Abstract
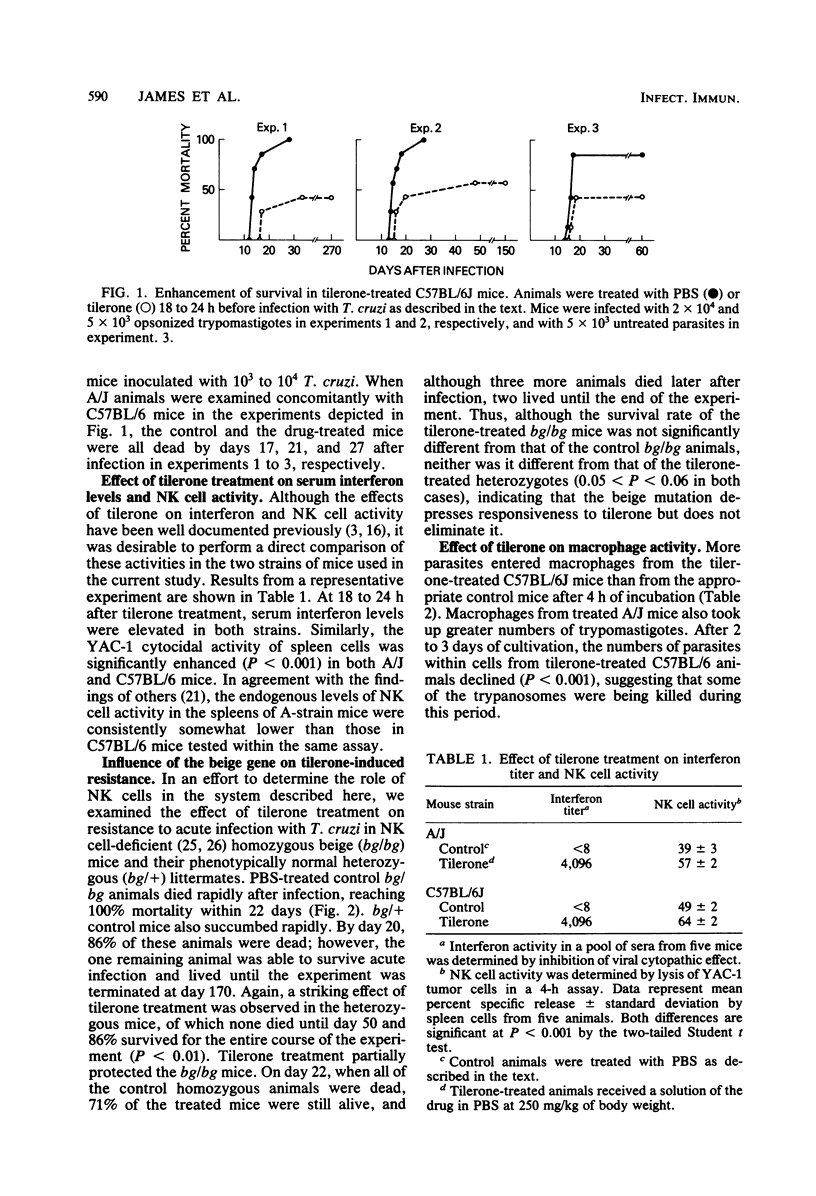
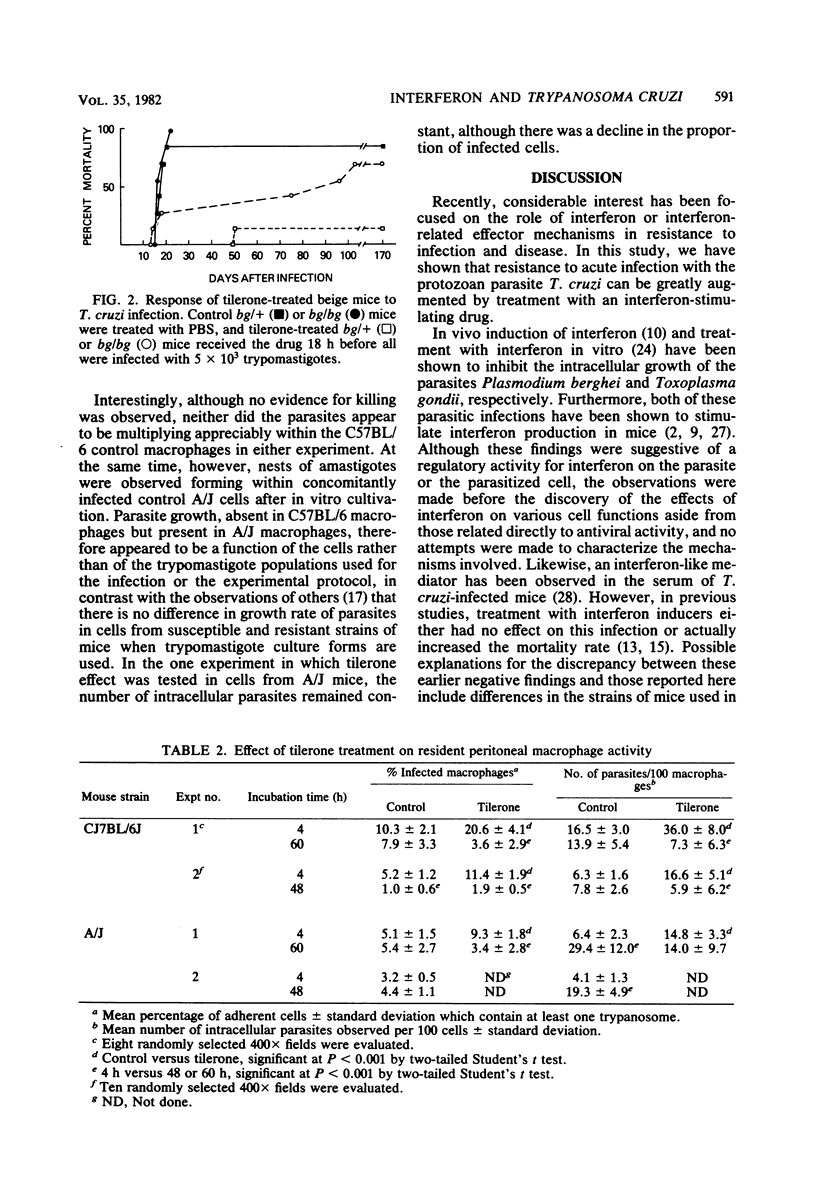
For an exploration of the effects of interferon-inducible resistance mechanisms in acute American trypanosomiasis, the synthetic interferon inducer tilerone hydrochloride was administered to mice of the C57BL/6J strain, which is highly resistant to Trypanosoma cruzi, 18 to 24 h before infection with a potentially lethal dose of bloodstream trypomastigotes. Although all of the control mice died within 30 days of the acute infection, approximately 50% of the tilerone-treated animals were able to survive indefinitely (P less than 0.05). The tilerone-treated mice demonstrated significant levels of serum interferon and splenic natural killer cells at the time of infection. Macrophages isolated from the peritoneal cavities of tilerone-treated C57BL/6J mice appeared to kill significant numbers of trypanosomes during 2 to 3 days of in vitro culture, indicating that activated macrophages may contribute to the enhanced resistance to T. cruzi infection in these mice. Beige mice treated with tilerone did not survive T. cruzi infection as well as tilerone-treated heterozygotes did, suggesting a role for natural killer cells in interferon-induced resistance. These results suggest that interferon or effector mechanisms enhanced by interferon induction can play a significant role in influencing resistance to T. cruzi infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eugui E. M., Allison A. C. Malaria infections in different strains of mice and their correlation with natural killer activity. Bull World Health Organ. 1979;57 (Suppl 1):231–238. [PMC free article] [PubMed] [Google Scholar]
- Freshman M. M., Merigan T. C., Remington J. S., Brownlee I. E. In vitro and in vivo antiviral action of an interferon-like substance induced by Toxoplasma gondii. Proc Soc Exp Biol Med. 1966 Dec;123(3):862–866. doi: 10.3181/00379727-123-31625. [DOI] [PubMed] [Google Scholar]
- Gidlund M., Orn A., Wigzell H., Senik A., Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978 Jun 29;273(5665):759–761. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Gober L. L., Friedman-Kien A. E., Havell E. A., Vilcek J. Suppression of the intracellular growth of Shigella flexneri in cell cultures by interferon preparations and polyinosinic-polycytidylic acid. Infect Immun. 1972 Mar;5(3):370–376. doi: 10.1128/iai.5.3.370-376.