Background: Differences in ligand potency and selectivity are observed between human and mouse FFA2 and FFA3 orthologs.
Results: Potency differences result from differential constitutive activity between species.
Conclusion: An “ionic lock” between extracellular loop 2 and the ligand binding pocket regulates constitutive activity.
Significance: Understanding species differences in FFA2 and FFA3 function is critical to future studies with these receptors.
Keywords: 7-Helix Receptor, Fatty Acid, G Protein-coupled Receptors (GPCR), Heterotrimeric G Proteins, Signal Transduction, Constitutive Activity, Species Orthologs
Abstract
Free fatty acid receptors 2 and 3 (FFA2 and FFA3) are G protein-coupled receptors for short chain free fatty acids (SCFAs). They respond to the same set of endogenous ligands but with distinct rank-order of potency such that acetate (C2) has been described as FFA2-selective, whereas propionate (C3) is non-selective. Although C2 was confirmed to be selective for human FFA2 over FFA3, this ligand was not selective between the mouse orthologs. Moreover, although C3 was indeed not selective between the human orthologs, it displayed clear selectivity for mouse FFA3 over mouse FFA2. This altered selectivity to C2 and C3 resulted from broad differences in SCFAs potency at the mouse orthologs. In studies to define the molecular basis for these observations, marked variation in ligand-independent constitutive activity was identified using a [35S]GTPγS assay. The orthologs with higher potency for the SCFAs, human FFA2 and mouse FFA3, displayed high constitutive activity in this assay, whereas the orthologs with lower potency for the agonist ligands, mouse FFA2 and human FFA3, did not. Sequence alignments of the second extracellular loop identified single negatively charged residues in FFA2 and FFA3 not conserved between species and predicted to form ionic lock interactions with arginine residues within the FFA2 or FFA3 agonist binding pocket to regulate constitutive activity and SCFA potency. Reciprocal mutation of these residues between species orthologs resulted in the induction (or repression) of constitutive activity and in most cases also yielded corresponding changes in SCFA potency.
Introduction
Variations in the function, pharmacology, and regulation of species orthologs of proteins can provide considerable insight into structural elements and even individual amino acids which play key roles in defining the level of activity of the protein under study. G protein-coupled receptors (GPCRs)2 are the largest family of transmembrane signal transducing polypeptides (1), and because of their contribution to the control of key physiological systems they are the most targeted group of proteins for the development of small molecule therapeutic medicines (2, 3). The need to assess the function and efficacy of potential new medicines in a number of species and in animal models that inform on the likely impact of such ligands in human disease means that comparisons between species orthologs of a GPCR are commonplace within the drug development process (4, 5). Given the importance of rodent species to such analyses, then direct comparisons between human and either mouse and/or rat orthologs is a routine starting point (4–6).
In recent times it has become apparent that a number of endogenously produced molecules that were hitherto regarded only as metabolic intermediates are able to produce a wide range of effects via activation of GPCRs. Among such ligands are free fatty acids. A group of three related GPCRs, initially designated GPR40, GPR43, and GPR41, are now named, respectively FFA1, FFA2, and FFA3 (7). FFA1 responds selectively to medium and longer-chain free fatty acids and is considered an interesting potential therapeutic target for the treatment of diabetes (7–9). Indeed, an agonist at this receptor is currently undergoing late stage clinical trials (10). By contrast, both FFA2 and FFA3 are activated selectively by a common group of short chain free fatty acids (SCFAs) (7, 8, 11). These receptors are also attracting considerable interest and are currently being considered as novel therapeutic targets to treat metabolic disorders including diabetes and adiposity as well as to modulate inflammatory processes (7, 8, 11, 12). Although FFA2 and FFA3 are activated by the same group of SCFAs with chain length C1-C5, there is a distinct rank order of potency that has been established for human FFA2 and FFA3. Here, at FFA2 C2 = C3 > C4 > C5 = C1, whereas at FFA3 C3 = C4 = C5 > C2 > C1 (11). On the basis that acetate is reported to be some 20-fold more potent at human FFA2 than human FFA3, whereas propionate is essentially equipotent at the two receptors (13, 14), acetate is often used as a FFA2 selective ligand (15, 16), and effects of acetate in animal models are sometimes considered to reflect specific activation of FFA2 (15, 16). This is despite a limited data set on direct comparisons of ligand potency at human versus rodent FFA2 or FFA3, which suggests that the C1-C5 fatty acids are in general some 3–5-fold more potent at rat FFA3 than the human ortholog (17).
