Background: The intracrine nature of endothelin-1 is largely correlated with nuclear signaling events.
Results: In endothelial cells, endothelin-1 acting on endolysosomal ETB receptors increases cytosolic Ca2+ and nitric oxide.
Conclusion: Endolysosomal ETB receptors are functional.
Significance: We identify a new pathway for ET-1-induced intracrine signaling and provide the first evidence that intracellular G protein-coupled receptors are involved in redox signaling.
Keywords: Calcium Imaging, Endothelium, Lysosomes, Receptors, Redox Signaling, Intracellular Microinjection, Microautophagy
Abstract
Endothelin-1 exerts its actions via activation of ETA and ETB Gq/11 protein-coupled receptors, located in the plasmalemma, cytoplasm, and nucleus. Although the autocrine/paracrine nature of endothelin-1 signaling has been extensively studied, its intracrine role has been largely attributed to interaction with receptors located on nuclear membranes and the nucleoplasm. Because ETB receptors have been shown to be targeted to endolysosomes, we used intracellular microinjection and concurrent imaging methods to test their involvement in Ca2+ signaling and subsequential NO production. We provide evidence that microinjected endothelin-1 produces a dose-dependent elevation in cytosolic calcium concentration in ETB-transfected cells and endothelial cells; this response is sensitive to ETB but not ETA receptor blockade. In endothelial cells, the endothelin-1-induced Ca2+ response is abolished upon endolysosomal but not Golgi disruption. Moreover, the effect is prevented by inhibition of microautophagy and is sensitive to inhibitors of the phospholipase C and inositol 1,4,5-trisphosphate receptor. Furthermore, intracellular endothelin-1 increases nitric oxide via an ETB-dependent mechanism. Our results indicate for the first time that intracellular endothelin-1 activates endolysosomal ETB receptors and increase cytosolic Ca2+ and nitric oxide production. Endothelin-1 acts in an intracrine fashion on endolysosomal ETB to induce nitric oxide formation, thus modulating endothelial function.
Introduction
Compelling evidence indicates that in addition to the classical localization and signaling at the plasma membrane, a wide variety of G protein-coupled receptors (GPCRs)3 are expressed and fully functional intracellularly (1–3). Endothelin-1 (ET-1) acts on ETA and ETB receptors, Gq/11-coupled GPCRs, exerting opposing effects on vascular functions. In the cardiovascular human and rodent cells, ETA and ETB are localized to the plasma membrane, cytoplasm, nuclear membranes, and nucleoplasm (4–6). ET-1 elevates both cytosolic and nuclear Ca2+ concentrations; the rise in cysotolic Ca2+ concentration, [Ca2+]i, in response to ET-1 is seen in both intact and membrane-perforated cells (5). Moreover, ET-1 is involved in nitric oxide (NO) and reactive oxygen species generation both in the cytosol and in the nucleus (5).
Although the stimulation of nuclear membrane receptors elicits events largely restricted to the nucleus (3, 5), receptors located on the membrane of other organelles trigger signaling cascades within the cytoplasm, resulting in rapid cell responses. Because ETB receptors were identified in the endolysosomal system (7–9), in this study, we examined whether or not endolysosomal ETB receptors are involved in Ca2+ signaling and NO production in rat pulmonary microvascular endothelial cells (RPMVEC), which express ETB receptors (10–12).
EXPERIMENTAL PROCEDURES
Chemicals
Endothelin-1, bafilomycin A1, brefeldin A, BQ-123 sodium salt, BQ-788 sodium salt, ryanodine, and xestospongin C were purchased from EMD Millipore (Billerica, MA), U-73122, l-NAME, and heparin were from Sigma-Aldrich, and Ned-19 was from Tocris Bioscience (R&D Systems, Minneapolis, MN).
Cell Culture, DNA Constructs, and Transfection
RPMVEC were cultured in M199 medium (Thermo Fisher Scientific) containing 15% fetal bovine serum (Atlanta Biologicals, Inc., Lawrenceville, GA), 1% GlutaMax, 1% penicillin-streptomycin-amphotericin B (both from Invitrogen), 50 μg/ml endothelial cell growth supplement) (BD Biosciences), 1% non-essential amino acids (ATCC, Manassas, VA) on glass coverslips coated with gelatin. U2OS cells (ATCC) were cultured in DMEM (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Atlanta Biologicals). U2OS cells were transiently transfected with GFP-tagged ETB (rat) cDNA or pCMV6-AC-mGFP vector (OriGene Technologies Inc, Rockville, MD) using Turbofectin 8 transfection reagent according with the manufacturer's instructions (OriGene Technologies Inc). Cells were used 24–48 h after transfection.
Immunocytochemistry
RPMVEC were transiently transfected with GFP-tagged ETB (rat) using Turbofectin 8. The cells were incubated with LysoTracker Red (1 μm) (Invitrogen) following pretreatment with either the vehicle (dimethyl sulfoxide 0.1% (v/v)), bafilomycin A1 (1 μm) or glycyl-l-phenylalanine 2-naphthylamide (100 μm) for 1 h, as reported (13). Cells were fixed in 4% paraformaldehyde, mounted with DAPI Fluoromount G (Southern Biotech, Birmingham, AL), and examined under a confocal scanning microscope (Leica TCS SP5) with excitation wavelengths set to 405 nm for DAPI, 488 nm for GFP, and 561 nm for LysoTracker Red, in the sequential mode.
