.02 mm isopropyl β-d-thiogalactopyranoside for 24 h at 20 °C in the dark. The following purification procedures were carried out at 0–4 °C under dim red light. The cells were collected by centrifugation and stored at −80 °C until use. The cells were resuspended in the extraction buffer containing 20 mm HEPES-NaOH, pH 7.5, 500 mm NaCl, 10% glycerol, and 1 mm PMSF. The cells were lysed by sonication and centrifuged (100,000 × g for 30 min at 4 °C). The supernatant was loaded onto a HisTrap column (nickel-Sepharose high performance; GE Healthcare) and washed with the extraction buffer containing 30 mm imidazole. The sample was eluted with the buffer containing 500 mm imidazole. The eluted sample was purified by size exclusion column chromatography (Superdex 200 pg; GE Healthcare) with the reaction buffer (20 mm Tris-HCl, pH 7.8, 100 mm NaCl, 10% (w/v) glycerol, and 1 mm EGTA). The samples were stored at −80 °C until use.
The E. coli BL21 (DE3) strain transformed with the pGEX_P2Nt vector was precultured at 37 °C in LB medium containing 30 μg ml−1 ampicillin overnight. The preculture was moved to 1 liter of LB medium and shaken for 8 h at 37 °C. Then the culture was incubated with 0.1 mm isopropyl β-d-thiogalactopyranoside for 24 h at 20 °C in the dark. The cells were harvested by centrifugation (5,000 × g for 10 min at 4 °C) and stored at −80 °C. The cells were sonicated with extraction buffer (20 mm Tris-HCl, pH 7.8, 100 mm NaCl, 10% (w/v) glycerol, and 1 mm EGTA) and then centrifuged (50,000 × g for 30 min at 4 °C). The supernatant was incubated with GST-resin (GST-Sepharose; GE Healthcare) for 30 min at 4 °C. The resin was washed with extraction buffer three times, and the P2Nt was eluted with extraction buffer containing 20 mm reduced GSH. The buffer was exchanged to extraction buffer to remove the GSH with HiTrap desalting (GE Healthcare). The P2Nt was stored at −80 °C until use. For the preparation of P1L2K and P1Nt, see Ref. 26.
Spectroscopy
UV and visible absorption spectra were recorded with a spectrophotometer (model U2300; Hitachi-hitec) with a thermostat controller (model 131-0305; Hitachi-hitec). The samples were excited in the cell using a handmade illuminator with a blue LED (LUXEON star, Lumileds Lighting, λmax = 465 nm). The BL-excited spectra were recorded in the dark under the blue illumination. The reversion of S390 to D450 in the dark was monitored by the absorption changes at 445 nm at the temperatures indicated. For flash excitation, a combination of the illuminator and an electronic shutter (COPAL, release time < 50 ms) was used.
Phosphorylation Assay
P1L2K or P2L2K was incubated with either P1Nt or P2Nt substrate in a kinase reaction buffer (20 mm Tris-HCl, pH 7.8, 100 mm NaCl, 1 mm Na2EGTA, 10% glycerol, 10 mm MgCl2, 20 μm ATP, and 37 kBq of [γ-32P]ATP) at the temperatures indicated. The effect of blue light on phosphorylation was measured by either irradiation with a blue LED (ISL-150X150-88; CCS Inc.; λmax at 475 nm) or mock irradiation. The intensity of the irradiation was varied with ND filters. The reaction was terminated by the addition of 3× SDS-PAGE sample buffer followed by boiling for 3 min. Then the samples were analyzed on an SDS-PAGE gel and stained with Coomassie Brilliant Blue. The phosphorylated bands were visualized with imaging plates (Fuji Film) and a STORM (GE Healthcare) scanner. The signal intensities were measured by an imaging software.
RESULTS
Spectroscopic Properties of P2L2K
The purity of P2L2K was estimated at 90% by CBB staining of the SDS-PAGE gel (Fig. 2A) and was comparable with that of P1L2K (26). The UV-visible absorption spectrum of P2L2K in the ground state (D450) showed three absorption peaks at 446, 473, and 370 nm (Fig. 2B) characteristic of LOV2 (13, 31). The spectrum was almost the same as those of the At 2xLOV2 fusion protein (31), At phot2 LOV2, or LOV2-linker (16). The exception was the larger absorption at 280 nm resulting from the protein moiety attached to the kinase domain. This signal indicated that the addition of the kinase domain did not significantly perturb the electrostatic environment around the FMN chromophore. Upon BL excitation, absorption at 450 nm decreased, and that at 390 nm increased, indicating the formation of a cysteinyl-flavin adduct (S390; Fig. 2B). The absorption at 450 nm returned in the dark (supplemental Fig. S2), demonstrating a characteristic photocycle for the LOV cores.
FIGURE 2.
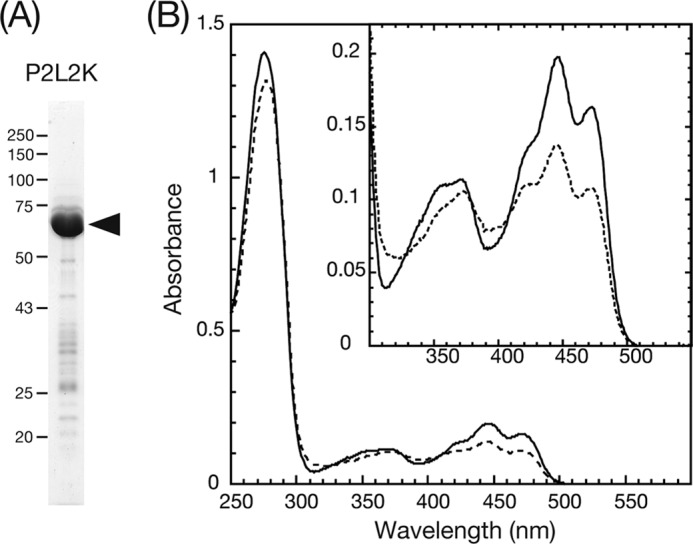
A, SDS-PAGE gel of purified P2L2K stained with CBB. The black arrowhead indicates the position of the native protein. B, absorption spectra of P2L2K in a solution containing 20 mm Tris-HCl, pH 7.8, 100 mm NaCl, 10% (w/v) glycerol, and 1 mm Na2EGTA at 20 °C. The solid and the dashed spectra were measured after dark adaptation and under BL irradiation, respectively.