During studies to define the relative potencies of both endogenous SCFAs and a series of synthetic small carboxylic acids (SCAs) at human and rodent FFA2 and FFA3, we noted marked variation in constitutive, ligand-independent activity between the human and rodent forms. We have now examined this systematically, explored the molecular basis, and provide evidence that orthologs of both FFA2 and FFA3 with low constitutive activity likely form an extracellular ionic lock between a key arginine residue(s) of the orthosteric SCFA binding pocket and an acidic residue in the second extracellular loop, whereas those with high constitutive activity lack this potential. Confirmation of this concept was provided by the capacity to interchange the extent of constitutive activity by breaking or engineering such a lock.
EXPERIMENTAL PROCEDURES
Materials and Compounds
Tissue culture reagents were from Invitrogen. Molecular biology enzymes and reagents were from Promega (Southampton, UK). The SCFAs (formic acid (C1), acetic acid (C2), propionic acid (C3), and butyric acid (C4)) and the SCAs (1-methylcyclopropanecarboxylic acid (1-MCPC) and trans-2-methylcrotonic acid (tiglic acid)) were purchased from Sigma. 4-Chloro-α-(1-methylethyl)-N-2-thiazolylbenzeneacetamide (4-CMTB) was synthesized as described previously (18). The radiochemical [35S]GTPγS was from PerkinElmer Life Sciences. All other experimental reagents were from Sigma.
Plasmids and Site-directed Mutagenesis
Human (h) FFA2 or FFA3 was fused via the C terminus to enhanced yellow fluorescent protein (eYFP) and subcloned into the pcDNA5/FRT/TO plasmid (Invitrogen) as described previously (19). Equivalent C-terminal eYFP-fused mouse (m) and rat (r) FFA2 and FFA3 constructs were generated by first PCR amplifying the desired receptor sequence without its stop codon from either mouse or rat genomic DNA (BioLine, London, UK). The PCR primers used incorporated either a HindIII (m- and rFFA2) or AflII (m- and rFFA3) site immediately upstream of the receptor sequence as well as an EcoRV site immediately after the receptor sequence. PCR products were then ligated into the hFFA2-eYFP pcDNA5/FRT/TO construct that had been cut with either HindIII/EcoRV (m- and rFFA2) or AflII/EcoRV (m- and rFFA3) to remove the hFFA2 sequence, and each of the resulting plasmids had its sequence verified. Primers used were: mFFA2, forward (AAGGAAGCTTATGACCCCAGACTGGCAC) and reverse (GGGGGATATCCTCGGTGACAAATTCAGAACTCT); mFFA3 (forward GGGGCTTAAGATGGGGACAAGCTTCTTTCTT) and reverse (CCTTGATATCGCTCGGACACTCCTTGGAT); rFFA2, forward (AAGGAAGCTTATGACCCCAGACTGGCAC) and reverse (AAAAGATATCCTCGGTGACAAAGTCAGAGC); rFFA3 (forward GGGGCTTAAGATGGACACAAGCTTCTTTCCC) and reverse (CCAAGATATCGCTCGGACATTCCTTGGA). Site-directed mutagenesis to generate hFFA2-G159E, hFFA3-D158N, mFFA2-E159G, and mFFA3-N154D was carried out according to the QuikChange method (Stratagene). In each case the mutation was incorporated into the eYFP-tagged form of the receptor encoded in the pcDNA5/FRT/TO plasmid.