Calcium Imaging
Measurements of [Ca2+]i were performed as described previously (13, 14). Cells were incubated with 5 μm Fura-2 AM (Invitrogen) in Hanks' balanced salt solution at room temperature for 45 min, in the dark, washed three times with dye-free Hanks' balanced salt solution, and then incubated for another 45 min to allow for complete de-esterification of the dye. Coverslips (25-mm diameter) were subsequently mounted in an open bath chamber (RP-40LP, Warner Instruments, Hamden, CT) on the stage of an inverted microscope Nikon Eclipse TiE (Nikon, Inc., Melville, NY). The microscope is equipped with a Perfect Focus System and a Photometrics CoolSnap HQ2 CCD camera (Photometrics, Tucson, AZ). During the experiments, the Perfect Focus System was activated. Fura-2 AM fluorescence (emission, 510 nm), following alternate excitation at 340 and 380 nm, was acquired at a frequency of 0.25 Hz. Images were acquired and analyzed using NIS-Elements AR software (version 3.1, Nikon, Inc.). The ratio of the fluorescence signals (340/380 nm) was converted to Ca2+ concentrations (15).
Intracellular Microinjection
Injections were performed using Femtotips II, InjectMan NI2, and FemtoJet systems (Eppendorf) as reported previously (16–19). Pipettes were back filled with an intracellular solution composed of 110 mm KCl, 10 mm NaCl, and 20 mm HEPES (pH 7.2) (20) or the specific chemicals. The injection time was 0.4 s at 60 hectoPascal with a compensation pressure of 20 hPa to maintain the microinjected volume to <1% of cell volume, as measured by microinjection of a fluorescent compound (Fura-2-free acid) (20). The intracellular concentration of chemicals was determined based on the concentration in the pipette and the volume of injection. The cellular volume was estimated to 1000 μm3 (21).
Measurement of NO Levels
Intracellular NO was monitored with DAF-FM (4-amino-5-methylamino-2′,7′-difluorofluorescein, Invitrogen), a pH-insensitive fluorescent dye, as described previously (22). Cells were incubated at room temperature for 45 min in Hanks' balanced salt solution containing a low concentration (0.5 μm) of DAF-FM. This condition significantly reduced the background autofluorescence and improved the signal-to-noise ratio of NO detection in single cells. After loading, cells were rinsed three times with saline. NO fluorescence was measured at a rate of 0.1 Hz using excitation/emission wavelengths of 488 nm/540 nm.
Data Analysis
Data were expressed as mean and S.E. One-way analysis of variance, followed by post hoc Bonferonni and Tukey tests, was used to assess significant differences between groups; p < 0.05 was considered statistically significant.
RESULTS
To avoid any possible plasmalemmal effect of ET-1, in all series of experiments, the cells were pretreated for 5 min with a mixture of ETA and ETB plasmalemmal non-permeant antagonists (BQ-123 and BQ-788, both 10−7 m).
Microinjection of ET-1 (10−10 m) produced small and non-significant increases in [Ca2+]i in untransfected or GFP-transfected U2OS cells (Δ[Ca2+]i was 22 ± 4 nm and 19 ± 3 nm, respectively, Fig. 1, A and B). Similar non-significant responses were observed upon intracellular administration of ETA antagonist BQ-123 or ETB antagonist BQ-788 (both 10−7 m) to ETB-GFP-transfected U2OS cells (Δ[Ca2+]i was 34 ± 4 nm and 32 ± 2 nm, respectively, Fig. 1, C and D) or to endothelial cells (Δ[Ca2+]i was 29 ± 4 nm and 27 ± 4 nm, respectively, Fig. 1, E and F). Six cells were injected in each of the above mentioned control experiments.
FIGURE 1.
Control experiments. A, averaged Ca2+ responses to intracellular administration of endothelin-1 (10−10 m) in untransfected (black) or GFP-transfected (gray) U2OS cells. B, comparison of the amplitudes of ET-1 effects on [Ca2+]i in untransfected and GFP-transfected U2OS control cells. C, averaged Ca2+ responses to BQ-123 (ETA antagonist, 10−7 m, black) or BQ-788 (ETB antagonist, 10−7 m, gray) microinjection in ETB-GFP-transfected U2OS cells. D, comparison of the Ca2+ responses of ETB-GFP-U2OS cells to intracellular administration of BQ-123 or BQ-788. E, averaged Ca2+ responses to BQ-123 (10−7 m, black) or BQ-788 (10−7 m, gray) microinjection in rat endothelial cells, endogenously expressing ETB. F, comparison of the Ca2+ responses of endothelial cells to intracellular administration of BQ-123 or BQ-788.
Intracellular Injection of ET-1 Elevates [Ca2+]i in ETB-expressing Cells
In U2OS cells transiently transfected with ETB-GFP, microinjection of ET-1 (10−11 m, 10−10 m, and 10−9 m final concentrations inside the cell) produced a concentration-dependent elevation of [Ca2+]i by 361 ± 7 nm, 706 ± 16 nm, and 1394 ± 28 nm, respectively (n = 6 for each concentration tested) (Fig. 2, A, B, and D). Intracellular microinjection of control buffer in ETB-expressing cells resulted in a small and non-significant increase in [Ca2+]i, by 34 ± 4 nm (n = 6 cells) (Fig. 2, A–C). The small increase in [Ca2+]i, followed by a rapid return and steady Ca2+ base line after control microinjections indicate the optimization of injection parameters to avoid any potential artifacts produced by cell damage at the level of organelles or plasma membrane. Untransfected and GFP-transfected U2OS cells did not respond to ET-1 microinjection (Fig. 1, A and B). When the cells were co-injected with the ETA antagonist BQ-123 (10−7 m), the rise in [Ca2+]i in response to ET-1 (10−10 m) was 698 ± 12 nm (n = 6), largely similar to that produced by ET-1 (10−10 m) alone (Fig. 2, A, B, D, and E). However, co-administration of the ETB antagonist BQ-788 (10−7 m) and ET-1 (10−10 m) prevented ET-1-induced response, elevating [Ca2+]i only by 22 ± 3 nm (n = 6), a response resembling that of control buffer (Fig. 2, A, B, C, and F). ETA and ETB antagonists alone did not affect [Ca2+]i when injected into U2OS-ETB-GFP (Fig. 1, C and D).
FIGURE 2.