Kinase Activity of P2L2K and P1L2K on P1Nt and P2Nt Substrate
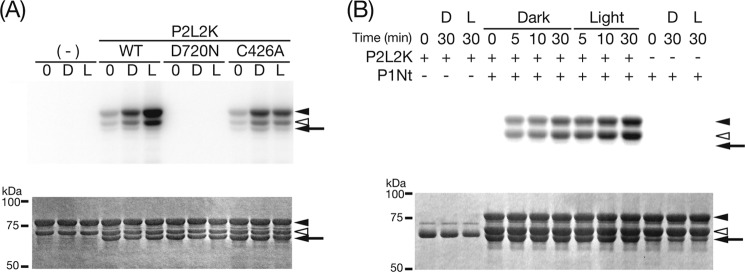
Because P1L2K successfully phosphorylated its N-terminal polypeptide, P1Nt (26), harboring six phosphorylation sites (24, 27), the activity of P2L2K on its N-terminal polypeptide, P2Nt, harboring 25 phosphorylation sites (28), was measured. P2L2K phosphorylated P2Nt (Fig. 3A, left three lanes) and P1Nt (Fig. 3A, right three lanes), as well as their degradation products, in a light-dependent manner. These results indicated cross-phosphorylation activity with the phot1 substrate. In contrast to P1L2K, P2L2K showed phosphorylation in the dark. Interestingly, the degree of phosphorylation of P1Nt was much higher than that of P2Nt even in the dark. The cross-reactivity of P1L2K with P2Nt was examined for comparison. As reported previously (26), P1L2K phosphorylated P1Nt and its degraded products in a light-dependent manner (Fig. 3B, right three lanes). In addition, P1L2K cross-phosphorylated P2Nt, but to a lesser degree (Fig. 3B, middle three lanes). Phosphorylation of both P1Nt and P2Nt by P1L2K in the dark was undetectable (Fig. 3B, middle and left three lanes). Therefore, P1L2K and P2L2K are able to phosphorylate P1Nt and P2Nt, but the degree of phosphorylation of P1Nt is much greater than that of P2Nt for both kinases. Hereafter, P1Nt was used as a substrate for the phosphorylation assay. Although the number of the reported phosphorylation sites in P2Nt (28) is larger that in P1Nt (24, 27), the phosphorylation level of P1Nt was higher than that of P2Nt. It is to be elucidated if the reported phosphorylation sites can act as the phosphorylation sites for the P1L2K or P2L2K; however, this might reflect higher saturation of the phosphorylation sites in P2Nt than P1Nt by endogenous protein kinases during the expression in E. coli. Autophosphorylation was undetectable for both with either P1L2K (arrow in Fig. 3B) or P2L2K (arrow in Fig. 4B).
FIGURE 3.
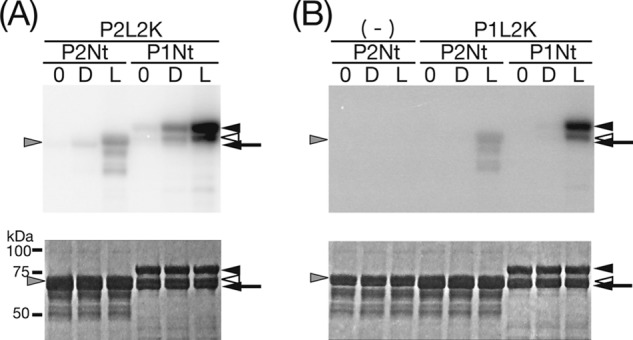
Kinase activity of P2L2K (A) and P1L2K (B) on P1Nt and P2Nt. The upper and lower panels indicate autoradiogram and CBB staining of SDS-PAGE gels, respectively. The arrows indicate the positions of the native kinase polypeptides of P1L2K (A) and P2L2K (B), respectively. The arrowheads indicate the positions of the substrates, native P1Nt and its major degradation product (right positioning filled and open arrowheads, respectively), and native P2Nt (left positioning gray arrowhead). (−) indicates the absence of the kinase polypeptides. 0, D, and L show that the SDS-PAGE samples were prepared immediately after incubation with mock or BL (200 μmol m−2 s−1) irradiation for 15 min, respectively, in a phosphorylation assay medium at 20 °C.
FIGURE 4.
Phosphorylation of P1Nt by P2L2K. The upper and lower panels indicate autoradiogram and CBB staining of SDS-PAGE gels, respectively. A, effect of amino acid substitutions on kinase activity. For details of the substitutions, see “Experimental Procedures.” (−), 0, D, and L are the same as in Fig. 3. B, time course of phosphorylation in the dark (Dark) and under BL (Light). (+) and (−) indicate the presence and absence, respectively, of the kinase and the substrate polypeptides. D and L are the same as in A. The arrows and the arrowheads are the same as in Fig. 3.
Phosphorylation of P1Nt by P2L2K
The kinase activity of P2L2K was next characterized by measuring phosphorylation of P1Nt by P2L2K and its amino acid substitutes. P2L2K phosphorylation of P1Nt in the dark was increased by 2-fold upon BL irradiation (Fig. 4A). This phosphorylation disappeared with substitution of Asp-720 to Asn, which abolished ATP binding and the kinase activity of At phot2 (25), indicating that phosphorylation in the dark and under BL was mediated by the Ser/Thr kinase in P2L2K. When the conserved Cys responsible for the adduct formation in LOV2 was substituted with Ala, the increase in phosphorylation under BL disappeared, indicating that this increase came from the photoreaction of LOV2 in P2L2K. The phosphorylation under BL increased with incubation time, as did background phosphorylation in the dark (Fig. 4B).
Dependence of Light-induced Kinase Activity of P1L2K and P2L2K on Light Intensity
To unravel the different photosensitivities of kinase activation between phot1 and phot2, photoactivation of the phot kinases was measured at various light intensities using P1Nt as the substrate (Fig. 5A). Kinase activity was quantified by phosphorimaging and expressed as a percentage of maximal phosphorylation activity relative to dark controls. The activation curves differed markedly between phot1 and phot2 (Fig. 5B). P1L2K exhibited 50% kinase activation at as low as 2 μmol m−2 s−1, and the activation exceeded 90% at 25 μmol m−2 s−1. In contrast, P2L2K showed 50% kinase activation at 20 μmol m−2 s−1, and the activation was saturated at 100 μmol m−2 s−1. Based on the light intensity for 50% activation, P1L2K was revealed to be ∼10 times more sensitive to light than P2L2K.
FIGURE 5.
