Background: The pleiotropic hormone H2 relaxin can bind to both RXFP1 and RXFP2 receptors.
Results: A-chain-modified H2 relaxin analogues retain RXFP1 activity and anti-fibrotic properties while ablating RXFP2 responses.
Conclusion: Residues within H2 relaxin A-chain play a prominent role in maintaining structural fold and directing the receptor specificity.
Significance: We identified A-chain residues critical for receptor-ligand interactions, useful for development of future therapeutics.
Keywords: G Protein-coupled Receptor (GPCR), Nuclear Magnetic Resonance, Peptide Chemical Synthesis, Peptide Hormones, Structural Biology, A-chain, H2 Relaxin, RXFP1-selective, RXFP2, Anti-fibrotic
Abstract
Human gene-2 (H2) relaxin is currently in Phase III clinical trials for the treatment of acute heart failure. It is a 53-amino acid insulin-like peptide comprising two chains and three disulfide bonds. It interacts with two of the relaxin family peptide (RXFP) receptors. Although its cognate receptor is RXFP1, it is also able to cross-react with RXFP2, the native receptor for a related peptide, insulin-like peptide 3. In order to understand the basis of this cross-reactivity, it is important to elucidate both binding and activation mechanisms of this peptide. The primary binding mechanism of this hormone has been extensively studied and well defined. H2 relaxin binds to the leucine-rich repeats of RXFP1 and RXFP2 using B-chain-specific residues. However, little is known about the secondary interaction that involves the A-chain of H2 relaxin and transmembrane exoloops of the receptors. We demonstrate here through extensive mutation of the A-chain that the secondary interaction between H2 relaxin and RXFP1 is not driven by any single amino acid, although residues Tyr-3, Leu-20, and Phe-23 appear to contribute. Interestingly, these same three residues are important drivers of the affinity and activity of H2 relaxin for RXFP2 with additional minor contributions from Lys-9, His-12, Lys-17, Arg-18, and Arg-22. Our results provide new insights into the mechanism of secondary activation interaction of RXFP1 and RXFP2 by H2 relaxin, leading to a potent and RXFP1-selective analog, H2:A(4–24)(F23A), which was tested in vitro and in vivo and found to significantly inhibit collagen deposition similar to native H2 relaxin.
Introduction
Human gene-2 (H2)7 relaxin, the major stored and circulating form of human relaxin, is a 53-amino acid peptide hormone that has structural characteristics common to the insulin/relaxin family members, namely two peptide chains stabilized by three disulfide bonds (1, 2). In most mammals, relaxin has long been regarded as a hormone of pregnancy and is involved in remodeling the connective tissue of the pelvic ligaments and female reproductive tract to facilitate delivery of the young. However, relaxin is a pleiotropic hormone with additional roles in several non-reproductive processes that are centered around its anti-fibrotic (3), vasodilatory, and cardioprotective effects (4). Based on the latter two actions, human H2 relaxin is currently in Phase III clinical trials for the treatment of patients with acute heart failure (5).
H2 relaxin interacts with G protein-coupled receptors, recently renamed relaxin family peptide receptors (RXFP). Although the native receptor for H2 relaxin is RXFP1 (6), it also strongly binds to and activates RXFP2, the native receptor for related peptide insulin-like peptide 3 (INSL3) (7). Both RXFP1 and -2 receptors are class C leucine-rich repeat (LRR)-containing G protein-coupled receptors. The large extracellular domain of these receptors consists of series of LRR strands forming a parallel β-sheet linked to a low density lipoprotein receptor type A module (8).
The receptors RXFP1 and -2 are currently known to possess high and low affinity binding sites (also referred to as primary and secondary interaction sites) by which they interact with their ligands (9, 10). The primary mode of ligand-receptor interaction has been extensively studied and well characterized. The binding cassette (RXXXRXXI) present within the mid-region of the B-chain is responsible for the primary binding interaction of H2 relaxin with the LRR of its native receptor RXFP1 (11). The corresponding residues in RXFP1 interacting with the B-chain binding motif were later identified by mutations of the LRR region of the receptor (12). A pair of acidic residues (Asp and Glu) within RXFP1 form hydrogen bonds with each conserved residue (Arg) of the B-chain, whereas the single isoleucine residue forms hydrophobic interactions with the corresponding hydrophobic cluster (Trp, Ile, and Leu) present in the inner sheets of the LRR. Similar to the interaction of H2 relaxin with RXFP1, residues, such as His-12, Arg-16, Val-19, Arg-20, and Trp-27, present within the INSL3 B-chain are responsible for its high affinity binding to the LRR of RXFP2 (13). The corresponding residues in RXFP2 interacting with the B-chain of INSL3 were also determined and well characterized by receptor mutation studies (14). The INSL3-RXFP2 interaction site is different to the site of relaxin-RXFP1 interaction. Interestingly, when H2 relaxin binds to and activates RXFP2 receptor, it utilizes a hybrid relaxin/INSL3 binding site consisting partly of the INSL3-RXFP2 and H2 relaxin-RXFP1 interactions (15).
The recent evidence strongly suggest that there is a secondary interaction between ligands (H2 relaxin and INSL3) and receptors (RXFP1 and RXFP2), which ultimately causes activation and downstream signaling. It is now believed that such interactions involve the A-chain of ligands and the transmembrane (TM) exoloops of the receptors (16). The studies involving chimeric peptides and receptors have provided some insights into the mechanism of secondary interaction between peptides and receptors. For example, the native H3 relaxin peptide, another member of the insulin/relaxin family that is a neuropeptide, strongly binds to and activates its cognate RXFP3 receptor but also RXFP1. However, the chimeric peptide R3/I5, where the A-chain of H3 relaxin was replaced with the INSL5 A-chain, displayed no activity at RXFP1 while maintaining full activation at RXFP3, indicating that H3 relaxin binds to and activates its native receptor (RXFP3) using B-chain-specific residues, whereas it requires both the A- and B-chains to interact with the RXFP1 receptor (17). This result is supported by the facts that a single H3 relaxin B-chain can act as an RXFP3 agonist (17, 18) and a modified single B-chain can act as an antagonist (19). The importance of the H3 relaxin A-chain for RXFP1 activity is further supported by the fact that H3 relaxin is unable to activate a chimeric receptor comprising the LRR from RXFP1 and TM exoloops from RXFP2 (9). The other chimeric peptides recently studied, H2A-INSL3B (20) and INSL3A-H2B (21), exhibited poor or partial activity at RXFP2. This was surprising, given the fact that both INSL3 and H2 relaxin are strong RXFP2 agonists. Interestingly, when the chimeric peptide H2A-INSL3B was tested against an RXFP2 chimeric receptor comprising of the TM domain from RXFP1, full activation of the receptor was observed. These results highlight that the A-chains of H2 relaxin and INSL3 play a key role in interacting with the TM exoloops of RXFP1 or RXFP2, and the mode by which H2 relaxin or INSL3 binds to the LRR of RXFP2 is not compatible with the interaction between the A-chain of these chimeric peptides and TM exoloops of RXFP2 (21).
Recently, the residues and domains in the INSL3 A-chain that are involved in secondary interactions with RXFP2 have been decoded (20). No single amino acid within the INSL3 A-chain was found to be critical for RXFP2 activity. However, the N terminus domain consisting of 5 residues was identified to be crucial for activation and not for binding. This provides an explanation of why truncations at the N terminus of INSL3 produced a high affinity RXFP2 antagonist (22). Unlike the INSL3-RXFP2 system, the secondary interactions in both H2-RXFP1 and H2-RXFP2 systems are less well characterized, and the residues involved in these interactions have not been fully determined. Therefore, we performed mutations and/or truncations of the H2 relaxin A-chain in this study in order to identify the key determinants required for secondary interaction.
We demonstrate here that the secondary interaction between H2 relaxin and RXFP1 is not driven by any individual amino acid in the A-chain. This led to the assembly of H2 relaxin A-chain partial chimeras with INSL5 domains to provide a better understanding of which residues or domains that may be crucial for RXFP1 binding and activation. Last, N terminus truncations and/or single amino acid mutations of the H2 relaxin A-chain were carried out that led to the identification of RXFP1-selective H2 relaxin analogues in parallel to determining the amino acid residues present in the A-chain that are responsible for native folding and thus conferring receptor selectivity. This study offers important insights into the secondary interactions between H2 relaxin and RXFP1 or RXFP2.
EXPERIMENTAL PROCEDURES
Solid-phase Peptide Synthesis
Individual A- and B-chains of H2 relaxin analogues with appropriate regioselective S-protection were synthesized using either continuous flow or microwave-assisted solid-phase methodologies on an automated PerSeptives Biosystems Pioneer peptide synthesizer and a CEM Liberty peptide synthesizer, respectively. Following simultaneous cleavage, side chain deprotection, and purification of crude A- and B-chains, stepwise formation of the three disulfide bonds were carried out via oxidation, thiolysis and iodolysis consecutively (23–26). Altogether, there were 16 H2 relaxin analogues synthesized in this study, 10 of which were A-chain alanine-substituted (A3 (Tyr → Ala), A9 (Lys → Ala), A12 (His → Ala), A16 (Thr → Ala), A17 (Lys → Ala), A18 (Arg → Ala), A19 (Ser → Ala), A20 (Leu → Ala), A22 (Arg → Ala), and A23 (Phe → Ala)) and three H2 relaxin analogues with amino acid substitutions and/or truncations (A(4–24), A(4–24)(Phe → Ala), and A(4–23)(ΔF)). In addition, three H2/INSL5 chimeric analogues with three different domains were prepared. All native H2 relaxin and its analogues comprised two chains (A- and B-chains) linked covalently by three disulfide bonds.