Intracellular administration of endothelin-1 elevates [Ca2+]i in U2OS cells expressing ETB receptors. A, averaged traces (n = 6) illustrating the Ca2+ responses to ET-1 (10−11, 10−10, 10−9 m) and to ET-1 co-injected with the ETA antagonist BQ-123 (10−7 m) or with the ETB antagonist BQ-788 (10−7 m); arrows indicate the time point of injection. B, comparison of the increases in [Ca2+]i produced by various concentrations of ET-1 in absence and presence of ETA or ETB antagonist; p < 0.05 compared with control (*) or to ET-1 10−10 m (#) microinjection. C–F, representative images showing ETB-GFP fluorescence (first panels) and Fura-2 AM fluorescence ratio (F340/F380 nm) before, during and 6 min after microinjection (inj; second, third, and fourth panels, respectively) of control buffer (C), ET-1 (10−10 m) alone (D), or in presence of BQ-123 (E), or BQ-788 (F); arrows indicate the injected cells; the fluorescence scale (0–3) is illustrated in each panel and magnified in the second panel of C.
Intracellular Injection of ET-1 Elevates [Ca2+]i in RPMVEC
ET-1 microinjection to RPMVEC, which endogenously express ETB (23), produced fast and transient increases in [Ca2+]i. RPMVEC responded to intracellular administration of ET-1 (10−11 m, 10−10 m, and 10−9 m final concentrations inside the cell) with [Ca2+]i increases of 170 ± 6 nm, 447 ± 9 nm, and 751 ± 14 nm, respectively (n = 6), whereas control buffer microinjection produced a minor and non-significant [Ca2+]i elevation, of 22 ± 4 nm (Fig. 3, A–D). In RPMVEC, co-administration of BQ-123 (10−7 m) with ET-1 (10−10 m) did not significantly affect ET-1-induced response (Δ[Ca2+]i for BQ-123 + ET-1 was 428 ± 7 nm versus 447 ± 9 nm for ET-1 alone, Fig. 3, A, B, D, and E), whereas concomitant microinjection of BQ-788 (10−7 m) and ET-1 (10−10 m) completely abolished the ET-1 response (Δ[Ca2+]i for BQ-788 + ET-1 was 24 ± 4 nm, similar to control buffer microinjection, Fig. 3, A–C, and F). Neither the ETA nor ETB antagonist had an effect on its own when injected into RPMVEC (Fig. 1, E and F).
FIGURE 3.
Endothelin-1 microinjection increases [Ca2+]i in RPMVEC via ETB receptor activation. A, averaged traces (n = 6) illustrating concentration-dependent effect of ET-1 (10−11–10−9 m) on [Ca2+]i and the Ca2+ responses of ET-1 (10−10 m) co-injected with the ETA antagonist BQ-123 (10−7 m) or with the ETB antagonist BQ-788 (10−7 m); ETB blockade abolished ET-1 effect. B, comparison of the amplitude of [Ca2+]i elevations produced by various concentrations of ET-1 in absence and presence of ETA or ETB antagonist; p < 0.05 compared with control (*) or to ET-1 10−10 m (#) microinjection. C–F, typical fluorescence images of Fura-2 AM-loaded endothelial cells before (left), during (middle), and 6 min after (right) intracellular administration of control buffer (C), ET-1 (10−10 m) alone (D), or in presence of BQ-123 (E) or BQ-788 (F); arrows indicate the injected cells; the fluorescence scale (0–3) is illustrated in each panel and magnified in the first panel of C. inj, injection.
Endolysosomal Localization of Functional ETB Receptors
In endothelial cells, the increase in [Ca2+]i induced by microinjected ET-1 (10−10 m) was insensitive to brefeldin A (10 μm, 1-h incubation), a Golgi apparatus disruptor (24) (Δ[Ca2+]i was 423 ± 7 nm in the presence versus 447 ± 9 nm in the absence of brefeldin A, n = 6; Fig. 4, A–D). Conversely, exposure to bafilomycin A1 (1 μm, 1 h), a V-type ATPase inhibitor (25), abolished the effect of microinjected ET-1 on endothelial [Ca2+]i (Δ[Ca2+]i = 28 ± 6 nm, n = 6, Fig. 4, A, B, and E). Furthermore, inhibition of microautophagy with rapamycin (30 μm, 1 h) (26) prevented ET-1 from eliciting a significant rise in [Ca2+]i (Δ[Ca2+]i = 14 ± 5 nm, n = 6, Fig. 4, A, B, and F).
FIGURE 4.
Lysosomal localization of functional ETB receptors in RPMVEC. A, the Ca2+ response to endothelin-1 is prevented by 1-h pretreatment with bafilomycin A1 (Baf, 1 μm) or rapamycin (Rap, 30 μm), but not brefeldin A (Bref, 10 μm); averaged traces from six experiments are shown. B, comparison of the increases in [Ca2+]i produced by microinjected ET-1 in absence or presence of brefeldin A, bafilomycin A1, or rapamycin; *, p < 0.05 compared with ET-1 alone. C–F, representative images depicting Fura-2 AM fluorescence ratio (F340/F380 nm) of endothelial cells before (left), during (middle), and 6 min after (right) ET-1 microinjection in absence (C) or presence of brefeldin A (D), bafilomycin A1 (E), or rapamycin (F) pretreatment. inj, injection.
In ETB-GFP-expressing RPMVEC, endolysosomes were identified with LysoTracker Red (Fig. 5A). Treatment with bafilomycin A1 (1 μm, 1 h), or glycyl-l-phenylalanine 2-naphthylamide (100 μm, 1 h), a basic amine inducing lysosomal permeabilization (27), markedly diminished the LysoTracker Red and GFP-ETB fluorescence (Fig. 5, B and C).
FIGURE 5.