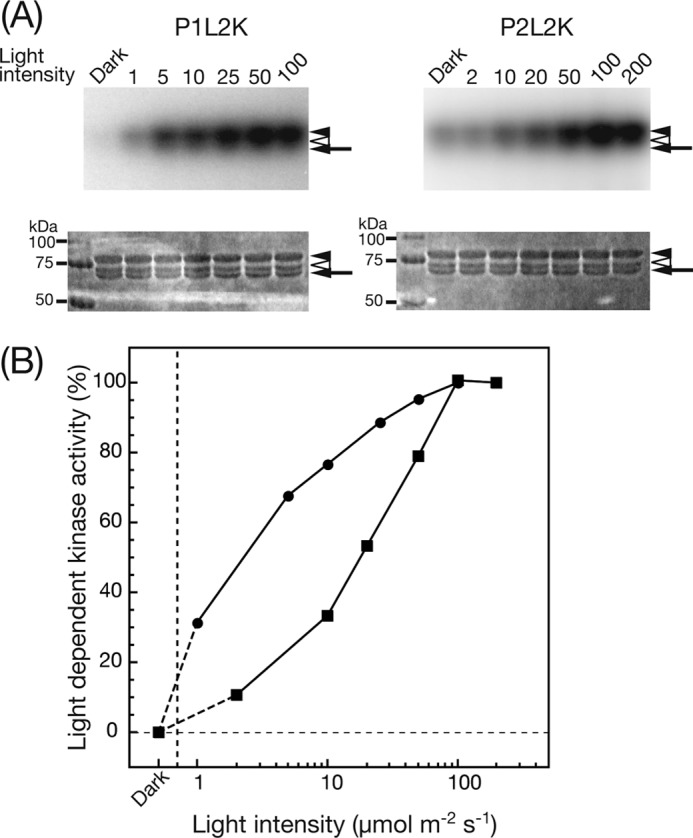
A, phosphorylation of P1Nt by P1L2K (left panels) and P2L2K (right panels) under BL at different light intensities. The upper and lower panels indicate autoradiogram and CBB staining of SDS-PAGE gels, respectively. The samples were incubated in a phosphorylation medium at 20 °C for 15 min under mock (Dark) or BL irradiation with the indicated light intensity (μmol m−2 s−1). The filled and open arrowheads indicate the positions of the native and the degraded polypeptides of P1Nt, respectively. The arrow indicates the position of P1L2K and P2L2K (see lane captions). B, dependence of light-induced kinase activity of P1L2K (filled circle) and P2L2K (filled square) on light intensity. The increase in phosphorylation signal upon BL irradiation relative to that of the mock irradiation was quantified by phosphorimaging and is plotted against the BL intensity, where the phosphorylation signals at the highest intensity (100 and 200 μmol m−2 s−1 for P1L2K and P2L2K, respectively) are normalized to 100%.
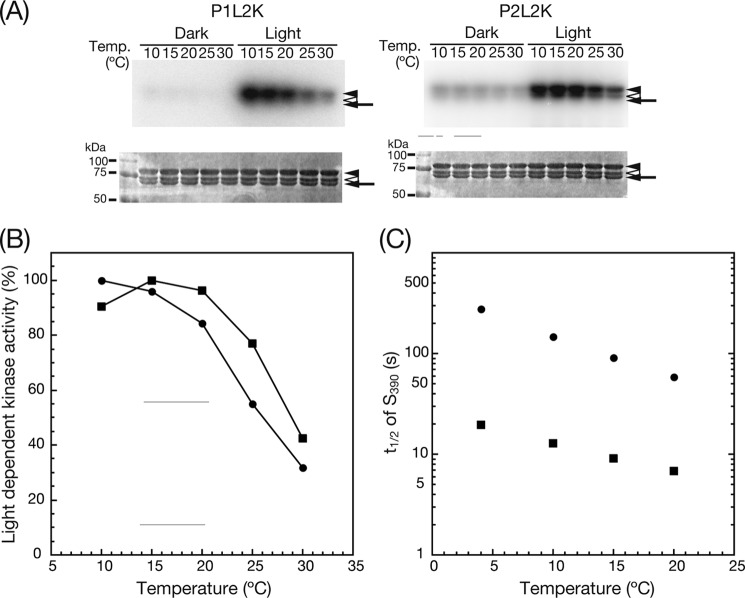
Dependence of Light-induced Kinase Activity of P1L2K and P2L2K on Temperature
To obtain further information regarding the different photosensitivities of kinase activation between P1L2K and P2L2K, the influence of reaction temperature was measured (Fig. 6). Interestingly, the degree of light-induced kinase activity of P1L2K was inversely proportional to the temperature (Fig. 6A, left panel), contrary to that of common enzymatic reactions, which show maximum activity at physiological temperature. P2L2K also showed a similar decrease with increased temperature (Fig. 6A, right panel). The control of phosphorylation by P2L2K in the dark was also slightly decreased, but no phosphorylation was detected for P1L2K in the dark at all temperatures tested. The kinase activity was quantified in a similar way to that in Fig. 5, and quantified phosphorimages were plotted against temperature (Fig. 6B). The P1L2K curve had an increasing negative slope with increasing temperature. On the other hand, the curve of P2L2K shifted ∼3 °C higher than P1L2K and had a maximum at 15 °C.
FIGURE 6.
A, phosphorylation of P1Nt by P1L2K (left panels) and P2L2K (right panels) at different incubation temperatures. The upper and lower panels indicate autoradiogram and CBB staining of SDS-PAGE gels, respectively. The samples were incubated in a phosphorylation medium at the indicated temperatures for 15 min under mock (Dark) or BL (Light) irradiation (10 and 100 μmol m−2 s−1 for P1L2K and P2L2K, respectively). The arrows and the arrowheads are the same as in Fig. 3. B, dependence of light-induced kinase activity of P1L2K (filled circles) and P2L2K (filled squares) on incubation temperature. The increase in phosphorylation signal upon BL irradiation relative to that of the mock irradiation was quantified by phosphorimaging and is plotted against the incubation temperature, where the phosphorylation signals at the highest intensity (10 and 15 °C for P1L2K and P2L2K, respectively) are normalized to 100%. C, dependence of the rate constants of the dark regeneration of D450 from S390 of P1L2K (filled circles) and P2L2K (filled squares) on temperature. Logarithms of the half-lives in Table 1 are plotted against temperature.
Efficiency of S390 Formation from D450 of P1L2K and P2L2K
Because the efficiency of S390 formation from D450 can be one of the causes for the different photosensitivities of P1L2K and P2L2K, this parameter was investigated. Equal concentrations of P1L2K and P2L2K were excited by a single flash pulse light with constant power, and the accompanied bleaching and regeneration of D450 were monitored (supplemental Fig. S1). The amounts of D450 bleached by a single pulse were 0.006 and 0.005 in terms of the absorption unit decrease at 450 nm for P1L2K and P2L2K with the same concentration of A450 = 0.2, respectively. This insignificant difference between P1L2K and P2L2K indicated that the efficiency of S390 formation from D450 contributed little to the origin of the different photosensitivities.