Peptide Characterization
The purity of individual synthetic peptides was assessed by analytical reversed-phase high performance liquid chromatography using a Vydac C18 column (250 × 4.6 mm, 300 Å, 5 μm) with a buffer system of 0.1% trifluoroacetic acid in water (buffer A) and 0.1% trifluoroacetic acid in acetonitrile (buffer B). The molecular weights of all analogues were determined by MALDI-TOF mass spectrometry using a Bruker AutoflexII instrument (Bremen, Germany) in the linear mode at 19.5 kV. Furthermore, the peptide content was quantitated by amino acid analysis using vapor-phase acid hydrolysis in 6 m hydrochloric acid containing 2% phenol at 110 °C over 24 h. The hydrolysate was then converted to stable, fluorescent derivatives using a Waters AccQTag kit (Sydney, Australia). The derivatized amino acids were separated using a Shim-Pak XR ODS column (3 × 75 mm, 2.2 μm) on a Shimadzu microbore reversed-phase HPLC system (Victoria, Australia).
Circular Dichroism (CD) Spectroscopy
CD spectra were obtained using a JASCO spectrophotometer (J-185) (JASCO, Tokyo, Japan) at 25 °C in a 1-mm path length cell. The peptides were dissolved at a concentration of 0.2 mg/ml in 10 mm phosphate buffer (pH 7.5).
NMR Structural Analysis
H2:A(Y3A) and H2:A(4–24)(F23A) were analyzed by solution NMR spectroscopy. Samples prepared comprised ∼1 mg of either H2:A(Y3A) or H2:A(4–24)(F23A) dissolved in 0.5 ml of 90% H2O, 10% D2O at pH ∼4. Two-dimensional homonuclear experiments, including TOCSY and NOESY, with a mixing time of 150 ms were recorded at 600 MHz using a Bruker Avance spectrometer. Additional two-dimensional experiments for H2:A(Y3A) were recorded at 900 MHz on a Bruker Avance II equipped with a cryogenic probe. Data sets were generally recorded with 4000 complex data points in the direct dimension and 512 increments in the indirect dimension, with the latter zero-filled to the 1000 data point, and both dimensions were multiplied by a sine square function prior to transformation. All data were recorded and processed using TOPSPIN 3.0 (Bruker) and analyzed using CARA (27). Data were referenced using the residual water signal at 4.77 ppm.
Ligand Binding Assay
Human embryonic kidney (HEK-293T) cells stably transfected with RXFP1 or RXFP2 were cultured in RPMI 1640 medium (Sigma) supplemented with 10% fetal calf serum, 100 μg/ml penicillin, 100 μg/ml streptomycin, and 2 mm l-glutamine and plated into 96-well plates precoated with poly-l-lysine for whole cell binding assays. Competition binding experiments were conducted with either europium-labeled H2 relaxin (28) or europium-labeled INSL3 (29) in the absence or presence of increasing concentrations of unlabeled H2 relaxin peptide analogues. Nonspecific binding was determined with an excess of unlabeled peptides (500 nm H2 relaxin or INSL3). Fluorescent measurements were recorded at an excitation wavelength of 340 nm and emission of 614 nm on a Victor plate reader (PerkinElmer Life Sciences). All data were are presented as the mean ± S.E. of the percentage of the total specific binding of triplicate wells, repeated in at least three separate experiments, and curves were fitted using one-site binding curves in GraphPad Prism 4 (GraphPad Inc, San Diego, CA). Statistical differences in pIC50 values were analyzed using one-way analysis of variance coupled to the Newman-Keuls multiple comparison test for multiple-group comparisons in GraphPad Prism 4.
Functional cAMP Assay
The ability of H2 relaxin and its analogues to stimulate cAMP response was evaluated using a cAMP reporter gene assay as described previously (10). HEK-293T cells co-transfected with either RXFP1 or RXFP2 and a pCRE β-galactosidase reporter plasmid were plated in 96-well plates (30). After 24 h, the co-transfected cells were incubated with increasing concentrations of H2 relaxin analogues in parallel to 10 nm H2 relaxin or INSL3 for RXFP1- or RXFP2-transfected cells, respectively. The amount of cAMP-driven β-galactosidase expression in each well was assessed with a colorimetric assay measuring absorbance at 570 nm on a Benchmark Plus microplate spectrophotometer (Bio-Rad). Ligand-induced cAMP stimulation was expressed as a percentage of maximal response of H2 relaxin or INSL3 for RXFP1 and RXFP2 cells, respectively. Each data point was measured in triplicate, and each experiment was conducted independently at least three separate times. Statistical differences in pEC50 values were analyzed using one-way analysis of variance coupled to the Newman-Keuls multiple comparison test for multiple group comparisons in GraphPad Prism 4.
Determination of Collagen Content
In Vitro Studies
Based on the ability of native H2 relaxin to inhibit TGF-β1-stimulated aberrant collagen deposition when applied to various types of fibroblasts (31–33) via RXFP1, the efficacy of the H2:A(4–24)(F23A) analog was compared with that of H2 relaxin via measurement of collagen deposition in BJ3 cells, a human dermal fibroblast cell line (34) that endogenously expresses RXFP1. BJ3 cells were maintained and plated for experimentation as detailed previously (35). TGF-β1 (2 ng/ml)-stimulated cells were treated for 72 h with either native H2 relaxin (100 ng/ml; 16.8 nm) or an equivalent concentration of the H2:A(4–24)(F23A) analog (16.8 nm), whereas TGF-β1-unstimulated cells treated with or without the H2:A(4–24)(F23A) analog were used as appropriate controls (from which basal collagen deposition could be measured). These treatments were carried out three or four separate times in duplicate or triplicate. The amount of collagen deposited by BJ3 cells into the matrix was then determined, as described before (35).
In Vivo Studies
Because H2 relaxin has consistently been found to prevent and/or reverse fibrosis progression in numerous mouse models of disease (through RXFP1 alone), regardless of etiology (3, 4, 32, 36), the efficacy of the H2:A(4–24)(F23A) analog was also compared with that of H2 relaxin via measurement of collagen deposition (fibrosis) in an isoproterenol (ISO)-induced murine model of myocardial injury (37). Briefly, 8–10-week-old male mice on a C57B6J × 129SV background (n = 15) were subcutaneously injected with isoprenaline hydrochloride (25 mg/kg; Sigma-Aldrich) once daily for 5 consecutive days and then left for a further 12 days for fibrosis progression to occur. Subgroups of animals (n = 5/group) received recombinant H2 relaxin (0.5 mg/mg/day; a dose that has been used previously to successful demonstrate its anti-fibrotic actions (3, 32, 36) and produces circulating levels of 20–40 ng/ml (38), which are well within those found in pregnant rodents). Alternatively, an equivalent dose of the H2:A(4–24)(F23A) analog (0.5 mg/kg/day) was administered via subcutaneously implanted osmotic minipumps (model 2002; Alzet, Cupertino, CA), which allowed for the continuous infusion of each peptide into the circulation of treated animals. A separate subgroup of mice (n = 5) that were not subjected to ISO or peptide treatment were used as untreated controls. Twelve days after the fifth ISO injection/17 days from the beginning of the study, all mice were weighed and then sacrificed for heart and left ventricular collection.
A similar portion of the left ventricle from each animal was then used for the determination of hydroxyproline content, as described before (39). Hydroxyproline values were then converted to collagen content as detailed previously (35, 39) and, in turn, divided by the dry weight of each corresponding left ventricular tissue assessed to yield collagen concentration (a measure of fibrosis).
RESULTS
Design and Synthesis of H2 Relaxin Analogues
A series of H2 relaxin analogues were designed and synthesized in this study (Table 1) in order to identify key residues involved in secondary interaction. Our well established regioselective disulfide formation chemical approach was utilized to assemble these two-chain peptides where three differential cysteine S-protecting groups (Trt, tBu, and Acm) were used to assist the direct formation of the three disulfide bonds. The successful formations of the peptides were achieved in good overall yields (10–14% relative to starting B-chain material) and were subjected to comprehensive characterization by MALDI-TOF mass spectrometry and reversed-phase HPLC, which confirmed their high purities.
TABLE 1.