Immunocytochemical localization of ETB receptors to lysosomes in RPMVEC. A, identification of ETB-GFP fluorescence, acidic organelles/lysosomes labeled with LysoTracker Red, and the nuclei labeled with DAPI in RPMVEC transiently transfected with GFP-labeled ETB. In the overlay image, the colocalization of intracellular ETB receptors with lysosomes is seen as orange fluorescence. B, pretreatment with bafilomycin A1 (Baf, 1 μm) markedly decreased both ETB-GFP and LysoTracker Red fluorescence. C, disruption of lysosomes by pretreatment with glycyl-l-phenylalanine 2-naphthylamide (GPN) (100 μm) also markedly reduced the ETB-GFP and LysoTracker Red fluorescence.
Lysosomal ETB Activation Releases Ca2+ from IP3-dependent Stores
In Ca2+-free saline, microinjection of ET-1 (10−10 m) to RPMVEC increased [Ca2+]i by 431 ± 11 (n = 6), largely similar to the Δ[Ca2+]i produced in Ca2+-containing Hanks' balanced salt solution (447 ± 9 nm, n = 6). This response was basically absent in cells pretreated with xestospongin C and heparin (36 ± 2 nm, n = 6, Fig. 6, A and B), which block inositol 1,4,5-trisphosphate (IP3) receptors (IP3R), or with the phospholipase C inhibitor U-73122 (21 ± 3 nm, n = 6, Fig. 6, A and B). Conversely, blocking NAADP-dependent Ca2+ release from endolysosomes with Ned-19 (5 μm, 15 min) (28) or inhibition of ryanodine receptors with ryanodine (1 μm, 15 min) was ineffective in counteracting ET-1 effect (424 ± 8 nm, n = 6; 435 ± 10 nm, n = 6, respectively; Fig. 6, A and B).
FIGURE 6.
Endothelin-1 mobilizes Ca2+ from the IP3-dependent stores. A, averaged Ca2+ responses (n = 6) to ET-1 microinjection in RPMVEC incubated in Ca2+-free saline with inhibitors of Ca2+ release from the lysosomes (Ned-19) and from the endoplasmic reticulum/lysosomal IP3R blockers xestospongin C (XeC) and heparin; endoplasmic reticulum ryanodine (Ry) receptor blocker ryanodine; or with the phospholipase C inhibitor U-73122. B, comparison of the [Ca2+]i increases in response to intracellular administration of ET-1 in Ca2+-containing and Ca2+-free saline in the absence and presence of the indicated antagonists; *, p < 0.05 compared with ET-1 (10−10 m). ET-1 effects were blocked by inhibition of phospholipase C or of IP3 receptors.
Lysosomal ETB-dependent NO Production in RPMVEC
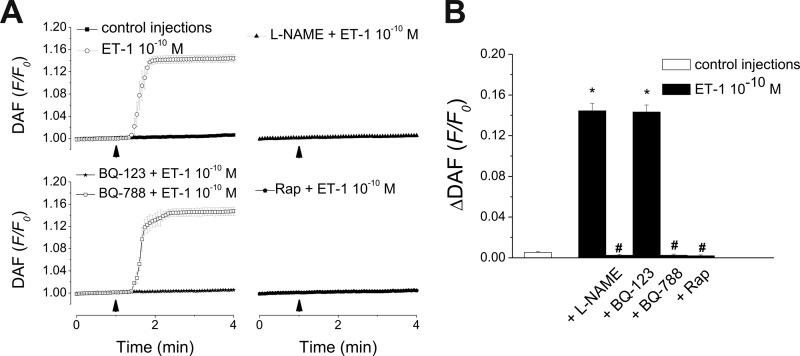
Cytosolic NO levels were measured in RPMVEC using DAF-FM fluorescence. Intracellular administration of ET-1 (10−10 m) significantly increased DAF-FM fluorescence by 14.45 ± 0.73% (p < 0.05; n = 6), whereas control buffer microinjection had a small and non-significant effect (0.53 ± 0.08%, n = 6, Fig. 7, A and B). The NO increase produced by ET-1 was not affected by BQ-123 (10−7 m, ΔDAF = 14.33 ± 0.7%, n = 6), which blocks ETA but was lost upon BQ-788 (10−7 m) treatment (ETB antagonist, ΔDAF reduced to 0.25 ± 0.12%, n = 6, Fig. 7, A and B). In the presence of the NO synthase inhibitor l-NAME (100 μm) or of the microautophagy blocker rapamycin (30 μm), intracellularly injected ET-1 no longer produced significant increases in NO levels (ΔDAF was 0.25 ± 0.09%, and 0.19 ± 0.09%, respectively; n = 6, Fig. 7, A and B).
FIGURE 7.
Intracellular administration of ET-1 elevates endothelial NO levels. A, averaged traces of DAF-FM fluorescence indicating increases in NO level in response to microinjection of control buffer or ET-1 (upper left panel), in cells incubated with either NO synthase inhibitor l-NAME (upper right panel) or microautophagy blocker rapamycin (lower right panel), or in response to ET-1 co-injected with ETA or ETB antagonists BQ-123 and BQ-788, respectively (lower left panel). B, quantification of the ΔDAF (F/Fo)-FM fluorescence increases in RPMVEC in each of the conditions listed in A; p < 0.05 compared control (*) or to ET-1 (10−10 m) (#). Rap, rapamycin.
DISCUSSION
ET-1, first identified as an endothelium-derived vasoconstrictor peptide, is an autocrine and paracrine signaling factor with extensive modulatory effects on vascular function (29). ET-1 acts also as an intracrine, activating intracellular cognate receptors (2). Stimulation of ET-1 receptors at the plasma or nuclear membrane induces Ca2+ release, NO, and reactive oxygen species formation in the cytosol or nucleus (5, 30). Cardiovascular disease pathogenesis involves oxidative stress and further alteration of ET-1 and NO signaling pathways (30, 31).
ET-1 immunoreactivity is expressed within the cytoplasm on the membranes of the endoplasmic reticulum (ER), mitochondria, and cytosolic vesicles of endothelial cells from human (4) and various rodent species, including rats (6). Moreover, the components responsible for ET-1 generation are present intracellularly in endothelial cells, as well as in other cardiovascular cells (6, 32, 33). These findings suggest that ET-1 is available within the cytoplasm to activate its intracellular receptors.