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgher R. R., Bertelli M. S., Petrillo-Peixoto M. L., Brener Z. Effect of interferon on the development of Trypanosoma cruzi in tissue culture "vero" cells. Mem Inst Oswaldo Cruz. 1980;75(1-2):157–160. doi: 10.1590/s0074-02761980000100015. [DOI] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Spontaneous lytic activity against allogeneic tumor cells and depression of specific cytotoxic responses in mice infected with Trypanosoma cruzi. J Immunol. 1981 Jun;126(6):2436–2442. [PubMed] [Google Scholar]
- Hoff R. A method for counting and concentrating living Trypanosoma cruzi in blood lysed with ammonium chloride. J Parasitol. 1974 Jun;60(3):527–528. [PubMed] [Google Scholar]
- Hoff R. Killing in vitro of Trypanosoma cruzi by macrophages from mice immunized with T. cruzi or BCG, and absence of cross-immunity on challege in vivo. J Exp Med. 1975 Aug 1;142(2):299–311. doi: 10.1084/jem.142.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. Y., Schultz W. W., Gordon F. B. Interferon induced by Plasmodium berghei. Science. 1968 Oct 4;162(3849):123–124. doi: 10.1126/science.162.3849.123. [DOI] [PubMed] [Google Scholar]
- Jahiel R. I., Nussenzweig R. S., Vilcek J., Vanderberg J. Protective effect of interferon inducers on Plasmodium berghei malaria. Am J Trop Med Hyg. 1969 Nov;18(6):823–835. doi: 10.4269/ajtmh.1969.18.823. [DOI] [PubMed] [Google Scholar]
- Kipnis T. L., David J. R., Alper C. A., Sher A., da Silva W. D. Enzymatic treatment transforms trypomastigotes of Trypanosoma cruzi into activators of alternative complement pathway and potentiates their uptake by macrophages. Proc Natl Acad Sci U S A. 1981 Jan;78(1):602–605. doi: 10.1073/pnas.78.1.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Worthington M., Tilles J. G., Abelmann W. H. Effect of the interferon stimulator polyinosinic-polycytidylic acid on experimental Trypanosoma cruzi infection. Proc Soc Exp Biol Med. 1971 Jul;137(3):884–888. doi: 10.3181/00379727-137-35686. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S. Algorithms in the diagnosis and management of exotic diseases. IV. American trypanosomiasis. J Infect Dis. 1975 Jul;132(1):121–124. doi: 10.1093/infdis/132.1.121. [DOI] [PubMed] [Google Scholar]