Amino acid sequences of native H2 relaxin and 16 analogues with both A- and B- chains linked by one intra- and two interdisulfide bonds were designed and prepared for this study
Effects of Single Alanine Substitutions of H2 Relaxin A-chain on RXFP1 and RXFP2
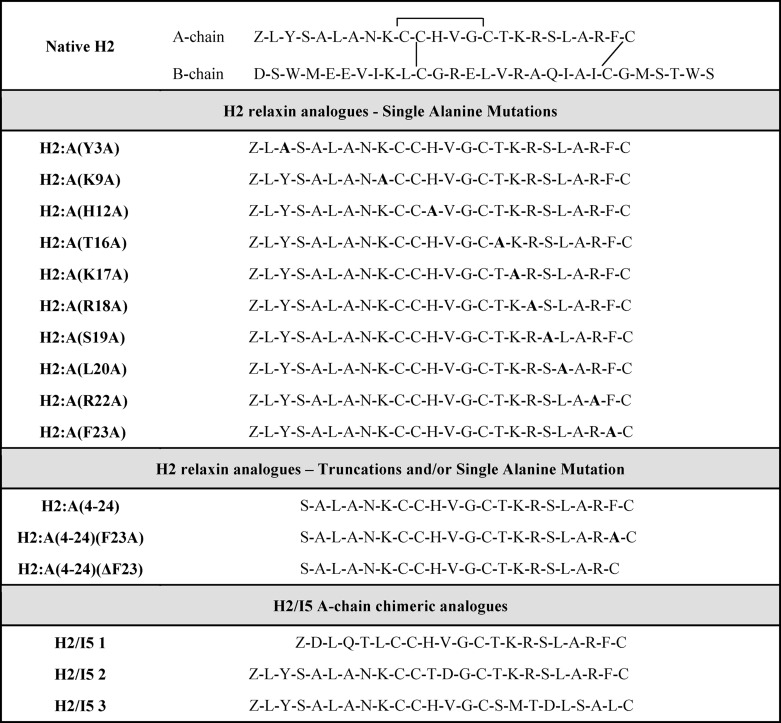
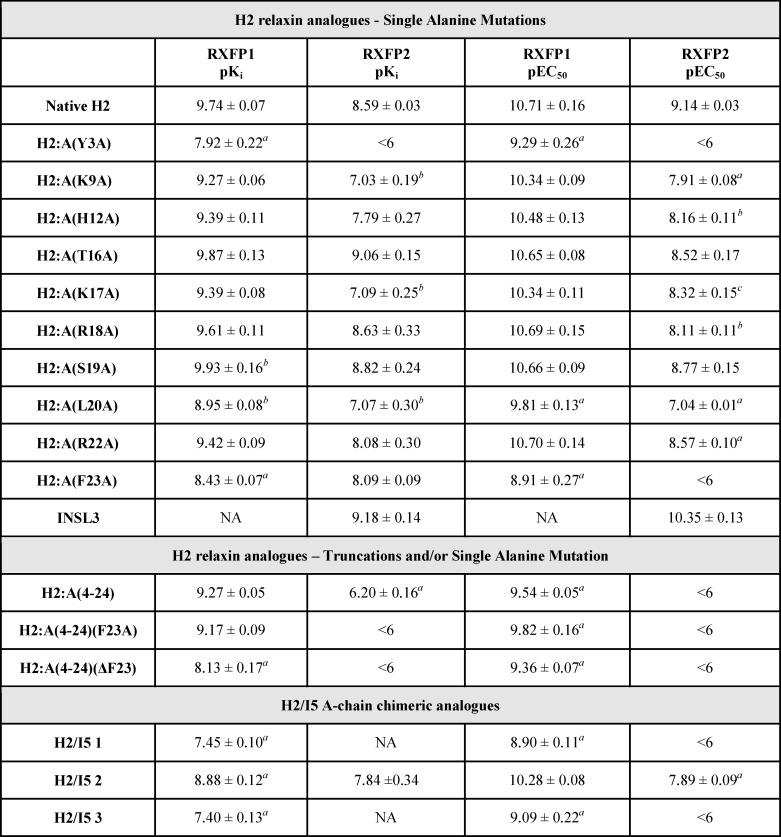
The importance of the A-chain structure and the specific contributions of individual amino acid residues within the A-chain toward receptor binding and activation were investigated by the preparation of H2 relaxin analogues that had individual amino acids within their A-chains replaced with alanine (Table 1). Cell-based assays were used to evaluate the ability of these analogues to bind and signal through RXFP1 and RXFP2 (Table 2). The single alanine substitutions within most of the A-chain analogues were found to retain similar affinity to that of native H2 relaxin for RXFP1. The corresponding activation response of these analogues correlated with the binding data suggesting that the side chains of these individual native residues do not greatly affect binding and activation at RXFP1 (Figs. 1 and 2). However, three H2 relaxin analogues, H2:A(Y3A), H2:A(L20A), and H2:A(F23A), displayed a decrease in affinity and potency for RXFP1 (Fig. 1 and Table 2). At RXFP2, most of the analogues displayed reduced binding affinity and potency (Fig. 2 and Table 2) compared with native H2 relaxin. Interestingly, H2:A(Y3A), H2:A(L20A), and H2:A(F23A) bound with remarkably lower affinity at RXFP2 and with correspondingly reduced activation response at RXFP2 (Fig. 2 and Table 2). This indicates that Tyr-3 at the N terminus and Leu-20 and Phe-23 at the C terminus of the A-chain are key residues for RXFP2 binding and activation.
TABLE 2.
Competition binding (pKi) and activation (pEC50) of RXFP1 and RXFP2 by H2 relaxin and analogues
a p < 0.001 versus H2 relaxin.
b p < 0.01 versus H2 relaxin.
c p < 0.05 versus H2 relaxin.
d NA, no activity.
FIGURE 1.
Activity of H2 analogues with single alanine mutations on the RXFP1 receptor. A, competition binding results of native H2 relaxin and single alanine-mutated H2 analogues (N terminus to mid-region of the A-chain) in the presence of a competitive ligand Eu3+-labeled H2 relaxin tested in HEK-293T cells stably expressing RXFP1 receptors. Data are expressed as a percentage of specific binding and are pooled data from at least three experiments performed in triplicate. B, cAMP activity of native H2 relaxin and single alanine-mutated H2 analogues (N terminus to mid-region of the A-chain) in HEK-293T cells expressing RXFP1 receptors using a pCRE-galactosidase reporter gene system. Data are expressed as a percentage of maximum H2 relaxin-stimulated cAMP response and are pooled data from at least three experiments performed in triplicate. C, competition binding results of native H2 relaxin and single alanine-mutated H2 analogues (mid-region to C terminus of the A-chain) in the presence of a competitive ligand Eu3+-labeled H2 relaxin tested in HEK-293T cells stably expressing RXFP1 receptors. Data are expressed as a percentage of specific binding and are pooled data from at least three experiments performed in triplicate. D, cAMP activity of native H2 relaxin and single alanine-mutated H2 analogues (mid-region to C terminus of the A-chain) in HEK-293T cells expressing RXFP1 receptors using a pCRE-galactosidase reporter gene system. Data are expressed as a percentage of maximum H2 relaxin-stimulated cAMP response and are pooled data from at least three experiments performed in triplicate. Error bars, S.E.
FIGURE 2.
Activity of H2 analogues with single alanine mutations on the RXFP2 receptor. A, competition binding results of native H2 relaxin and single alanine-mutated H2 analogues (N terminus to mid-region of the A-chain) in the presence of a competitive ligand Eu3+-labeled INSL3 tested in HEK-293T cells stably expressing RXFP2 receptors. Data are expressed as a percentage of specific binding and are pooled data from at least three experiments performed in triplicate. B, cAMP activity of native H2 relaxin and single alanine-mutated H2 analogues (N terminus to mid-region of the A-chain) in HEK-293T cells expressing RXFP2 receptors using a pCRE-galactosidase reporter gene system. Data are expressed as a percentage of maximum INSL3-stimulated cAMP response and are pooled data from at least three experiments performed in triplicate. C, competition binding results of native H2 relaxin and single alanine-mutated H2 analogues (mid-region to C terminus of the A-chain) in the presence of a competitive ligand Eu3+-labeled INSL3 tested in HEK-293T cells stably expressing RXFP2 receptors. Data are expressed as a percentage of specific binding and are pooled data from at least three experiments performed in triplicate. D, cAMP activity of native H2 relaxin and single alanine-mutated H2 analogues (mid-region to C terminus of the A-chain) in HEK-293T cells expressing RXFP2 receptors using a pCRE-galactosidase reporter gene system. Data are expressed as a percentage of maximum INSL3-stimulated cAMP response and are pooled data from at least three experiments performed in triplicate. Error bars, S.E.