We and others (17, 34–36) have previously reported that stimulation of intracellular GPCRs such as angiotensin II AT1 receptors, CB1, and CB2 cannabinoid receptors (16, 37, 38), or estrogen receptor GPER/GPR30 (18, 19, 39) leads to [Ca2+]i elevation. Using a similar approach in this study, we tested the hypothesis that ET-1 may act in an intracrine fashion on intracellular ETB receptors to modulate endothelial functions. Using imaging methods and concurrent intracellular injection of ET-1, we provide the first evidence of functionality of intracellular ETB receptors in cells transiently transfected with the receptor. Because ET-1 is an endothelium-derived peptide, and ETB is the predominant type of endothelin receptor in endothelial cells, the next series of experiments were designed to evaluate whether intracellular ETB receptors are functional in RPMVEC. Similar to ETB-transfected cells, RPMVEC responded to ET-1 microinjection with a dose-dependent increase in [Ca2+]i. Blocking intracellular ETB receptors abolished the effect of ET-1, whereas ETA antagonism did not affect it.
Interestingly, a previous study demonstrating cytoplasmic distribution of both ETA and ETB in aortic human vascular endothelial cells also showed that the responses initiated by ET-1 at the plasma membrane are not dependent on plasmalemmal ETB but on ETA (4). According to the present study, the reverse is true in RPMVEC where the effects of microinjected ET-1 are mediated through intracellular ETB receptors. Our findings are particularly relevant considering the ability of endothelial cells to synthesize ET-1 (40–42) and make it available within the cytoplasm (32, 33, 40) to activate its intracellular targets. Importantly, a majority of the responses elicited by ET-1 in the endothelium, including the release of vasorelaxant factors such as NO, prostacyclin, and endothelium-derived hyperpolarizing factor are ETB receptor-dependent (11).
We further examined the intracellular location of functional ETB receptors in endothelial cells. Previous studies have identified that ETB, similar to other GPCRs, may be targeted to the endolysosomes (7–9) or Golgi apparatus (43). Disruption of the Golgi apparatus did not affect the response of endothelial cells to intracellular ET-1, whereas inhibition of lysosomal acidification (25) completely abolished it. Our results indicate that degradation is not the only fate of endolysosomal ETB; they are also functional and involved in ET-1 signaling. This may correlate with the remarkable stability of ETB receptors in these organelles (44).
In addition to playing a role in cellular degradation, increasing evidence supports lysosomes as key regulators of cell homeostasis (45) and as platforms for continued receptor-mediated signaling (1). Similar to the plasma membrane, the membrane of the endocytic vesicles is organized into specialized domains, working as a platform for the assembly of specific signaling complexes; these features allow the endolysosomal targeted receptors to initiate signaling from this intracellular compartment (46). Accordingly, various types of receptors, including GPCRs, have been reported to trigger signal transduction pathways upon their endolysosomal activation (1, 47). However, the ET-1 binding pocket on the ETB receptor is located on the N-terminal side, thus within the lysosomal lumen (11). We have previously demonstrated that angiotensin II is transferred inside the endolysosomal vesicles via microautophagy (17), a process in which soluble cytosolic molecules are engulfed (48). Thus, we further tested whether a similar mechanism was applicable to endothelin. Indeed, rapamycin, an inhibitor of the final uptake reaction in the microautophagic process (26), prevented the cellular responses to microinjected endothelin-1. Two major events may be delimited in the microautophagic process: lysosomal membrane invagination/formation of autophagic tubes and vesicle scission (48). Although the former may occur with a 30-min lag, the latter is very rapid, occurring in seconds (26). Given that microautophagy is an ongoing process, important in housekeeping and in the maintenance of cytoplasmic mass (48), membrane invaginations are formed continuously and cytosolic components may be rapidly uptaken into the endolysosomal lumen. Indeed, activation of lysosomal ETB receptors occurred readily in response to microinjected ET-1.
Next, we defined the Ca2+ pools mobilized by ET-1 in endothelial cells. The ET-1-induced rise in [Ca2+]i was not modified in Ca2+-free saline, suggesting that Ca2+ release, but not Ca2+ influx, is the main mechanism underlying the ET-1-induced effect. The response to ET-1 was not affected by preventing lysosomal Ca2+ release with the two-pore channel blocker Ned-19 (28, 49); thus, despite their localization, activation of ETB receptors does not mobilize Ca2+ via two-pore channel activation. Conversely, the effect of intracellular ET-1 was sensitive to IP3R but not ryanodine receptor blockade, pointing to the IP3-responsive stores as the major Ca2+ pool mobilized upon activation of lysosomal ETB. It is largely accepted that the IP3Rs are located on the endoplasmic reticulum. However, it has been shown that IP3R subtypes 2 and 3 are located on acid-filled Ca2+ stores (50). Based on these findings, we cannot exclude the participation of both types of Ca2+ pools. Moreover, we found that the phospholipase C inhibitor U-73122 abolished the effect of ET-1; this is not surprising, given that ETB receptors couple to Gq/11 (11) and that phospholipase C is present in the lysosomal membrane (51). Immunocytochemical experiments confirmed the endolysosomal location of ETB receptors.
To further probe for the physiological relevance of the Ca2+ release in response to activation of endolysosomal ETB receptors, we examined the effect of microinjected endothelin on NO production. In endothelial cells, an elevation of [Ca2+]i results in activation of endothelial NO synthase via Ca2+/calmodulin binding (52, 53) and endothelial NO synthase phosphorylation (54), which leads to the release of the vasorelaxant mediator NO. Indeed, intracellular administration of ET-1 resulted in NO release, an effect that was completely contingent on endothelial NO synthase. Our results also indicate that microautophagy and ETB activation are critical steps for NO production in response to intracellular ET-1.