Kinetics of Dark Regeneration of D450 from S390 in P1L2K and P2L2K
To examine the relationship of the lifetime of S390 to the different photosensitivities of P1L2K and P2L2K, the dark regeneration of S450 from S390 during the photoreaction cycle of LOV2 was measured. The dark regeneration curves at 20 °C fit well with a single exponential curve for both P1L2K and P2L2K (Fig. 2C in Ref. 26 and supplemental Fig. S2, respectively). Their half-lives were calculated as 58.7 and 6.8 s, respectively. The half-lives and rate constants at four different temperatures were measured and are summarized in Table 1. The dark regeneration of P2L2K was ∼10 times faster than that of P1L2K at all the temperatures, indicating that the lifetime of S390 of P1L2K was ∼10 times longer than that of P2L2K. From the Arrhenius plots of these rate constants (supplemental Fig. S3), the activation energies and the pre-exponential factors for dark regeneration were calculated as 65.3 and 22.4 kJ mol−1 for P1L2K and 45.6 and 16.5 kJ mol−1 for P2L2K, respectively.
TABLE 1.
Rate constants (k) and half-lives (t1/2) of the dark regeneration of D450 from S390 of P1L2K and P2L2K at four different temperatures
| Temperature | P1L2K |
P2L2K |
||
|---|---|---|---|---|
| k | t1/2 | k | t1/2 | |
| s−1 | s | s−1 | s | |
| 4 °C (277 K) | 0.0025 | 275.9 | 0.0351 | 19.7 |
| 10 °C (283 K) | 0.0047 | 147.9 | 0.0540 | 12.8 |
| 15 °C (288 K) | 0.0076 | 91.8 | 0.0759 | 9.1 |
| 20 °C (293 K) | 0.0118 | 58.7 | 0.1013 | 6.8 |
Photochemistry of P1L2K with a Substitution of Arg-513 with Lys
phot LOV1 of Chlamydomonas reinhardtii was reported to show accelerated dark regeneration of D450 from S390 when Arg-58 was substituted by Lys. The half-lives of the native phot and the R58K substitute were 204 and 73 s, respectively (32). To alter the lifetime of S390 in P1L2K, Arg-513 (supplemental Fig. S4) was substituted with Lys (R513K), and its effect on the photochemistry was studied. Arg-513 resides next to the photoactive Cys, and its side chain interacts with ribitol and phosphate of the FMN chromophore via hydrogen bonding.
The purity of P1L2K-R513K was estimated at 80% by CBB staining of the SDS-PAGE gel (Fig. 7A). Its UV-visible absorption spectrum of D450 showed absorption peaks at 446, 473 and 376 nm (Fig. 7B) and was almost the same as the native sample. The P1L2K-R513K exhibited a larger absorption at 280 nm than the native P1L2K, indicating decreased FMN binding. The portion of P1L2K-R513K was roughly estimated at ∼⅓ of the native P1L2K judging from the ratio of the absorptions at 450 nm to that at 280 nm.
FIGURE 7.
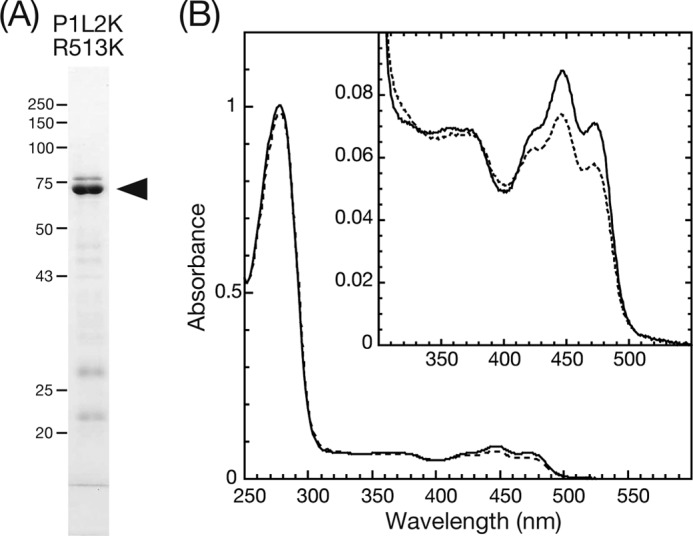
A, SDS-PAGE gel of purified P1L2K-R513K stained with CBB. The black arrowhead indicates the position of the native protein. B, absorption spectra of P1L2K-R513K in a solution of 20 mm Tris-HCl, pH 7.8, 100 mm NaCl, 10% (w/v) glycerol, and 1 mm Na2EGTA at 15 °C. The solid and dashed spectra were measured after dark adaptation and under BL irradiation, respectively.
P1L2K-R513K showed a characteristic photocycle for the LOV cores forming a transient cysteinyl-flavin adduct, S390, upon BL excitation (Fig. 7B, inset). The rate constants and half-lives of the regeneration of D450 from S390 at four different temperatures are summarized in Table 2. The half-life was 3.9 s at 15 °C or ∼15 times shorter than that of the native P1L2K. From the Arrhenius plots of these rate constants, the activation energy and the pre-exponential factor for dark regeneration were 68.4 and 26.4 kJ mol−1, respectively (supplemental Fig. S3). These were slightly larger than the 65.3 and 22.4 kJ mol−1 of the native P1L2K. The efficiency of S390 formation from D450 measured by a single flash pulse excitation was ∼70% of the native sample (data not shown). This again indicated that the efficiency of the S390 formation could not be a major cause for the different photosensitivities of kinase activation between native P1L2K and P1L2K-R513K.
TABLE 2.
Rate constants (k) and half-lives (t1/2) of the dark regeneration of D450 from S390 of P1L2K-R513K at four different temperatures
| Temperature | P1L2K-R513K |
|
|---|---|---|
| k | t1/2 | |
| s−1 | s | |
| 4 °C (277 K) | 0.0378 | 18.4 |
| 10 °C (283 K) | 0.0705 | 9.8 |
| 15 °C (288 K) | 0.1161 | 6.0 |
| 20 °C (293 K) | 0.1912 | 3.6 |
Dependence of Light-induced Kinase Activity of P1L2K-R513K on Light Intensity
The effect of the shortened S390 state in P1L2K-R513K on the photoactivation of the kinase was studied. Fig. 8 shows the dependence of the photoactivation of the P1L2K-R513K kinase on the irradiation light intensity. The kinase assay was performed at 15 °C instead of 20 °C because P1L2K-R513K was less stable than P1L2K and tended to form aggregates at 20 °C. Native P1L2K showed 50% kinase activation below 2 μmol m−2 s−1, and the activation was saturated above 10 μmol m−2 s−1 at 15 °C (compare the filled circle curve of Fig. 8B with that of Fig. 5B). This indicated that the photoactivation of the P1L2K kinase at 15 °C was more sensitive to light than that at 20 °C, in accordance with the results shown in Fig. 6. The R513K mutation shifted the photoactivation curve to higher light intensity, with its 50% kinase activation estimated at 20 μmol m−2 s−1. Based on the light intensity at 50% activation, the photosensitivity of P1L2K was ∼10 times lower than the R513K mutant.