Effects of Truncation with/without Single Alanine Substitutions of H2 Relaxin A-chain on RXFP1 and RXFP2
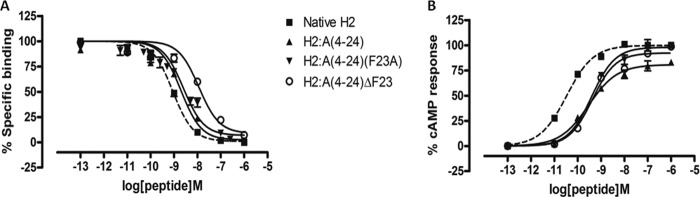
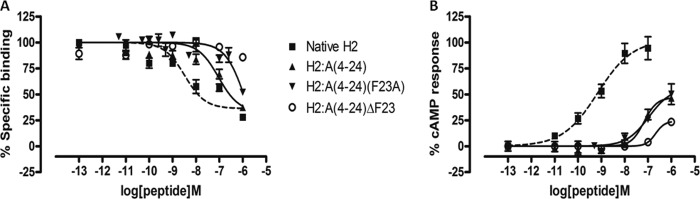
We have recently designed and created a truncated relaxin analog, H2:(A4–24)(B7–24), having an active core of 38 amino acids that displayed high RXFP1 selectivity over RXFP2 (35). Because of the numerous truncations, it was unclear which specific residue(s) contributed to the complete loss of RXFP2 activity, although Trp-28 is believed to be one of them (14, 35). The alanine scanning results described above now suggest that Tyr-3 in the A-chain is another important residue for RXFP2 activity. This observation is also supported by an A-chain shortened analog H2:A(5–24), which exhibited full RXFP1 affinity and significantly reduced affinity at RXFP2 (40). Hence, we hypothesized that truncation of the first few residues, including Tyr-3 of the A-chain together with a single alanine substitution at Phe-23, could aid in retaining close to native RXFP1 affinity while completely abolishing RXFP2 actions. Therefore, we first synthesized H2:A(4–24) as a control to confirm if this peptide is fully active at RXFP1, followed by our target analog H2:A(4–24)(F23A) (Table 1). We showed that both analogues retained almost similar binding and cAMP responses to native H2 relaxin at RXFP1 (Fig. 3 and Table 2) but were able to significantly reduce binding and activation at RXFP2 (Fig. 4 and Table 2). This series of analogues further confirmed the importance of the N- and C termini regions of the A-chain in directing RXFP2 specificity. To further prove our hypothesis that both Tyr-3 and Phe-23 are key residues for RXFP2 activity, we prepared another analog, H2:A(4–24)ΔF23, by removing both Tyr-3 and Phe-23 (Table 1) from the A-chain. It was found that H2:A(4–24)ΔF23 was able to completely abolish RXFP2 binding and cAMP responses but the responses at RXFP1 were compromised with a 1.5-fold log unit decreased shift in binding capacity (Fig. 4 and Table 2).
FIGURE 3.
Activity of H2 analogues with truncations and/or single alanine mutation on the RXFP1 receptor. A, competition binding results of native H2 relaxin and H2 analogues with truncation and/or single alanine mutation in the presence of a competitive ligand Eu3+-labeled H2 relaxin tested in HEK-293T cells stably expressing RXFP1 receptors. Data are expressed as a percentage of specific binding and are pooled data from at least three experiments performed in triplicate. B, cAMP activity of native H2 relaxin and H2 analogues with truncation and/or single alanine mutation in HEK-293T cells expressing RXFP1 receptors using a pCRE-galactosidase reporter gene system. Data are expressed as a percentage of maximum H2 relaxin-stimulated cAMP response and are pooled data from at least three experiments performed in triplicate. Error bars, S.E.
FIGURE 4.
Activity of H2 analogues with truncations and/or single alanine mutation on the RXFP2 receptor. A, competition binding results of native H2 relaxin and H2 analogues with truncation and/or single alanine mutation in the presence of a competitive ligand Eu3+-labeled INSL3 tested in HEK-293T cells stably expressing RXFP2 receptors. Data are expressed as a percentage of specific binding and are pooled data from at least three experiments performed in triplicate. B, cAMP activity of native H2 relaxin and H2 analogues with truncation and/or single alanine mutation in HEK-293T cells expressing RXFP2 receptors using a pCRE-galactosidase reporter gene system. Data are expressed as a percentage of maximum INSL3-stimulated cAMP response and are pooled data from at least three experiments performed in triplicate. Error bars, S.E.
Effects of Chimeric H2/INSL5 A-chains on RXFP1 and RXFP2
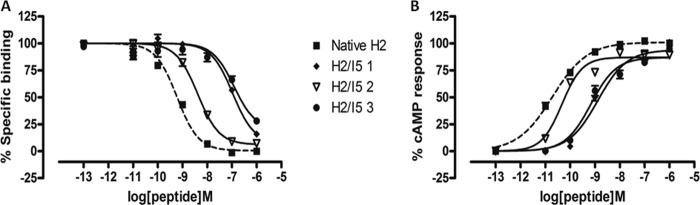
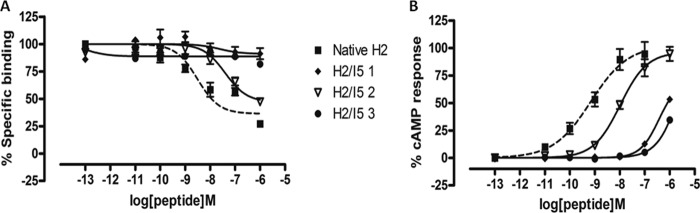
Chimeric H2 relaxin analogues were designed and synthesized (Table 1) to introduce the INSL5 A-chain in three different domains within the native H2 relaxin A-chain that were linked to native H2 relaxin B-chain. This was done in order to study the role of native H2 relaxin A-chain with different domains because no single amino acid was found to be very critical for RXFP1 action. Using such a strategy, we have recently identified a domain of 5–9 residues at the N terminus of the INSL3 A-chain that is critical for RXFP2 activation (20). In this study, three partial chimeric peptides were successfully prepared and tested. Chimera 1 had the first 9 residues replaced with the first 6 residues of the INSL5 A-chain; chimera 2 had the middle 3 residues present between the 2 cysteine residues replaced; and last, chimera 3 had the C-terminal region of native H2 relaxin swapped with INSL5 (Table 1). Functional studies showed that chimeras 1 and 3 resulted in a significant loss of binding and activation at both RXFP1 (Fig. 5 and Table 2) and RXFP2 (Fig. 6 and Table 2), whereas chimera 2 was able to retain some native affinity and potency at RXFP1 (Fig. 5A). This could be due to the fact that the native A-chain domain replacements with INSL5 A-chain residues led to the disruption of the native structure, thus resulting in poor affinity and potency at RXFP1 because of its inability to bind to the receptor in the proper conformation. In addition, the INSL5 A-chain is not a natural ligand for either RXFP1 or RXFP2. This explains the hypothesis that although single alanine substitutions did not cause a dramatic effect on binding and activation, it is important to have native A-chain residues at both the N- and C-terminal domains to retain the structure that is essential for effective ligand-receptor interactions.
FIGURE 5.
Activity of chimeric H2/INSL5 A-chains on the RXFP1 receptor. A, competition binding results of native H2 relaxin and chimeric H2/I5 A-chain relaxin analogues in the presence of a competitive ligand Eu3+-labeled H2 relaxin tested in HEK-293T cells stably expressing RXFP1 receptors. Data are expressed as a percentage of specific binding and are pooled data from at least three experiments performed in triplicate. B, cAMP activity of native H2 relaxin and H2 analogues with truncation and/or single alanine mutation in HEK-293T cells expressing RXFP1 receptors using a pCRE-galactosidase reporter gene system. Data are expressed as a percentage of maximum H2 relaxin-stimulated cAMP response and are pooled data from at least three experiments performed in triplicate. Error bars, S.E.
FIGURE 6.
Activity of chimeric H2/INSL5 A-chains on the RXFP2 receptor. A, competition binding results of native H2 relaxin and chimeric H2/I5 A-chain relaxin analogues in the presence of a competitive ligand Eu3+-labeled INSL3 tested in HEK-293T cells stably expressing RXFP2 receptors. Data are expressed as a percentage of specific binding and are pooled data from at least three experiments performed in triplicate. B, cAMP activity of native H2 relaxin and H2 analogues with truncation and/or single alanine mutation in HEK-293T cells expressing RXFP2 receptors using a pCRE-galactosidase reporter gene system. Data are expressed as a percentage of maximum INSL3-stimulated cAMP response and are pooled data from at least three experiments performed in triplicate. Error bars, S.E.
Structural Studies of H2 Relaxin Analogues
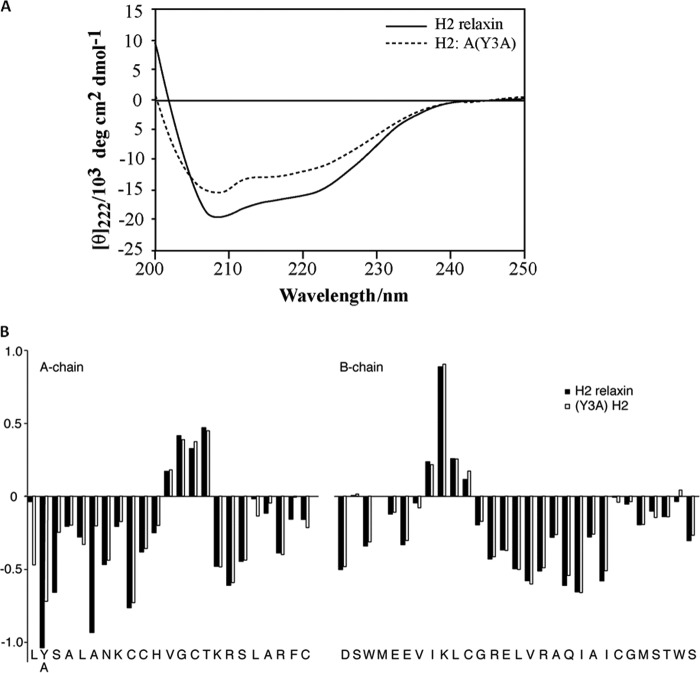
To understand the influence of A-chain mutation on the conformation of H2 relaxin peptides, CD spectroscopy was employed for both native H2 relaxin and one of the mutated analogues, H2:A(Y3A). The CD spectra of both native and analog peptides revealed a typical α-helical pattern with double minima at 208 and 222 nm. The mean residual weight ellipticity values at 222 nm, [θ]222, were used to determine the helix content of the peptides. The [θ]222 value for native H2 relaxin is −15,056.9 and corresponds to an α-helix content of 42%. A single amino acid substitution at the N terminus of the A-chain, H2:A(Y3A), resulted in a decrease of the [θ]222 value, −11,283.5, corresponding to 32% helicity content (Fig. 7A).