Synthesis of (S)-3-(2-(3-Chlorophenyl)acetamido)-4-(4-(trifluoromethyl)phenyl)butanoic acid (CATPB)
A solution of 2-(3-chlorophenyl)acetic acid (72 mg, 0.42 mmol), 1-hydroxybenzotriazole (58 mg, 0.46 mmol), and diisopropylethylamine (0.17 ml, 0.96 mmol) in dry CH2Cl2 (3 ml) at 0 °C was treated with N,N′-diisopropylcarbodiimide (0.071 ml, 0.46 mmol) and stirred for 30 min. A solution of (S)-methyl 3-amino-4-(4-(trifluoromethyl)phenyl)butanoate (100 mg, 0.38 mmol) in CH2Cl2 (1 ml) was then added, and the reaction mixture was stirred for 12 h at room temperature. The reaction mixture was diluted with CH2Cl2 (10 ml) and washed with brine. The organic phase was dried (Na2SO4) and concentrated. The crude product was purified by column chromatography (SiO2, 30% ethyl acetate in petroleum ether) to obtain (S)-methyl 3-(2-(3-chlorophenyl)acetamido)-4-(4-(trifluoromethyl)phenyl)butanoate (47 mg, 30%) as a white solid: 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 8.0 Hz, 2H), 7.29–7.23 (m, 2H), 7.20–7.16 (m, 3H), 7.05–7.01 (m, 1H), 6.09 (d, J = 8.4 Hz, 1H), 4.51–4.42 (m, 1H), 3.66 (s, 3H), 3.45 (s, 2H), 2.93 (dd, J = 13.7, 7.4 Hz, 1H), 2.84 (dd, J = 13.7, 7.2 Hz, 1H), 2.56–2.44 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 171.8, 169.5, 141.4, 136.5, 134.6, 130.1, 129.5, 129.3, 129.2, 128.9, 127.5, 127.4, 125.5, 125.4, 122.8, 51.8, 47.2, 43.4, 39.4, 36.9.
To a solution of the methyl ester (43 mg, 0.10 mmol) in THF (1.5 ml), aqueous lithium hydroxide (9 mg, 0.25 mmol, 0.5 ml) was added at 0 °C, and the reaction mixture was stirred for overnight at room temperature. The reaction mixture was diluted with ethyl acetate and extracted. The aqueous layer was neutralized with 2 n HCl and extracted with CH2Cl2 (3 × 5 ml). The combined organic layers were dried (Na2SO4) and concentrated under reduced pressure to procure the target compound CATPB (39 mg, 94%) as a white solid: 1H NMR (400 MHz, DMSO-d6) δ 8.13 (d, J = 8.4 Hz, 1H), 7.56 (d, J = 8.1 Hz, 2H), 7.31 (d, J = 7.9 Hz, 2H), 7.27–7.25 (m, 2H), 7.20 (br s, 1H), 7.07–7.03 (m, 1H), 4.27–4.21 (m, 1H), 2.89 (dd, J = 13.4, 4.7 Hz, 1H), 2.76 (dd, J = 13.4, 8.7 Hz, 1H), 2.47–2.36 (m, 2H); 13C NMR (101 MHz, DMSO-d6) δ 172.1, 168.8, 143.3, 138.6, 132.5, 129.9, 129.7, 128.7, 127.5, 126.1, 124.7, 47.1, 41.7, 39.1, 38.9. High resolution mass spectrometry calculated for C19H17ClF3NO3 (M+Na)+ was 422.0747; found was 422.0741.