FIGURE 8.
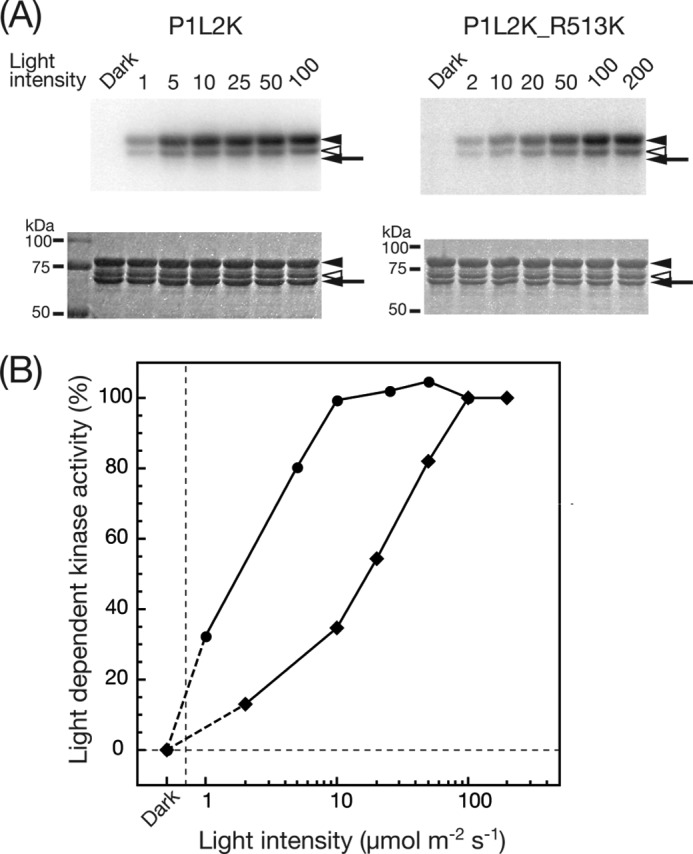
A, phosphorylation of P1Nt by P1L2K (left panels) and P1L2K-R513K (right panels) under BL of different light intensities. The upper and lower panels indicate autoradiogram and CBB staining of SDS-PAGE gels, respectively. The samples were incubated in a phosphorylation medium at 15 °C for 15 min under mock (Dark) or BL irradiation with the indicated light intensity (μmol m−2 s−1). The arrows and arrowheads are the same as in Fig. 3. B, dependence of light-induced kinase activity of P1L2K (filled circles) and P1L2K-R513K (filled diamonds) on light intensity. The increase in phosphorylation signal upon BL irradiation relative to that of mock irradiation was quantified by phosphorimaging and is plotted against the BL intensity, where the phosphorylation signals at the highest intensity (10 and 100 μmol m−2 s−1 for P1L2K and P1L2K-R513K, respectively) are normalized to 100%.
DISCUSSION
P2L2K as a BL-regulated Kinase with Dark Activity
P1L2K was previously shown to serve as a BL-regulated protein kinase (26). In the present study, P2L2K was revealed to also act as a photoactive kinase. In contrast to P1L2K, which showed little dark kinase activity on either of the artificial substrates, P1Nt and P2Nt, P2L2K significantly phosphorylated both of them in the dark (Figs. 3A and 4). The dark kinase activity was significant because it disappeared when the kinase was inactivated by the amino acid substitution D720A. This was consistent with the previous observation that GST fusion proteins of At phot2 LOV2- and LOV1-LOV2-linker-kinase domain phosphorylated casein, an artificial substrate, in the dark (25). In the case of autophosphorylation, both At phot1 and phot2 expressed in insect cells showed phosphorylation in the dark, with phot2 showing greater phosphorylation than phot1 (22, 30). Observed differences in the dark kinase activities (i.e., very low dark kinase activity for P1L2K and marked dark kinase activity for P2L2K) might therefore be characteristic of substrate phosphorylation but not autophosphorylation.
LOV2 is a major inhibitor of the phot kinases in the dark state (22, 25, 30, 31). The different degrees of substrate phosphorylation and autophosphorylation in the dark between the Atphot1 and phot2 proteins suggested that the modes of kinase inhibition by LOV2 might differ between phot1 and phot2. Amino acid sequences in the linker regions including the Jα helix and the C-terminal end region of kinase domain are not well conserved between At phot1 and phot2 despite the high homology in the LOV2 and other regions of kinase domain. This could cause the different dark kinase activities through different molecular conformations between LOV2 and kinase domain.
The dark kinase activity of phot2 might have a physiological significance because At phot2 is reported to be involved in the positioning of chloroplasts for accumulation at the bottom of mesophyll cells in the dark (33). Complementation analyses of At phot2-mediated chloroplast relocation by truncated phot2 showed that the phot2 fragment corresponding to P2L2K complemented the dark positioning observed with the wild type, an effect that disappeared when the phot2 kinase was inactivated by amino acid substitution (34). Furthermore, substitution of the two phosphorylation sites, Ser-761 and Ser-763, in the activation loop of At phot2 with Ala abolished the dark positioning in addition to the accumulation and avoidance responses of chloroplast (28). Thus, the observed dark kinase activity might be biologically significant, possibly through relocation of chloroplasts in the dark.
Cross-phosphorylation between phot1 and phot2
The present results showed phosphorylation of P1Nt by P2L2K and vice versa, suggesting cross-phosphorylation between phot1 and phot2. Cross-phosphorylation between At phot1 and phot2 has been reported in the past using an insect cell expression system (30). Full-length phot2 also phosphorylated kinase-inactivated full-length phot1 in a light-dependent manner, indicating that phot2 could phosphorylate phot1 in vitro. Considering that P2L2K phosphorylated P1Nt and that P1Nt includes six of nine autophosphorylation sites of phot1 (27), it is probable that phot2 phosphorylates phot1 at these six sites from the C terminus to the hinge between LOV1 and LOV2 (Fig. 1, At phot1) in vitro. The present observation that autophosphorylation of P1L2K and P2L2K themselves was not observable (Figs. 3B and 4B) supported this proposal. However, amino acid substitution of phot1 showed that the phosphorylation at these sites did not contribute to the phot1-mediated physiological responses, such as stomatal opening, phototropism, chloroplast accumulation, and leaf flattening (27). Thereafter, phosphorylation of phot1 by phot2 in vivo and its biological meaning remain unknown.