FIGURE 7.
Structural studies of H2 relaxin analogues. A, CD spectra result of native H2 relaxin in comparison with H2:A(Y3A). H2 relaxin peptide analogues for CD were performed in 10 mm phosphate buffer at pH 7.5. B, secondary Hα chemical shifts of H2 relaxin and H2:A(Y3A). Secondary shifts (i.e. differences between observed chemical shifts and the random coil shifts) are sensitive indicators of secondary structure. Three distinct regions of negative numbers corresponding to the three helical segments and two stretches of positive numbers accounting for the two β-sheets are seen from both H2 relaxin and H2:A(Y3A). Although secondary structural elements appear conserved, NOEs appeared to be weak, and lines were generally broader, indicating an increase in flexibility of the overall fold, which suggests a destabilized structure.
To further confirm the effect of the mutations on overall structure, two of the analogues with altered pharmacological profile, H2:A(Y3A) and H2:A(4–24)(F23A), were analyzed by solution NMR spectroscopy. The H2:A(4–24)(F23A) variant showed in comparison with native H2 relaxin significantly poorer signal dispersion and generally broad lines, suggesting a rather compromised overall fold not amenable to resonance assignments. In contrast, the H2:A(Y3A) variant showed good resonance dispersion, consistent with an ordered structure in solution, albeit with somewhat broader lines than native H2 relaxin. Nonetheless, using ultra-high field two-dimensional NMR data recorded at 900 MHz using a cryoprobe, nearly complete resonance assignments of the peptide backbone and most side chain resonances could be achieved through standard homonuclear sequential assignment strategies (41). Secondary Hα shifts (i.e. differences between observed chemical shifts and the chemical shifts expected for corresponding amino acids in random coil type structure) are sensitive indicators of secondary structure (42). The secondary shifts for H2:A(Y3A) are reported in Fig. 7B and compared with corresponding values for native H2 relaxin (43). From this, it is clear that all elements of secondary structure, including the three α-helical segments as well as two extended regions forming a small anti-parallel β-sheet, are conserved, evident from stretches of negative and positive values, respectively. Further support for a retained overall fold comes from the presence of key long range NOEs that are hallmarks of the fold, including interchain NOEs across the β-sheet. However, most of these NOEs are weak, and the lines are generally broader, suggesting an increase in flexibility of the fold, presumably due to a destabilized hydrophobic core in the absence of Tyr-3 within the A-chain. This result correlates with the CD data indicating that H2:A(Y3A) exhibited less helical structure compared with native H2 relaxin (Fig. 7A).
Determination of Collagen Content (Fibrosis)
From the results obtained from both ligand binding and cAMP activity assays for all H2 relaxin analogues, the peptide H2:A(4–24)(F23A), which displayed the most selectivity for RXFP1 over RXFP2, was selected for further functional characterization in both in vitro and in vivo experimental models of fibrosis.
In Vitro Studies
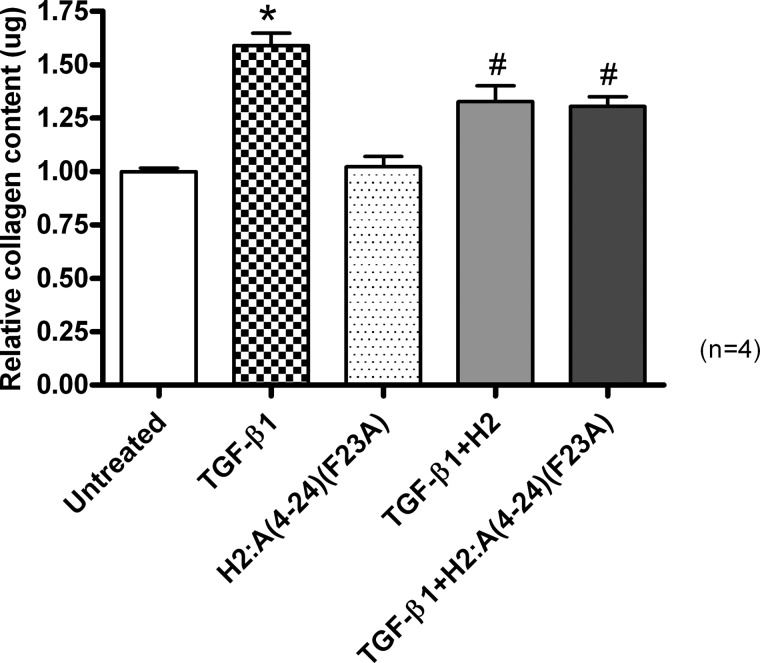
TGF-β1 is a potent stimulator of fibroblast differentiation and collagen production and was found to significantly (p < 0.001) promote collagen deposition from BJ3 human dermal fibroblasts (at a dose of 2 ng/ml) over a 72-h culture period, by ∼60% of that measured in untreated cultures (Fig. 8). Consistent with H2 relaxin's previously documented ability to prevent TGF-β1-stimulated collagen deposition from various fibroblast cultures, regardless of their origin (31–33, 35), co-administration of native H2 relaxin (16.8 nm) to TGF-β1-stimulated BJ3 cells was able to inhibit this aberrant deposition of collagen by 45–50% (p < 0.01 versus TGF-β1 treatment alone; Fig. 8) over the same time period. Similarly, co-administration of H2:A(4–24)(F23A) to TGF-β1-stimulated cells prevented collagen deposition to the same extent as native H2 relaxin (p < 0.01 versus TGF-β1 treatment alone; Fig. 8) without affecting basal collagen levels when administered to unstimulated fibroblasts.
FIGURE 8.
The relative mean ± S.E. (error bars) collagen content from untreated BJ3 fibroblasts and from cells treated with TGF-β1 (2 ng/ml) alone, with H2:A(4–24)(F23A) alone, or with native H2 relaxin (16.8 nm) or H2:A(4–24)(F23A) (16.8 nm) in TGF-β1 (2 ng/ml)-stimulated cells. All data are expressed as the relative ratio of collagen content measured in the untreated group, which was expressed as 1, whereas the numbers in parentheses represent the number of separate experiments that were conducted for each group in triplicate. *, p < 0.001 versus the untreated group; #, p < 0.01 versus the TGF-β1 alone-treated group.
In Vivo Studies
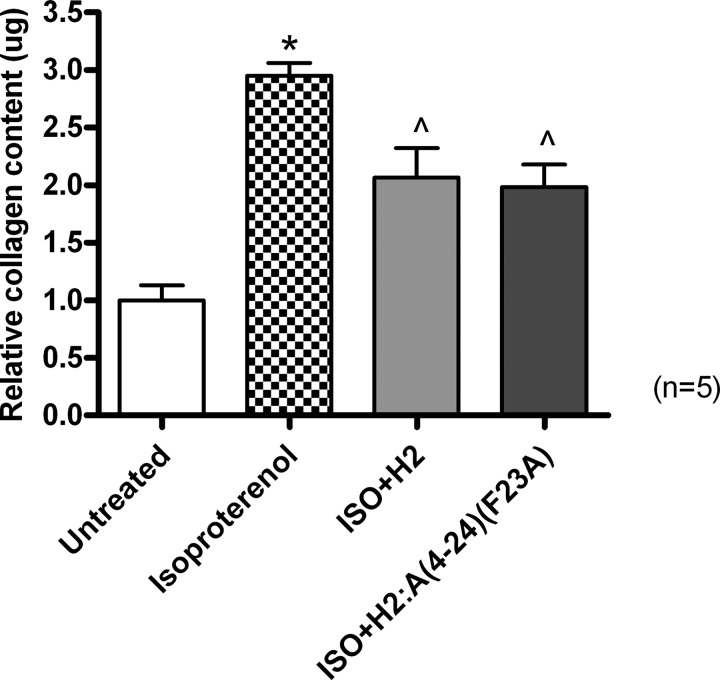
Additionally, in an ISO-induced murine model of cardiac toxicity and fibrosis, collagen concentration (percentage of collagen content/dry weight tissue) was significantly increased by ∼2-fold in animals given five daily subcutaneous injections of 25 mg/kg ISO before being left for a further 12 days for fibrosis progression to accumulate compared with that measured in untreated control animals (p < 0.001 versus control group; Fig. 9). Again, this increase in aberrant collagen deposition was significantly prevented in separate subgroups of mice co-administered with ISO and native H2 relaxin (0.5 mg/kg/day) or ISO and the H2:A(4–24)(F23A) analog (0.5 mg/kg/day) by 45–50% (both p < 0.05 versus the ISO alone group; Fig. 9) to a comparable extent.
FIGURE 9.