Thus, we propose a new pathway for ET-1-induced intracrine signaling: intracellular ET-1 is transferred to the endolysosomal lumen via microautophagy to trigger ETB-phospholipase C-dependent IP3 generation. IP3 further activates specific Ca2+ release channels (IP3R) from the endoplasmic reticulum or endolysosomes; [Ca2+]i elevation stimulates endothelial NO synthase, resulting in endothelial NO accumulation (Fig. 8). This mode of action of ET-1 is canonical, in that it is associated with its receptor, ETB, is second messenger-dependent, and occurs at a membranous compartment (55, 56).
FIGURE 8.
Proposed signaling of endothelin-1 via endolysosomal ETB receptor activation. Cytosolic endothelin-1 (ET-1), transferred to the endolysosomal lumen (Endo-Lys) via microautophagy, stimulates endolysosomal ETB receptors, which in turn activate phospholipase C (PLC) located in the membrane of endolysosomal Ca2+ stores. Thus, IP3 is released from membrane phosphoinositides and activates IP3 receptors from the endoplasmic reticulum (ER) or endolysosomes. The subsequent IP3-induced increase in cytosolic Ca2+ activates endothelial NO synthase (eNOS) to produce NO. The NO released from the endothelial cells leads to relaxation of the vascular smooth muscle.
ET-1 released in the circulation is a very potent vasoconstrictor that has been implicated in the pathophysiology of systemic and pulmonary hypertension and in atherosclerosis (57, 58). Although ET-1 acts on the vascular smooth muscle to produce vasoconstriction, activation of endothelial ETB receptors promotes vasorelaxation via NO release (59). ET-1 involvement in atherosclerosis is also dual. ET-1 promotes vasoprotection through ETB receptor-dependent generation of NO and reactive oxygen species in endothelial cells (58). However, ET-1 stimulates vascular smooth muscle cell proliferation, inducing vascular hypertrophy and endothelial dysfunction, effects that are sensitive to ETA receptor blockade (58). Specific trapping of ET-1 to vascular endothelial cells may produce selective activation of lysosomal ETB, Ca2+ mobilization and NO generation; this may shift its overall effects toward vasorelaxation/vasoprotection and prevent its potentially detrimental, vasoconstrictor/proliferative activity on adjacent vascular smooth muscle cells. Endothelial cell-specific ETB knock-out mice exhibit decreased endogenous release of NO and increased plasma endothelin-1 (60). Innovative approaches previously employed to demonstrate that another intracrine, angiotensin II, increases blood pressure upon intracellular trapping at the kidney level (55, 61), may also prove useful in the case of ET-1. Should our findings be supported by in vivo data, selective targeting of ETB agonists to the endothelial cytosol may prove therapeutically beneficial in cardiovascular disorders associated with dysfunction of ET-1/NO pathway.
To summarize, this study provides the first evidence that activation of endolysosomal ETB receptors increases cytosolic Ca2+ concentration and nitric oxide production. Furthermore, we show here for the first time that intracellular receptors (namely, ETB) may be involved in redox signaling. Our study suggests a new mechanism for ETB-mediated endothelium-dependent vasorelaxation and extends the current knowledge on intracrine signaling.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1HL90804 (to E. B.).
- GPCR
- G protein-coupled receptor
- [Ca2+]i
- intracellular calcium ions concentration
- DAF-FM
- 4-amino-5-methylamino-2′,7′-difluorofluorescein (NO indicator)
- ET-1
- endothelin-1
- ETA
- endothelin receptor type A
- ETB
- endothelin receptor type B
- IP3
- inositol 1,4,5-trisphosphate
- IP3R
- inositol 1,4,5-trisphosphate receptor
- l-NAME
- l-NG-nitroarginine methyl ester
- RPMVEC
- rat pulmonary microvascular endothelial cells.
REFERENCES
- 1. Calebiro D., Nikolaev V. O., Lohse M. J. (2010) Imaging of persistent cAMP signaling by internalized G protein-coupled receptors. J. Mol. Endocrinol. 45, 1–8 [DOI] [PubMed] [Google Scholar]
- 2. Re R. N., Cook J. L. (2007) Mechanisms of disease: Intracrine physiology in the cardiovascular system. Nat. Clin. Pract. Cardiovasc. Med. 4, 549–557 [DOI] [PubMed] [Google Scholar]
- 3. Tadevosyan A., Vaniotis G., Allen B. G., Hébert T. E., Nattel S. (2012) G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J. Physiol. 590, 1313–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Avedanian L., Riopel J., Bkaily G., Nader M., D'Orleans-Juste P., Jacques D. (2010) ETA receptors are present in human aortic vascular endothelial cells and modulate intracellular calcium. Can. J. Physiol. Pharmacol. 88, 817–829 [DOI] [PubMed] [Google Scholar]
- 5. Bkaily G., Avedanian L., Al-Khoury J., Provost C., Nader M., D'Orléans-Juste P., Jacques D. (2011) Nuclear membrane receptors for ET-1 in cardiovascular function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R251–263 [DOI] [PubMed] [Google Scholar]
- 6. Loesch A. (2005) Localisation of endothelin-1 and its receptors in vascular tissue as seen at the electron microscopic level. Curr. Vasc. Pharmacol. 3, 381–392 [DOI] [PubMed] [Google Scholar]
- 7. Abe Y., Nakayama K., Yamanaka A., Sakurai T., Goto K. (2000) Subtype-specific trafficking of endothelin receptors. J. Biol. Chem. 275, 8664–8671 [DOI] [PubMed] [Google Scholar]
- 8. Bremnes T., Paasche J. D., Mehlum A., Sandberg C., Bremnes B., Attramadal H. (2000) Regulation and intracellular trafficking pathways of the endothelin receptors. J. Biol. Chem. 275, 17596–17604 [DOI] [PubMed] [Google Scholar]