The present results also indicated phosphorylation of phot2 by phot1. It is possible that this phosphorylation occurs in the P2Nt region similarly for phot1 phosphorylation by phot2. However, complementation analyses of phot2-mediated responses by truncated phot2 showed that the amino acid region from the N terminus to the hinge between LOV1 and LOV2 was not essential for phot2 signaling in Adiantum (35) and Arabidopsis (34). Again, phosphorylation of phot2 by phot1 in vivo and its biological significance have not yet been elucidated.
Involvement of the Lifetime of S390 in the Photosensitivity of Kinase Photoactivation
To date, phot kinase is thought to be activated through conformational changes induced mainly in the linker region following adduct formation. Concerning the photoreaction of LOV, time-resolved (12) and low temperature (36) UV-visible absorption spectroscopy detected the distinct photointermediates L660 and S390. However, UV-visible absorption reflects the electronic environments around the isoalloxazine ring of the FMN chromophore and cannot sense the conformational changes in the protein moiety. We therefore proposed denoting these photointermediates with a UV-visible absorption spectrum of S390 and conformational changes in the protein moiety detected by NMR (15), SAXS (16), FTIR (17, 18), or transient grating (19) as S390II (37). In the present investigation, the phot proteins consisting of LOV2-linker-kinase also exhibited production of a photointermediate (S390) similar to that of LOV cores accompanied by kinase activation. Fig. 9 illustrates the relationship between photointermediates and kinase activation. Sa and Si are designated as the molecular species with active and inactive kinase forms, respectively. Formation and decay of S390 (inner circle) precedes activation and inactivation of the kinases (outer circle), respectively. There exists a time lag between the S390 formation and the conformational changes that enable the kinase activated. Because time resolution of the assay for BL-induced kinase activity change is poor, it is hard to measure these delays at this stage. BL induces cancelation of kinase inhibition by LOV2 possibly through changes in the interaction between LOV2 and the kinase domain. One of these changes was observed with P2L2K by SAXS, although it lost kinase activity because of the substitution of Asp-720 with Gln. In this case, LOV2 is proposed to move away from the N-terminal lobe of the kinase domain upon BL excitation (20). Furthermore, FTIR detected a BL-induced loss of helicity in the activation loop region of the kinase domain in Chlamydomonas full-length phototropin (38).
FIGURE 9.
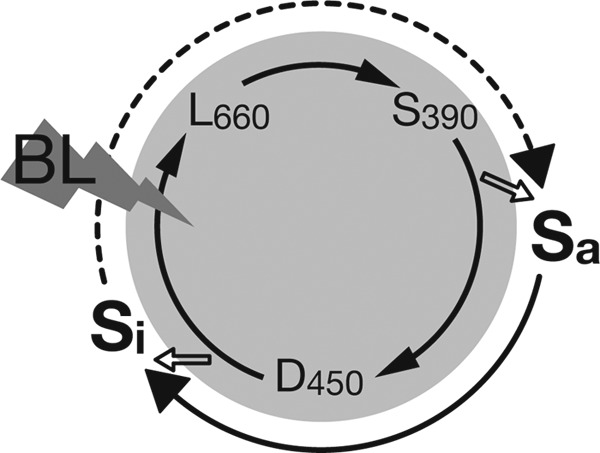
Schematic of the relationship between photocycle and kinase activation in kinase-containing phot proteins. The inner circle indicates photocycle expressed by a ground state D450, its singlet excited state D450*, and two photointermediates (L660 and S390) detectable by UV-visible absorption spectroscopy. The outer circle shows active (Sa) and inactive (Si) states of the kinase.
Activity of the phot kinase is expected to be proportional to the number of phot kinase molecules in the Sa state. Because S390 reverts to D450 spontaneously in the dark followed by kinase inactivation, the degree of kinase activation can be expressed as a product of the number of incident photons (light intensity of BL), the quantum efficiency of Sa formation, and the lifetime of Sa. Using this expression for kinase activity, we compared the degree of kinase photoactivation of P1L2K and P2L2K. Because the efficiency of Sa formation approximated by the amount of S390 formed by a single flashlight is comparable between the two kinases, the lifetime of Sa becomes a rate-limiting element for the degree of kinase photoactivation. Kinetic analyses of D450 regeneration from S390 showed that P1L2K had a 10 times longer lifetime than P2L2K (Table 1). Because the time required for the transition from S390 to Sa is short enough to be considered negligible and the lifetime of Sa can be approximated by that of S390, the degree of photoactivation of P1L2K is suggested to be ∼10 times greater than that of P2L2K. This, in turn, indicated that ∼10 times more photons are required for P2L2K to produce the same degree of kinase photoactivation as that of P1L2K. The dependence of light-induced kinase activity on the irradiation light intensity (Fig. 5) clearly showed that P1L2K was 10 times more photosensitive than P2L2K for kinase photoactivation.
This result was further confirmed by an amino acid substitution experiment of P1L2K to shorten the Sa lifetime to approximate that of S390 (Fig. 8). Introduction of an R513K substitution to P1L2K reduced both the S390 lifetime and the photosensitivity of the kinase photoactivation to one-tenth of the original, supporting the idea that the photosensitivity of the kinase photoactivation is regulated by the lifetime of S390. Similar results were obtained with phot2 by introducing R427K substitution corresponding to R513K of phot1 (supplemental Fig. S5). The substitution accelerates the dark regeneration, and the t½ came from 6.8 to 0.4 s at 20 °C. Photoactivation of the phosphorylation required much more intense light. Obvious photoactivation was detected under 800 μmol m−2 s−1 in which the photodamage by the intense light irradiation upon the light activation of the kinase was checked by the preirradiation and was not observed. The shortened lifetime again desensitized the photoactivation of the kinase.
The above idea was further supported by the temperature effects on kinase photoactivation and S390 lifetime. The rate of S390 formation for both LOV1 and LOV2 of Avena sativa phot1 were reported to be unaffected by lowering the temperature from room temperature to ice temperature (11). Meanwhile, kinase photoactivation of both P1L2K and P2L2K was inversely proportional to temperature, which could be explained by the prolonged lifetime of S390 at lower temperatures (Fig. 6). The present results demonstrated that the lifetime of S390 in LOV2 is one of the key factors regulating the photosensitivity of photoactivation of phot kinases.