The relative mean ± S.E. (error bars) collagen content from untreated group and from mice treated with ISO alone or with native H2 relaxin or H2:A(4–24)(F23A) in ISO-induced myocardial infarction. All data are expressed as the relative ratio of collagen content measured in the untreated group, which was expressed as 1, whereas the numbers in parentheses represent the number of separate experiments that were conducted for each group in triplicate. *, p < 0.001 versus the untreated group; ∧, p < 0.05 versus the ISO alone-treated group.
DISCUSSION
Since the discovery that the native receptor for H2 relaxin is the G protein-coupled receptor, RXFP1 (8), increasing efforts have been made to better understand how H2 relaxin interacts with not only RXFP1 but also RXFP2, for which it acts as a ligand. These studies have involved investigations into its primary mode of action through the B-chain (12) and a secondary interaction that involves the A-chain of which the intricate details of the ligand-binding interaction remain unclear (44). Park et al. (45) has recently performed mutations at the C-terminal helix of the H2 relaxin A-chain in order to understand the role of this region in binding and activation. They have identified some residues important for secondary interactions and suggested that Lys-17 is important for H2-RXFP1 interaction, whereas Phe-23 and Thr-16 are important for H2-RXFP2 interaction. However, the nature of H2-RXFP1/RXFP2 interactions was not clearly realized because no reduction in the affinity for RXFP1 or RXFP2 was observed upon mutation of Thr-16 or Lys-17 to alanine residues. Furthermore, Phe-23 within the A-chain was thought to be interacting directly with the receptor. Thus, the reduction of RXFP2 activity by the Phe-23-mutated analog was speculated to be caused by a residue side chain effect, whereas the secondary effect relating to structural perturbation was not considered. Clearly, further mutational studies targeting the entire A-chain were required to understand the mechanism of secondary interaction, particularly for the development of RXFP1-selective H2 relaxin agonists or antagonists. Hence, we have successfully designed and chemically synthesized 16 mutants, with all mutations in the A-chain of the H2 relaxin peptide (Table 1). We demonstrated that no single amino acid dominates in driving the secondary interaction between H2 relaxin and RXFP1, although some contributions were observed from Tyr-3, Leu-20, and Phe-23. In addition, these same residues predominantly drive the affinity and activity of H2 relaxin at RXFP2. We also propose a new mechanism in contrast to Park et al. (45), by which the decreased RXFP2 activity observed from the Phe-23-mutated analog was due to a structural effect rather than specific side chain-driven influences.
Insights into the Mechanism of Secondary Interaction of RXFP1 and RXFP2 by H2 Relaxin
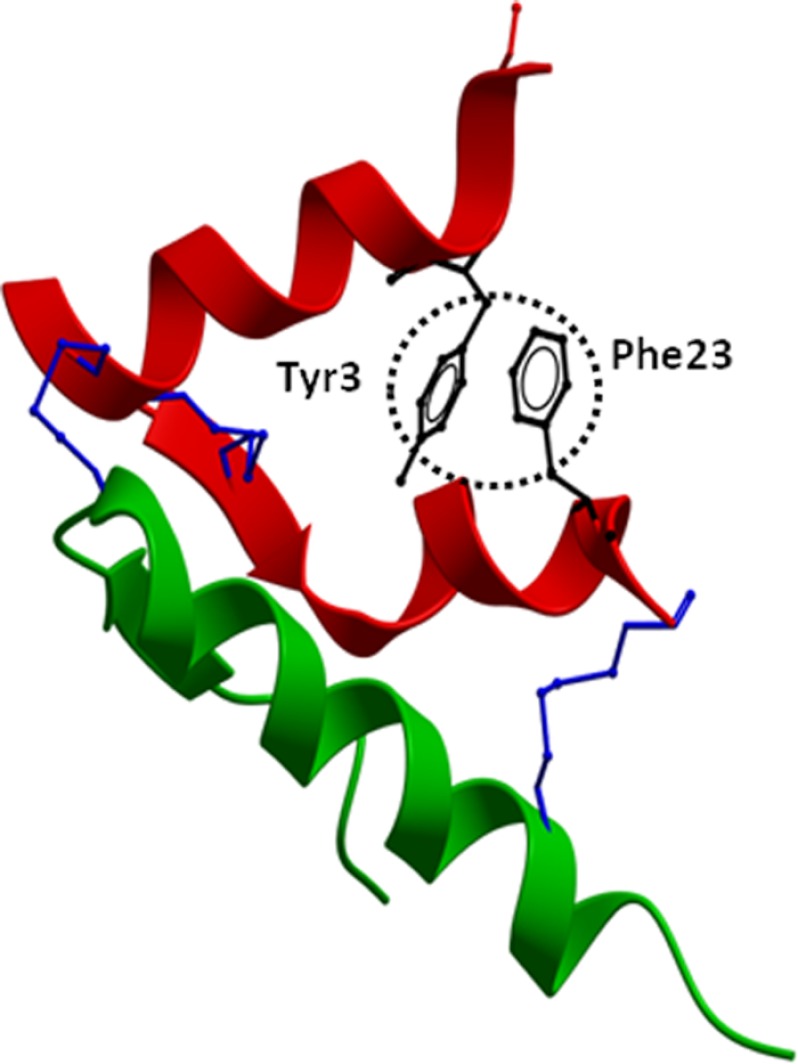
Mutational studies for H2 relaxin A-chain residues were conducted on those residues that had side chains that could affect binding and activation at RXFP1 and RXFP2. The majority of the H2 relaxin analogues mutated from the N to C terminus did not have significant differences in binding and activation at RXFP1. These alanine substitutions reveal that no individual residues from Lys-9 to Arg-22 are highly critical for RXFP1 activity. However, H2:A(Y3A) and H2:A(F23A) at the far end of the N and C termini, respectively, had reduced affinity and potency at RXFP1 and RXFP2 and were able to almost completely abolish RXFP2 binding and activation. This demonstrates that both Tyr-3 and Phe-23 play an important role in directing RXFP2 specificity, and by replacing these residues, RXFP2 responses were severely compromised, with H2:A(Y3A) giving a slightly greater effect than H2:A(F23A). In addition, the truncated analog H2:A(4–24) lacking three residues from the N terminus of the A-chain displayed reduced activity for RXFP2 but was able to maintain close to native binding and activation at RXFP1, further confirming that the Tyr-3 residue is responsible for the reduction in RXFP2 responses. Complete ablation of RXFP2 binding and cAMP was achieved with H2:A(4–24)ΔF23, in the absence of both Tyr-3 and Phe-23 residues, affirming that both residues are crucial for RXFP2 binding and activation. The third most important amino acid found in the A-chain was Leu-20, which is also hydrophobic in nature. It is most likely that the hydrophobic interaction between two aromatic residues, along with the Leu-20 residue inside the core, may stabilize the structure of H2 relaxin, which might be very important for RXFP2 activity but not so for RXFP1. Intriguingly, the truncated analog, H2:A(4–24), showed higher RXFP1 activity compared with H2:A(Y3A), possibly due to its induced fit conformation for RXFP1. Finally, having the truncation at the N terminus and the Phe-23 mutation at the C terminus, we obtained an analog, H2:A(4–24)(F23A), that exhibited very high activity at RXFP1 but no activity at RXFP2 at nanomolar concentrations. This result supports our previous hypothesis that “it should be possible to construct a H2 relaxin analog with high affinity for RXFP1 but no affinity for RXFP2 by judicious mutations within the A-chain of the peptide” (21). As seen from the structural representation of the H2 relaxin A-chain (Fig. 10), both Tyr-3 and Phe-23 are in close proximity to each other, although one is present at the N terminus and the other at the C terminus. We hypothesize that the hydrophobic or π-π interactions between these side chains of two aromatic residues are essential for maintenance of native folding. The removal or mutation of the Tyr-3 and/or Phe-23 residue(s) may destabilize the structure of H2 relaxin, causing a loss of affinity and activity. This hypothesis is supported by our CD and NMR structural data, where the H2:A(Y3A) and H2:A(4–24)(F23A) variants exhibited compromised overall fold.
FIGURE 10.
Structure of native H2 relaxin. The crystal structure of H2 relaxin (Protein Data Bank code 6RLX) is shown with the side chains of Tyr-3 and Phe-23 (circled in black) that are present at opposite ends of the relaxin A-chain in close proximity. The A-chain is shown in red, the B-chain in green, and the conserved disulfide bridges in blue. The absence of Tyr-3 and/or Phe-23 contributes to the destabilization of the overall fold of the peptide. This causes loss of RXFP2 binding and activation because the peptide is unable to adopt an optimized orientation for binding to the receptor.