- 9. Oksche A., Boese G., Horstmeyer A., Furkert J., Beyermann M., Bienert M., Rosenthal W. (2000) Late endosomal/lysosomal targeting and lack of recycling of the ligand-occupied endothelin B receptor. Mol. Pharmacol. 57, 1104–1113 [PubMed] [Google Scholar]
- 10. Eguchi S., Hirata Y., Imai T., Marumo F. (1995) Endothelin-1 as an autocrine growth factor for endothelial cells. J. Cardiovasc. Pharmacol. 26, S279–283 [PubMed] [Google Scholar]
- 11. Mazzuca M. Q., Khalil R. A. (2012) Vascular endothelin receptor type B: Structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 84, 147–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiffrin E. L. (2005) Vascular endothelin in hypertension. Vascul. Pharmacol. 43, 19–29 [DOI] [PubMed] [Google Scholar]
- 13. Brailoiu G. C., Brailoiu E., Parkesh R., Galione A., Churchill G. C., Patel S., Dun N. J. (2009) NAADP-mediated channel “chatter” in neurons of the rat medulla oblongata. Biochem. J. 419, 91–97, 92 p following 97 [DOI] [PubMed] [Google Scholar]
- 14. Brailoiu G. C., Deliu E., Tica A. A., Chitravanshi V. C., Brailoiu E. (2012) Urocortin 3 elevates cytosolic calcium in nucleus ambiguus neurons. J. Neurochem. 122, 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grynkiewicz G., Poenie M., Tsien R. Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260, 3440–3450 [PubMed] [Google Scholar]
- 16. Brailoiu G. C., Oprea T. I., Zhao P., Abood M. E., Brailoiu E. (2011) Intracellular cannabinoid type 1 (CB1) receptors are activated by anandamide. J. Biol. Chem. 286, 29166–29174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deliu E., Tica A. A., Motoc D., Brailoiu G. C., Brailoiu E. (2011) Intracellular angiotensin II activates rat myometrium. Am. J. Physiol. Cell Physiol. 301, C559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tica A. A., Dun E. C., Tica O. S., Gao X., Arterburn J. B., Brailoiu G. C., Oprea T. I., Brailoiu E. (2011) G protein-coupled estrogen receptor 1-mediated effects in the rat myometrium. Am. J. Physiol. Cell Physiol. 301, C1262–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deliu E., Brailoiu G. C., Arterburn J. B., Oprea T. I., Benamar K., Dun N. J., Brailoiu E. (2012) G protein-coupled estrogen receptor 1-mediated effects in the rat myometrium. J. Pain 13, 742–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guse A. H., de Wit C., Klokow T., Schweitzer K., Mayr G. W. (1997) Unique properties of the capacitative Ca2+-entry antagonist LU 52396: its inhibitory activity depends on the activation state of the cells. Cell Calcium 22, 91–97 [DOI] [PubMed] [Google Scholar]
- 21. Rubin D. B., Drab E. A., Bauer K. D. (1989) Endothelial cell subpopulations in vitro: cell volume, cell cycle, and radiosensitivity. J. Appl. Physiol. 67, 1585–1590 [DOI] [PubMed] [Google Scholar]
- 22. Brailoiu G. C., Gurzu B., Gao X., Parkesh R., Aley P. K., Trifa D. I., Galione A., Dun N. J., Madesh M., Patel S., Churchill G. C., Brailoiu E. (2010) Acidic NAADP-sensitive calcium stores in the endothelium: agonist-specific recruitment and role in regulating blood pressure. J. Biol. Chem. 285, 37133–37137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang L., Luo B., Patel R. P., Ling Y., Zhang J., Fallon M. B. (2007) Modulation of pulmonary endothelial endothelin B receptor expression and signaling: implications for experimental hepatopulmonary syndrome. Am. J. Physiol. Lung. Cell Mol. Physiol. 292, L1467–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Betina V. (1992) Biological effects of the antibiotic brefeldin A (decumbin, cyanein, ascotoxin, synergisidin): a retrospective. Folia Microbiol. 37, 3–11 [DOI] [PubMed] [Google Scholar]
- 25. Bowman E. J., Siebers A., Altendorf K. (1988) Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. 85, 7972–7976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kunz J. B., Schwarz H., Mayer A. (2004) Determination of four sequential stages during microautophagy in vitro. J. Biol. Chem. 279, 9987–9996 [DOI] [PubMed] [Google Scholar]
- 27. Berg T. O., Strømhaug E., Løvdal T., Seglen O., Berg T. (1994) Use of glycyl-L-phenylalanine 2-naphthylamide, a lysosome-disrupting cathepsin C substrate, to distinguish between lysosomes and prelysosomal endocytic vacuoles. Biochem. J. 300, 229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naylor E., Arredouani A., Vasudevan S. R., Lewis A. M., Parkesh R., Mizote A., Rosen D., Thomas J. M., Izumi M., Ganesan A., Galione A., Churchill G. C. (2009) Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 5, 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schneider M. P., Boesen E. I., Pollock D. M. (2007) Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu. Rev. Pharmacol. Toxicol. 47, 731–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bourque S. L., Davidge S. T., Adams M. A. (2011) The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1288–1295 [DOI] [PubMed] [Google Scholar]
- 31. Black S. M., Fineman J. R. (2006) Oxidative and nitrosative stress in pediatric pulmonary hypertension: roles of endothelin-1 and nitric oxide. Vascul. Pharmacol. 45, 308–316 [DOI] [PubMed] [Google Scholar]
- 32. Jafri F., Ergul A. (2003) Nuclear localization of endothelin-converting enzyme-1: subisoform specificity. Arterioscler. Thromb. Vasc. Biol. 23, 2192–2196 [DOI] [PubMed] [Google Scholar]
- 33. Russell F. D., Davenport A. P. (1999) Evidence for intracellular endothelin-converting enzyme-2 expression in cultured human vascular endothelial cells. Circ. Res. 84, 891–896 [DOI] [PubMed] [Google Scholar]