Implication for Physiological Responses
It has been well established that phot1 acted in response to low and high light, whereas phot2 responded only to high light (9). Although phot1 responds to high light as well as to low light, the high and low photosensitivity of phot1 and phot2, respectively, coincided well with the high and low photosensitivities in kinase photoactivation of P1L2K and P2L2K, respectively. This raised an interesting question of whether the lifetime of S390 was involved in the different photosensitivities of phot1 and phot2. Such an analysis was reported with Adiantum capillus-veneris phot2 (35). Specifically, the half-life of the signal in chloroplast avoidance movement in Adiantum was estimated to be 67 s by cessation of movement after switching off BL and 167 s by timing backward movement in the dark. These times were similar to the half-lives of activated A. diantum capillus-veneris phot2-LOV1+LOV2 obtained by spectroscopy. Furthermore, it was shown that At phot1 retained autophosphorylation activity in the dark after the cessation of a light pulse (4, 39) and that its time course was similar to the dark regeneration of D450 of At phot1 (13). The dark regeneration in At phot1 was also shown to correlate with the recovery of phototropic sensitivity following a saturating pulse of light (40).
These results suggested that the lifetime of the activated state is one of the most important factors controlling signal transmission downstream through autophosphorylation and/or phosphorylation of substrates involved in the phot-mediated responses. More experiments in vivo are required to fully understand this mechanism, including variation of the lifetime of the activated state. To vary the lifetime, amino acid substitution is an effective technique that is easy to apply to the present kinase assay systems. Such an assay will provide useful information that can be used to guide the in vivo assay systems. It should be noted that plants or algae might have acquired this type of amino acid substitution during the evolutionary process to fine-tune the lifetime of the activated state and to adapt to different light environments. In addition to the lifetime of the activated state, there may be other factors involved in the photosensitivity of the physiological responses, such as the communication of phot with its interacting proteins, such as NPH3 (41), RPT2 (42), PKSs (43, 44), and ABCB19 (29).
Acknowledgment
We thank Mihoko Nakajima of Osaka Prefecture University for technical assistance.
This work was supported by Grant-in-Aid for Scientific Research on Priority Areas 17084008 (to S. T.), Grant-in-Aid for Scientific Research on Innovative Areas 22120005 (to S. T.), and Grant-in-Aid for Exploratory Research 23657105 (to S. T.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.

This article contains supplemental Figs. S1–S5.
- BL
- blue light
- Ac
- Adiantum capillus-veneris
- At
- A. thaliana
- CBB
- Coomassie Brilliant Blue
- FMN
- flavin mononucleotide
- LOV
- light oxygen voltage-sensing domain
- P1L2K
- LOV2-linker-kinase of phototropin1
- P2L2K
- LOV2-linker-kinase of phototropin2
- phot
- phototropin
- SAXS
- small-angle x-ray scattering.
REFERENCES
- 1. Franklin K. A., Quail P. H. (2010) Phytochrome functions in Arabidopsis development. J. Exp. Bot. 61, 11–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu H., Liu B., Zhao C., Pepper M., Lin C. (2011) The action mechanisms of plant cryptochromes. Trends Plant Sci. 16, 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christie J. M. (2007) Phototropin blue-light receptors. Annu. Rev. Plant Biol. 58, 21–45 [DOI] [PubMed] [Google Scholar]
- 4. Christie J. M., Reymond P., Powell G. K., Bernasconi P., Raibekas A. A., Liscum E., Briggs W. R. (1998) Arabidopsis NPH1. A flavoprotein with the properties of a photoreceptor for phototropism. Science 282, 1698–1701 [DOI] [PubMed] [Google Scholar]
- 5. Kagawa T., Sakai T., Suetsugu N., Oikawa K., Ishiguro S., Kato T., Tabata S., Okada K., Wada M. (2001) Arabidopsis NPL1. A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291, 2138–2141 [DOI] [PubMed] [Google Scholar]
- 6. Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K. (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414, 656–660 [DOI] [PubMed] [Google Scholar]
- 7. de Carbonnel M., Davis P., Roelfsema M. R., Inoue S., Schepens I., Lariguet P., Geisler M., Shimazaki K., Hangarter R., Fankhauser C. (2010) The Arabidopsis phytochrome kinase substrate 2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 152, 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kozuka T., Kong S. G., Doi M., Shimazaki K., Nagatani A. (2011) Tissue-autonomous promotion of palisade cell development by phototropin 2 in Arabidopsis. Plant Cell 23, 3684–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sakai T., Kagawa T., Kasahara M., Swartz T. E., Christie J. M., Briggs W. R., Wada M., Okada K. (2001) Arabidopsis nph1 and npl1. Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. U.S.A. 98, 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christie J. M., Salomon M., Nozue K., Wada M., Briggs W. R. (1999) LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1). Binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. U.S.A. 96, 8779–8783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salomon M., Christie J. M., Knieb E., Lempert U., Briggs W. R. (2000) Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39, 9401–9410 [DOI] [PubMed] [Google Scholar]
- 12. Swartz T. E., Corchnoy S. B., Christie J. M., Lewis J. W., Szundi I., Briggs W. R., Bogomolni R. A. (2001) The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J. Biol. Chem. 276, 36493–36500 [DOI] [PubMed] [Google Scholar]
- 13. Kasahara M., Swartz T. E., Olney M. A., Onodera A., Mochizuki N., Fukuzawa H., Asamizu E., Tabata S., Kanegae H., Takano M., Christie J. M., Nagatani A., Briggs W. R. (2002) Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii. Plant Physiol. 129, 762–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crosson S., Moffat K. (2001) Structure of a flavin-binding plant photoreceptor domain. Insights into light-mediated signal transduction. Proc. Natl. Acad. Sci. U.S.A. 98, 2995–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harper S. M., Neil L. C., Gardner K. H. (2003) Structural basis of a phototropin light switch. Science 301, 1541–1544 [DOI] [PubMed] [Google Scholar]
- 16. Nakasako M., Iwata T., Matsuoka D., Tokutomi S. (2004) Light-induced structural changes of LOV domain-containing polypeptides from Arabidopsis phototropin 1 and 2 studied by small-angle x-ray scattering. Biochemistry 43, 14881–14890 [DOI] [PubMed] [Google Scholar]