It has been reported that there are very intricate and important interactions within both the A- and B-chains of the native peptide that contribute to RXFP1 and RXFP2 receptor binding and activation (44). This ranges from a single amino acid with an important side chain to a series of amino acid residues, which constitute a domain region necessary to maintain the correct fold and structure of the peptide. With reference to the recent INSL3 study (20), we have produced three partial chimeras of H2 relaxin and INSL5 in order to find out if a domain (series of residues) was critical for RXFP1 activity because no single amino acid in the A-chain was found to be. In stark contrast to the INSL3-INSL5 partial chimera, the INSL5-H2 partial chimera analogues exhibited loss of both affinity and activity. This ascertains that the secondary binding site requires specific domains of the H2 relaxin A-chain to be in the correct precise orientation for subsequent affinity and cAMP responses. The precise orientation is disrupted, possibly due to the change in overall structure, which is supported by our CD and NMR studies on two analogues we discussed above. Hence, unlike INSL3, it is not possible to separate the binding and activation events of H2 relaxin, which confirms our previous hypothesis that both interactions (binding and activation) at H2-RXFP1 and H2-RXFP2 are closely related (40). This is again supported by the fact that the loss of H2-RXFP1/RXFP2 activity is accompanied by a reduction in affinity (Table 2) either with a single amino acid mutation, single amino acid deletion, or domain replacement. This is in contrast to the INSL3-RXFP2 system, where binding and activation are two separate events (35). Additionally, in contrast to H2 relaxin, the A-chain of structurally related insulin peptide directly engages the key signaling element of the receptor α-subunit (46), and mutations within the A-chain of insulin directly create marked perturbations to this interaction (47). The differences between the relative roles of the A- and B-chains of insulin versus the relaxin family peptide members fundamentally reflect the different classification of receptors, which ultimately has broad implications for the evolution and divergence within these respective ligand families.
The Relaxin Analog H2:A(4–24)(F23A) Is Very Potent in Vitro and in Vivo
Because native H2 relaxin possesses potent antifibrotic effects and could potentially be developed as a therapy for fibrotic disorders (2–4, 31–33, 38), it would be interesting to see whether the RXFP1-selective H2 analog, H2:A(4–24)(F23A), exhibits similar potency to native relaxin both in vitro and in vivo. The analog was tested in human BJ3 cells in vitro, which natively express endogenous RXFP1 receptors, and found to be able to comparably prevent TGF-β1-stimulated collagen deposition. Moreover, when tested in an experimental mouse model of fibrosis in vivo, the outcomes paralleled those of the in vitro fibroblast culture study, where H2:A(4–24)(F23A) was able to significantly and similarly prevent aberrant cardiac fibrosis progression to that of native H2 relaxin. This result also confirms our previous findings that anti-fibrotic effects of relaxin are mediated through RXFP1 receptor (48, 49).
In conclusion, we have identified key A-chain residues spanning from the N to C termini that are important for native structure and function. Our results provide new biochemical insights into the mechanism of secondary interaction between H2 relaxin and RXFP1 or RXFP2. Although no single amino acid in the A-chain was found to be very critical for full RXFP1 binding and activation, Tyr-3, Leu-20, and Phe-23 were found to be essential for peptide folding and thus for RXFP2 activity. This led to the creation of an RXFP1-selective analog, H2:A(4–24)(F23A), that mimics the actions of native H2 relaxin in diminishing aberrant collagen deposition (anti-fibrotic) in both in vitro and in vivo experimental models of fibrosis. Because H2 relaxin is currently in Phase III clinical trials for the treatment of acute heart failure, the advantage of having this RXFP1-selective analog will be beneficial to avoid potential undesired side effects, if any, mediated through RXFP2. Last, this RXFP1 analog will unquestionably serve as a valuable template for the development of future clinical compounds.
Acknowledgments
We are grateful to Tania Ferraro for assistance with biochemical assays and to Feng Lin for amino acid analysis.
This research was funded by National Health and Medical Research Council (NHMRC) (Australia) Project Grants 508995 and 1023078 (to J. D. W., M. A. H., and R. A. D. B.). Studies at the Florey Neuroscience Institutes were supported by the Victorian Government's Operational Infrastructure Support Program.
- H2
- human gene-2
- RXFP
- relaxin family peptide(s)
- INSL3
- insulin-like peptide 3
- LRR
- leucine-rich repeat
- TM
- transmembrane
- ISO
- isoproterenol.
REFERENCES
- 1. Bryant-Greenwood G. D., Schwabe C. (1994) Human relaxins. Chemistry and biology. Endocr. Rev. 15, 5–26 [DOI] [PubMed] [Google Scholar]
- 2. Chan L. J., Hossain M. A., Samuel C. S., Separovic F., Wade J. D. (2011) The relaxin peptide family. Structure, function, and clinical applications. Protein Pept. Lett. 18, 220–229 [DOI] [PubMed] [Google Scholar]
- 3. Samuel C. S., Cendrawan S., Gao X. M., Ming Z., Zhao C., Kiriazis H., Xu Q., Tregear G. W., Bathgate R. A., Du X. J. (2011) Relaxin remodels fibrotic healing following myocardial infarction. Lab. Invest. 91, 675–690 [DOI] [PubMed] [Google Scholar]
- 4. Du X. J., Bathgate R. A., Samuel C. S., Dart A. M., Summers R. J. (2010) Cardiovascular effects of relaxin. From basic science to clinical therapy. Nat. Rev. Cardiol. 7, 48–58 [DOI] [PubMed] [Google Scholar]
- 5. Teerlink J. R., Metra M., Felker G. M., Ponikowski P., Voors A. A., Weatherley B. D., Marmor A., Katz A., Grzybowski J., Unemori E., Teichman S. L., Cotter G. (2009) Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF). A multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 373, 1429–1439 [DOI] [PubMed] [Google Scholar]
- 6. Bathgate R. A., Ivell R., Sanborn B. M., Sherwood O. D., Summers R. J. (2006) International Union of Pharmacology LVII. Recommendations for the nomenclature of receptors for relaxin family peptides. Pharmacol. Rev. 58, 7–31 [DOI] [PubMed] [Google Scholar]
- 7. Kumagai J., Hsu S. Y., Matsumi H., Roh J. S., Fu P., Wade J. D., Bathgate R. A., Hsueh A. J. (2002) INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J. Biol. Chem. 277, 31283–31286 [DOI] [PubMed] [Google Scholar]
- 8. Hsu S. Y., Nakabayashi K., Nishi S., Kumagai J., Kudo M., Sherwood O. D., Hsueh A. J. (2002) Activation of orphan receptors by the hormone relaxin. Science 295, 671–674 [DOI] [PubMed] [Google Scholar]
- 9. Sudo S., Kumagai J., Nishi S., Layfield S., Ferraro T., Bathgate R. A., Hsueh A. J. (2003) H3 relaxin is a specific ligand for LGR7 and activates the receptor by interacting with both the ectodomain and the exoloop 2. J. Biol. Chem. 278, 7855–7862 [DOI] [PubMed] [Google Scholar]
- 10. Scott D. J., Layfield S., Yan Y., Sudo S., Hsueh A. J., Tregear G. W., Bathgate R. A. (2006) Characterization of novel splice variants of LGR7 and LGR8 reveals that receptor signaling is mediated by their unique low density lipoprotein class A modules. J. Biol. Chem. 281, 34942–34954 [DOI] [PubMed] [Google Scholar]
- 11. Büllesbach E. E., Schwabe C. (2000) The relaxin receptor-binding site geometry suggests a novel gripping mode of interaction. J. Biol. Chem. 275, 35276–35280 [DOI] [PubMed] [Google Scholar]
- 12. Büllesbach E. E., Schwabe C. (2005) The trap-like relaxin-binding site of the leucine-rich G-protein-coupled receptor 7. J. Biol. Chem. 280, 14051–14056 [DOI] [PubMed] [Google Scholar]
- 13. Rosengren K. J., Zhang S., Lin F., Daly N. L., Scott D. J., Hughes R. A., Bathgate R. A., Craik D. J., Wade J. D. (2006) Solution structure and characterization of the LGR8 receptor binding surface of insulin-like peptide 3. J. Biol. Chem. 281, 28287–28295 [DOI] [PubMed] [Google Scholar]
- 14. Scott D. J., Wilkinson T. N., Zhang S., Ferraro T., Wade J. D., Tregear G. W., Bathgate R. A. (2007) Defining the LGR8 residues involved in binding insulin-like peptide 3. Mol. Endocrinol. 21, 1699–1712 [DOI] [PubMed] [Google Scholar]
- 15. Scott D. J., Tregear G. W., Bathgate R. A. (2009) Modeling the primary hormone-binding site of RXFP1 and RXFP2. Ann. N.Y. Acad. Sci. 1160, 74–77 [DOI] [PubMed] [Google Scholar]
- 16. Kong R. C., Shilling P. J., Lobb D. K., Gooley P. R., Bathgate R. A. (2010) Membrane receptors. Structure and function of the relaxin family peptide receptors. Mol. Cell. Endocrinol. 320, 1–15 [DOI] [PubMed] [Google Scholar]