- Martinez-Silva R., Lopez V. A., Chiriboga J. Effects of poly I-C on the course of infection with Trypanosoma cruzi. Proc Soc Exp Biol Med. 1970 Jul;134(3):885–888. doi: 10.3181/00379727-134-34904. [DOI] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. A. Trypanosoma cruzi: in vitro induction of macrophage microbicidal activity. J Exp Med. 1978 Jul 1;148(1):288–300. doi: 10.1084/jem.148.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C. T. Cultivation of the leishmaniform stage of Trypanosoma cruzi in cell-free media at different temperatures. Am J Trop Med Hyg. 1968 Nov;17(6):823–832. doi: 10.4269/ajtmh.1968.17.823. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Manejias R. E., Russo M., Abbey E. E. Increased spreading of macrophages from mice treated with interferon inducers. Cell Immunol. 1977 Mar 1;29(1):86–95. doi: 10.1016/0008-8749(77)90277-5. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Merigan T. C. Interferon: protection of cells infected with an intracellular protozoan (Toxoplasma gondii). Science. 1968 Aug 23;161(3843):804–806. doi: 10.1126/science.161.3843.804. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Wigzell H. The beige mutation in the mouse. II. Selectivity of the natural killer (NK) cell defect. J Immunol. 1979 Nov;123(5):2174–2181. [PubMed] [Google Scholar]
- Roder J. C. The beige mutation in the mouse. I. A stem cell predetermined impairment in natural killer cell function. J Immunol. 1979 Nov;123(5):2168–2173. [PubMed] [Google Scholar]
- Rytel M. W., Jones T. C. Induction of interferon in mice infected with Toxoplasma gondii. Proc Soc Exp Biol Med. 1966 Dec;123(3):859–862. doi: 10.3181/00379727-123-31624. [DOI] [PubMed] [Google Scholar]
- Rytel M. W., Marsden P. D. Induction of an interferon-like inhibitor by Trypanosoma cruzi infection in mice. Am J Trop Med Hyg. 1970 Nov;19(6):929–931. doi: 10.4269/ajtmh.1970.19.929. [DOI] [PubMed] [Google Scholar]
- Schultz R. M., Chirigos M. A. Similarities among factors that render macrophages tumoricidal in lymphokine and interferon preparations. Cancer Res. 1978 Apr;38(4):1003–1007. [PubMed] [Google Scholar]
- Stutman O., Cuttito M. J. Normal levels of natural cytotoxic cells against solid tumours in NK-deficient beige mice. Nature. 1981 Mar 19;290(5803):254–257. doi: 10.1038/290254a0. [DOI] [PubMed] [Google Scholar]
- Trischmann T., Tanowitz H., Wittner M., Bloom B. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp Parasitol. 1978 Aug;45(2):160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]