- 17. Alexandre M. T., Domratcheva T., Bonetti C., van Wilderen L. J., van Grondelle R., Groot M. L., Hellingwerf K. J., Kennis J. T. (2009) Primary reactions of the LOV2 domain of phototropin studied with ultrafast mid-infrared spectroscopy and quantum chemistry. Biophys. J. 97, 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koyama T., Iwata T., Yamamoto A., Sato Y., Matsuoka D., Tokutomi S., Kandori H. (2009) Different role of the Jα helix in the light-induced activation of the LOV2 domains in various phototropins. Biochemistry 48, 7621–7628 [DOI] [PubMed] [Google Scholar]
- 19. Eitoku T., Nakasone Y., Matsuoka D., Tokutomi S., Terazima M. (2005) Conformational dynamics of phototropin 2 LOV2 domain with the linker upon photoexcitation. J. Am. Chem. Soc. 127, 13238–13244 [DOI] [PubMed] [Google Scholar]
- 20. Takayama Y., Nakasako M., Okajima K., Iwata A., Kashojiya S., Matsui Y., Tokutomi S. (2011) Light-induced movement of the LOV2 domain in an Asp720Asn mutant LOV2-kinase fragment of Arabidopsis phototropin 2. Biochemistry 50, 1174–1183 [DOI] [PubMed] [Google Scholar]
- 21. Harper S. M., Neil L. C., Day I. J., Hore P. J., Gardner K. H. (2004) Conformational changes in a photosensory LOV domain monitored by time-resolved NMR spectroscopy. J. Am. Chem. Soc. 126, 3390–3391 [DOI] [PubMed] [Google Scholar]
- 22. Christie J. M., Swartz T. E., Bogomolni R. A., Briggs W. R. (2002) Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J. 32, 205–219 [DOI] [PubMed] [Google Scholar]
- 23. Salomon M., Knieb E., von Zeppelin T., Rüdiger W. (2003) Mapping of low- and high-fluence autophosphorylation sites in phototropin 1. Biochemistry 42, 4217–4225 [DOI] [PubMed] [Google Scholar]
- 24. Sullivan S., Thomson C. E., Lamont D. J., Jones M. A., Christie J. M. (2008) In vivo phosphorylation site mapping and functional characterization of Arabidopsis phototropin 1. Mol. Plant. 1, 178–194 [DOI] [PubMed] [Google Scholar]
- 25. Matsuoka D., Tokutomi S. (2005) Blue light-regulated molecular switch of Ser/Thr kinase in phototropin. Proc. Natl. Acad. Sci. U.S.A. 102, 13337–13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okajima K., Matsuoka D., Tokutomi S. (2011) LOV2-linker-kinase phosphorylates LOV1-containing N-terminal polypeptide substrate via photoreaction of LOV2 in Arabidopsis phototropin1. FEBS Lett. 585, 3391–3395 [DOI] [PubMed] [Google Scholar]
- 27. Inoue S., Kinoshita T., Matsumoto M., Nakayama K. I., Doi M., Shimazaki K. (2008) Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. U.S.A. 105, 5626–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inoue S., Matsushita T., Tomokiyo Y., Matsumoto M., Nakayama K. I., Kinoshita T., Shimazaki K. (2011) Functional analyses of the activation loop of phototropin2 in Arabidopsis. Plant Physiol. 156, 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christie J. M., Yang H., Richter G. L., Sullivan S., Thomson C. E., Lin J., Titapiwatanakun B., Ennis M., Kaiserli E., Lee O. R., Adamec J., Peer W. A., Murphy A. S. (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol. 9, e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cho H. Y., Tseng T. S., Kaiserli E., Sullivan S., Christie J. M., Briggs W. R. (2007) Physiological roles of the light, oxygen, or voltage domains of phototropin 1 and phototropin 2 in Arabidopsis. Plant Physiol. 143, 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaiserli E., Sullivan S., Jones M. A., Feeney K. A., Christie J. M. (2009) Domain swapping to assess the mechanistic basis of Arabidopsis phototropin 1 receptor kinase activation and endocytosis by blue light. Plant Cell 21, 3226–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Losi A., Kottke T., Hegemann P. (2004) Recording of blue light-induced energy and volume changes within the wild-type and mutated phot-LOV1 domain from Chlamydomonas reinhardtii. Biophys. J. 86, 1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suetsugu N., Kagawa T., Wada M. (2005) An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol. 139, 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kong S. G., Kinoshita T., Shimazaki K., Mochizuki N., Suzuki T., Nagatani A. (2007) The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses. Plant J. 51, 862–873 [DOI] [PubMed] [Google Scholar]
- 35. Kagawa T., Kasahara M., Abe T., Yoshida S., Wada M. (2004) Function analysis of phototropin 2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant Cell Physiol. 45, 416–426 [DOI] [PubMed] [Google Scholar]
- 36. Zikihara K., Iwata T., Matsuoka D., Kandori H., Todo T., Tokutomi S. (2006) Photoreaction cycle of the light, oxygen, and voltage domain in FKF1 determined by low-temperature absorption spectroscopy. Biochemistry 45, 10828–10837 [DOI] [PubMed] [Google Scholar]
- 37. Matsuoka D., Iwata T., Zikihara K., Kandori H., Tokutomi S. (2007) Primary Processes During the Light-signal Transduction of Phototropin. Photochem. Photobiol. 83, 470. [DOI] [PubMed] [Google Scholar]
- 38. Pfeifer A., Mathes T., Lu Y., Hegemann P., Kottke T. (2010) Blue light induces global and localized conformational changes in the kinase domain of full-length phototropin. Biochemistry 49, 1024–1032 [DOI] [PubMed] [Google Scholar]
- 39. Salomon M., Zacherl M., Rudiger W. (1996) Changes in blue-light-dependent protein phosphorylation during the early development of etiolated oat seedlings. Planta 199, 336–342 [DOI] [PubMed] [Google Scholar]
- 40. Briggs W. R., Christie J. M., Salomon M. (2001) Phototropins. A new family of flavin-binding blue light receptors in plants. Antioxid. Redox Signal. 3, 775–788 [DOI] [PubMed] [Google Scholar]
- 41. Motchoulski A., Liscum E. (1999) Arabidopsis NPH3. A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286, 961–964 [DOI] [PubMed] [Google Scholar]
- 42. Sakai T., Wada T., Ishiguro S., Okada K. (2000) RPT2. A signal transducer of the phototropic response in Arabidopsis. Plant Cell 12, 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lariguet P., Schepens I., Hodgson D., Pedmale U. V., Trevisan M., Kami C., de Carbonnel M., Alonso J. M., Ecker J. R., Liscum E., Fankhauser C. (2006) Phytochrome kinase substrate 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. U.S.A. 103, 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Demarsy E., Schepens I., Okajima K., Hersch M., Bergmann S., Christie J., Shimazaki K., Tokutomi S., Fankhauser C. (2012) Phytochrome kinase substrate 4 is phosphorylated by the phototropin 1 photoreceptor. EMBO J. 31, 3457–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]