- 17. Liu C., Chen J., Kuei C., Sutton S., Nepomuceno D., Bonaventure P., Lovenberg T. W. (2005) Relaxin-3/insulin-like peptide 5 chimeric peptide, a selective ligand for G protein-coupled receptor (GPCR)135 and GPCR142 over leucine-rich repeat-containing G protein-coupled receptor 7. Mol. Pharmacol. 67, 231–240 [DOI] [PubMed] [Google Scholar]
- 18. Liu C., Eriste E., Sutton S., Chen J., Roland B., Kuei C., Farmer N., Jörnvall H., Sillard R., Lovenberg T. W. (2003) Identification of relaxin-3/INSL7 as an endogenous ligand for the orphan G-protein-coupled receptor GPCR135. J. Biol. Chem. 278, 50754–50764 [DOI] [PubMed] [Google Scholar]
- 19. Haugaard-Kedström L. M., Shabanpoor F., Hossain M. A., Clark R. J., Ryan P. J., Craik D. J., Gundlach A. L., Wade J. D., Bathgate R. A., Rosengren K. J. (2011) Design, synthesis, and characterization of a single-chain peptide antagonist for the relaxin-3 receptor RXFP3. J. Am. Chem. Soc. 133, 4965–4974 [DOI] [PubMed] [Google Scholar]
- 20. Bathgate R. A., Zhang S., Hughes R. A., Rosengren K. J., Wade J. D. (2012) The structural determinants of insulin-like peptide 3 activity. Front. Endocrinol. (Lausanne) 3, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hossain M. A., Wade J. D., Bathgate R. A. (2012) Chimeric relaxin peptides highlight the role of the A-chain in the function of H2 relaxin. Peptides 35, 102–106 [DOI] [PubMed] [Google Scholar]
- 22. Büllesbach E. E., Schwabe C. (2005) LGR8 signal activation by the relaxin-like factor. J. Biol. Chem. 280, 14586–14590 [DOI] [PubMed] [Google Scholar]
- 23. Bathgate R. A., Lin F., Hanson N. F., Otvos L., Jr., Guidolin A., Giannakis C., Bastiras S., Layfield S. L., Ferraro T., Ma S., Zhao C., Gundlach A. L., Samuel C. S., Tregear G. W., Wade J. D. (2006) Relaxin-3. Improved synthesis strategy and demonstration of its high-affinity interaction with the relaxin receptor LGR7 both in vitro and in vivo. Biochemistry 45, 1043–1053 [DOI] [PubMed] [Google Scholar]
- 24. Hossain M. A., Zhang S., Lin F., Ferraro T., Bathgate R. A., Tregear G. W., Wade J. D. (2006) Regioselective disulfide solid phase synthesis, chemical characterization and in vitro receptor binding activity of equine relaxin. Int. J. Pept. Res. Ther. 12, 211–215 [Google Scholar]
- 25. Akhter Hossain M., Bathgate R. A., Kong C. K., Shabanpoor F., Zhang S., Haugaard-Jönsson L. M., Rosengren K. J., Tregear G. W., Wade J. D. (2008) Synthesis, conformation, and activity of human insulin-like peptide 5 (INSL5). Chembiochem 9, 1816–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang S., Lin F., Hossain M. A., Shabanpoor F., Tregear G. W., Wade J. D. (2008) Simultaneous post-cysteine (S-Acm) group removal quenching of iodine and isolation of peptide by one step ether precipitation. Int. J. Pept. Res. Ther. 14, 301–305 [Google Scholar]
- 27. Keller R. L. (2004) The Computer-aided Resonance Assignment Tutorial. CANTINA Verlag, Goldau, Switzerland [Google Scholar]
- 28. Shabanpoor F., Bathgate R. A., Belgi A., Chan L. J., Nair V. B., Wade J. D., Hossain M. A. (2012) Site-specific conjugation of a lanthanide chelator and its effects on the chemical synthesis and receptor binding affinity of human relaxin-2 hormone. Biochem. Biophys. Res. Commun. 420, 253–256 [DOI] [PubMed] [Google Scholar]
- 29. Shabanpoor F., Hughes R. A., Bathgate R. A., Zhang S., Scanlon D. B., Lin F., Hossain M. A., Separovic F., Wade J. D. (2008) Solid-phase synthesis of europium-labeled human INSL3 as a novel probe for the study of ligand-receptor interactions. Bioconjug. Chem. 19, 1456–1463 [DOI] [PubMed] [Google Scholar]
- 30. Chen W., Shields T. S., Stork P. J., Cone R. D. (1995) A colorimetric assay for measuring activation of Gs- and Gq-coupled signaling pathways. Anal. Biochem. 226, 349–354 [DOI] [PubMed] [Google Scholar]
- 31. Unemori E. N., Amento E. P. (1990) Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J. Biol. Chem. 265, 10681–10685 [PubMed] [Google Scholar]
- 32. Samuel C. S., Unemori E. N., Mookerjee I., Bathgate R. A., Layfield S. L., Mak J., Tregear G. W., Du X. J. (2004) Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology 145, 4125–4133 [DOI] [PubMed] [Google Scholar]
- 33. Heeg M. H., Koziolek M. J., Vasko R., Schaefer L., Sharma K., Müller G. A., Strutz F. (2005) The antifibrotic effects of relaxin in human renal fibroblasts are mediated in part by inhibition of the Smad2 pathway. Kidney Int. 68, 96–109 [DOI] [PubMed] [Google Scholar]
- 34. Hahn W. C., Counter C. M., Lundberg A. S., Beijersbergen R. L., Brooks M. W., Weinberg R. A. (1999) Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 [DOI] [PubMed] [Google Scholar]
- 35. Hossain M. A., Rosengren K. J., Samuel C. S., Shabanpoor F., Chan L. J., Bathgate R. A., Wade J. D. (2011) The minimal active structure of human relaxin-2. J. Biol. Chem. 286, 37555–37565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hewitson T. D., Ho W. Y., Samuel C. S. (2010) Antifibrotic properties of relaxin. In vivo mechanism of action in experimental renal tubulointerstitial fibrosis. Endocrinology 151, 4938–4948 [DOI] [PubMed] [Google Scholar]
- 37. Brooks W. W., Conrad C. H. (2009) Isoproterenol-induced myocardial injury and diastolic dysfunction in mice. Structural and functional correlates. Comp. Med. 59, 339–343 [PMC free article] [PubMed] [Google Scholar]
- 38. Samuel C. S., Zhao C., Bathgate R. A., Bond C. P., Burton M. D., Parry L. J., Summers R. J., Tang M. L., Amento E. P., Tregear G. W. (2003) Relaxin deficiency in mice is associated with an age-related progression of pulmonary fibrosis. FASEB J. 17, 121–123 [DOI] [PubMed] [Google Scholar]
- 39. Samuel C. S. (2009) Determination of collagen content, concentration, and sub-types in kidney tissue. Methods Mol. Biol. 466, 223–235 [DOI] [PubMed] [Google Scholar]
- 40. Hossain M. A., Rosengren K. J., Haugaard-Jönsson L. M., Zhang S., Layfield S., Ferraro T., Daly N. L., Tregear G. W., Wade J. D., Bathgate R. A. (2008) The A-chain of human relaxin family peptides has distinct roles in the binding and activation of the different relaxin family peptide receptors. J. Biol. Chem. 283, 17287–17297 [DOI] [PubMed] [Google Scholar]
- 41. Wüthrich K. (1983) Sequential individual resonance assignments in the 1H NMR spectra of polypeptides and proteins. Biopolymers 22, 131–138 [DOI] [PubMed] [Google Scholar]
- 42. Wishart D. S., Bigam C. G., Holm A., Hodges R. S., Sykes B. D. (1995) 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. 1. Investigations of nearest-neighbor effects. J. Biomol. NMR 5, 67–81 [DOI] [PubMed] [Google Scholar]
- 43. Rosengren K. J., Bathgate R. A., Craik D. J., Daly N. L., Haugaard-Jönsson L. M., Hossain M. A., Wade J. D. (2009) Structural insights into the function of relaxins. Ann. N.Y. Acad. Sci. 1160, 20–26 [DOI] [PubMed] [Google Scholar]
- 44. Hartley B. J., Scott D. J., Callander G. E., Wilkinson T. N., Ganella D. E., Kong C. K., Layfield S., Ferraro T., Petrie E. J., Bathgate R. A. (2009) Resolving the unconventional mechanisms underlying RXFP1 and RXFP2 receptor function. Ann. N.Y. Acad. Sci. 1160, 67–73 [DOI] [PubMed] [Google Scholar]
- 45. Park J. I., Semyonov J., Yi W., Chang C. L., Hsu S. Y. (2008) Regulation of receptor signaling by relaxin A chain motifs. Derivation of pan-specific and LGR7-specific human specific analogs. J. Biol. Chem. 283, 32099–32109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whittaker J., Whittaker L. J., Roberts C. T., Jr., Phillips N. B., Ismail-Beigi F., Lawrence M. C., Weiss M. A. (2012) α-Helical element at the hormone-binding surface of the insulin receptor functions as a signaling element to activate its tyrosine kinase. Proc. Natl. Acad. Sci. U.S.A. 109, 11166–11171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang K., Chan S. J., Hua Q. X., Chu Y. C., Wang R. Y., Klaproth B., Jia W., Whittaker J., De Meyts P., Nakagawa S. H., Steiner D. F., Katsoyannis P. G., Weiss M. A. (2007) The A-chain of insulin contacts the insert domain of the insulin receptor. Photo-cross-linking and mutagenesis of a diabetes-related crevice. J. Biol. Chem. 282, 35337–35349 [DOI] [PubMed] [Google Scholar]
- 48. Hossain M. A., Wade J. D. (2010) The roles of the A- and B-chains of human relaxin-2 and -3 on their biological activity. Curr. Protein Pept. Sci. 11, 719–724 [DOI] [PubMed] [Google Scholar]
- 49. Hossain M. A., Man B. C., Zhao C., Xu Q., Du X. J., Wade J. D., Samuel C. S. (2011) H3 relaxin demonstrates antifibrotic properties via the RXFP1 receptor. Biochemistry 50, 1368–1375 [DOI] [PubMed] [Google Scholar]