- 34. Filipeanu C. M., Brailoiu E., Kok J. W., Henning R. H., De Zeeuw D., Nelemans S. A. (2001) Intracellular angiotensin II elicits Ca2+ increases in A7r5 vascular smooth muscle cells. Eur. J. Pharmacol. 420, 9–18 [DOI] [PubMed] [Google Scholar]
- 35. Haller H., Lindschau C., Erdmann B., Quass P., Luft F. C. (1996) Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ. Res. 79, 765–772 [DOI] [PubMed] [Google Scholar]
- 36. Zhuo J. L., Li X. C., Garvin J. L., Navar L. G., Carretero O. A. (2006) Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am. J. Physiol. Renal. Physiol. 290, F1382–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bénard G., Massa F., Puente N., Lourenço J., Bellocchio L., Soria-Gómez E., Matias I., Delamarre A., Metna-Laurent M., Cannich A., Hebert-Chatelain E., Mulle C., Ortega-Gutiérrez S., Martín-Fontecha M., Klugmann M., Guggenhuber S., Lutz B., Gertsch J., Chaouloff F., López-Rodríguez M. L., Grandes P., Rossignol R., Marsicano G. (2012) Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat. Neurosci. 15, 558–564 [DOI] [PubMed] [Google Scholar]
- 38. den Boon F. S., Chameau P., Schaafsma-Zhao Q., van Aken W., Bari M., Oddi S., Kruse C. G., Maccarrone M., Wadman W. J., Werkman T. R. (2012) Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. 109, 3534–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 40. Loesch A., Gajkowska B., Dashwood M. R., Fioretto E. T., Gagliardo K. M., Lima A. R., Ribeiro A. A. (2005) Endothelin-1 and endothelin receptors in the basilar artery of the capybara. J. Mol. Histol. 36, 25–34 [DOI] [PubMed] [Google Scholar]
- 41. O'Brien R. F., Robbins R. J., McMurtry I. F. (1987) Endothelial cells in culture produce a vasoconstrictor substance. J. Cell Physiol. 132, 263–270 [DOI] [PubMed] [Google Scholar]
- 42. Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332, 411–415 [DOI] [PubMed] [Google Scholar]
- 43. Tawfeek H. A., Abou-Samra A. B. (2004) Important role for the V-type H+-ATPase and the Golgi apparatus in the recycling of PTH/PTHrP receptor. Am. J. Physiol. Endocrinol. Metab. 286, E704–710 [DOI] [PubMed] [Google Scholar]
- 44. Foster N., Loi T. H., Owe-Young R., Stanley K. K. (2003) Lysosomal traffic of liganded endothelin B receptor. Biochim. Biophys. Acta 1642, 45–52 [DOI] [PubMed] [Google Scholar]
- 45. Boya P. (2012) Lysosomal function and dysfunction: mechanism and disease. Antioxid. Redox. Signal. 17, 766–774 [DOI] [PubMed] [Google Scholar]
- 46. Gruenberg J. (2001) The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2, 721–730 [DOI] [PubMed] [Google Scholar]
- 47. Sorkin A., von Zastrow M. (2009) Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li W. W., Li J., Bao J. K. (2012) Microautophagy: lesser-known self-eating. Cell Mol. Life Sci. 69, 1125–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Patel S., Marchant J. S., Brailoiu E. (2010) Two-pore channels: Regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium 47, 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gerasimenko J. V., Lur G., Sherwood M. W., Ebisui E., Tepikin A. V., Mikoshiba K., Gerasimenko O. V., Petersen O. H. (2009) Pancteatci protease activation by alcohol metabolite depends on Ca2+ release via acid store IP3 receptors. Proc. Natl. Acad. Sci. U.S.A. 106, 10758–10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matsuzawa Y., Hostetler K. Y. (1980) Properties of phospholipase C isolated from rat liver lysosomes. J. Biol. Chem. 255, 646–652 [PubMed] [Google Scholar]
- 52. Griffith O. W., Stuehr D. J. (1995) Nitric oxide synthases: properties and catalytic mechanism. Annu. Rev. Physiol. 57, 707–736 [DOI] [PubMed] [Google Scholar]
- 53. Nathan C., Xie Q. W. (1994) Nitric oxide synthases: roles, tolls, and controls. Cell 78, 915–918 [DOI] [PubMed] [Google Scholar]
- 54. Xiao Z., Wang T., Qin H., Huang C., Feng Y., Xia Y. (2011) Endoplasmic reticulum Ca2+ release modulates endothelial nitric-oxide synthase via extracellular signal-regulated kinase (ERK) 1/2-mediated serine 635 phosphorylation. J. Biol. Chem. 286, 20100–20108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cook J. L., Re R. N. (2012) Lessons from in vitro studies and a related intracellular angiotensin II transgenic mouse model. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Re R. N., Cook J. L. (2011) Noncanonical intracrine action. J. Am. Soc. Hypertens. 5, 435–448 [DOI] [PubMed] [Google Scholar]
- 57. Barton M., Yanagisawa M. (2008) Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 86, 485–498 [DOI] [PubMed] [Google Scholar]
- 58. Muller G., Morawietz H. (2009) Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid. Redox. Signal. 11, 1711–1731 [DOI] [PubMed] [Google Scholar]
- 59. Takayanagi R., Kitazumi K., Takasaki C., Ohnaka K., Aimoto S., Tasaka K., Ohashi M., Nawata H. (1991) Presence of non-selective type of endothelin receptor on vascular endothelium and its linkage to vasodilation. FEBS Lett. 282, 103–106 [DOI] [PubMed] [Google Scholar]
- 60. Bagnall A. J., Kelland N. F., Gulliver-Sloan F., Davenport A. P., Gray G. A., Yanagisawa M., Webb D. J., Kotelevtsev Y. V. (2006) Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension 48, 286–293 [DOI] [PubMed] [Google Scholar]
- 61. Li X. C., Cook J. L., Rubera I., Tauc M., Zhang F., Zhuo J. L. (2011) Intrarenal transfer of an intracellular fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am. J. Physiol. Renal. Physiol. 300, F1076–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]