Cell Culture and Generation of Flp-InTM T-RExTM 293 Cell Lines
All cells were maintained in Dulbecco's modified Eagle's medium without sodium pyruvate supplemented with 10% dialyzed fetal bovine serum, 1× penicillin/streptomycin mixture (Sigma Aldrich), and 5 μg/ml blasticidin at 37 °C in a humidified atmosphere containing 5% CO2. Inducible Flp-InTM T-RExTM 293 cells were generated for hFFA2-eYFP and hFFA3-eYFP as described previously (19). Inducible Flp-InTM T-RExTM 293 cell lines for mFFA2, mFFA3, rFFA2, rFFA3, hFFA2-G159E, hFFA3-D158N, mFFA2-E159G, and mFFA3-N154D were generated by co-transfecting each pcDNA5/FRT/TO plasmid containing the desired receptor with the pOG44 plasmid, encoding the Flp recombinase enzyme (Invitrogen) into Flp-InTM T-RExTM 293 cells using Lipofectamine (Invitrogen). 48 h after the transfection hygromycin B (200 μg/ml) was added to the culture medium, allowing for polyclonal selection of stable doxycycline (Dox)-inducible cell lines. Inducible expression was verified in each cell line by fluorescence microscopy for eYFP after 24 h of treatment with 500 ng/ml Dox.
[35S]GTPγS Incorporation Assay
Cell membrane preparations were made from Flp-InTM T-RExTM 293 cells either uninduced or treated with Dox (500 ng/ml unless otherwise indicated) to induce receptor expression, as described previously (19). [35S]GTPγS binding experiments were performed according to a previously described method (18). Briefly, cell membrane preparations containing 5 or 10 μg of protein were added to assay buffer (50 mm Tris-HCl, pH 7.4, 10 mm MgCl2, 100 mm NaCl, 1 mm EDTA, 1 μm GDP, and 0.1% fatty acid-free bovine serum albumin) containing the appropriate concentrations of ligand and allowed to reach equilibrium by preincubating for 15 min at 25 °C. To initiate the assay, 50 nCi of [35S]GTPγS was added to each tube, and the reaction was terminated by rapid filtration through GF/C glass filters using a 24-well Brandel cell harvester (Alpha Biotech, Glasgow, UK) after 1 h of incubation at 25 °C. Unbound radioligand was washed from filters by 3 washes with ice-cold wash buffer (50 mm Tris-HCl, pH 7.4, and 10 mm MgCl2) before the remaining bound [35S]GTPγS was measured by liquid scintillation spectrometry.
Relative Receptor Expression Assessed as eYFP Fluorescence
Relative receptor expression levels in isolated membrane preparations were quantified based on eYFP expression. For this, membranes containing 2.5 μg of protein in 50 μl of [35S]GTPγS assay buffer were dispensed in triplicate into a black 384-well plate. Fluorescence was measured from these samples using a PolarStar Omega plate reader (BMG Labtech) fitted with 500/10-nm excitation and 530/10-nm emission filters.
Immunoblot for Gαi/o Expression
Cell membrane preparations from Flp-InTM T-RExTM 293 cells induced to express the receptor of interest were separated by SDS-PAGE and transferred to nitrocellulose membranes. After blocking with 5% nonfat milk, membranes were incubated overnight at 4 °C with primary antibodies generated in house against either Gαi1/2, Gαi3, or Gαo. Membranes were washed then incubated with an HRP-conjugated secondary antibody for 1 h at room temperature. After a final wash, membranes were incubated in an enhanced chemiluminescent substrate (Thermo Scientific, Erembodegem, Belgium) and exposed to x-ray film, which was subsequently developed using a Kodak X-Omat developer.
Data Analysis and Curve-fitting
All data presented represent the means ± S.E. of at least three independent experiments. Data analysis and curve-fitting was carried out using the Graphpad Prism software package v5.0b. Concentration-response data were fit to three parameter sigmoidal concentration-response curves. To include basal unstimulated values on these curves, basal values were assigned concentrations one log unit lower than the lowest tested concentration of agonist. All statistical analysis of curve fit parameters was carried out by independently fitting the data from triplicate experiments and comparing the resulting curve fit values by t test or one-way analysis of variance as appropriate.
RESULTS
Short Chain Fatty Acids Display Distinct Selectivity at Human and Mouse Orthologs of FFA2 and FFA3
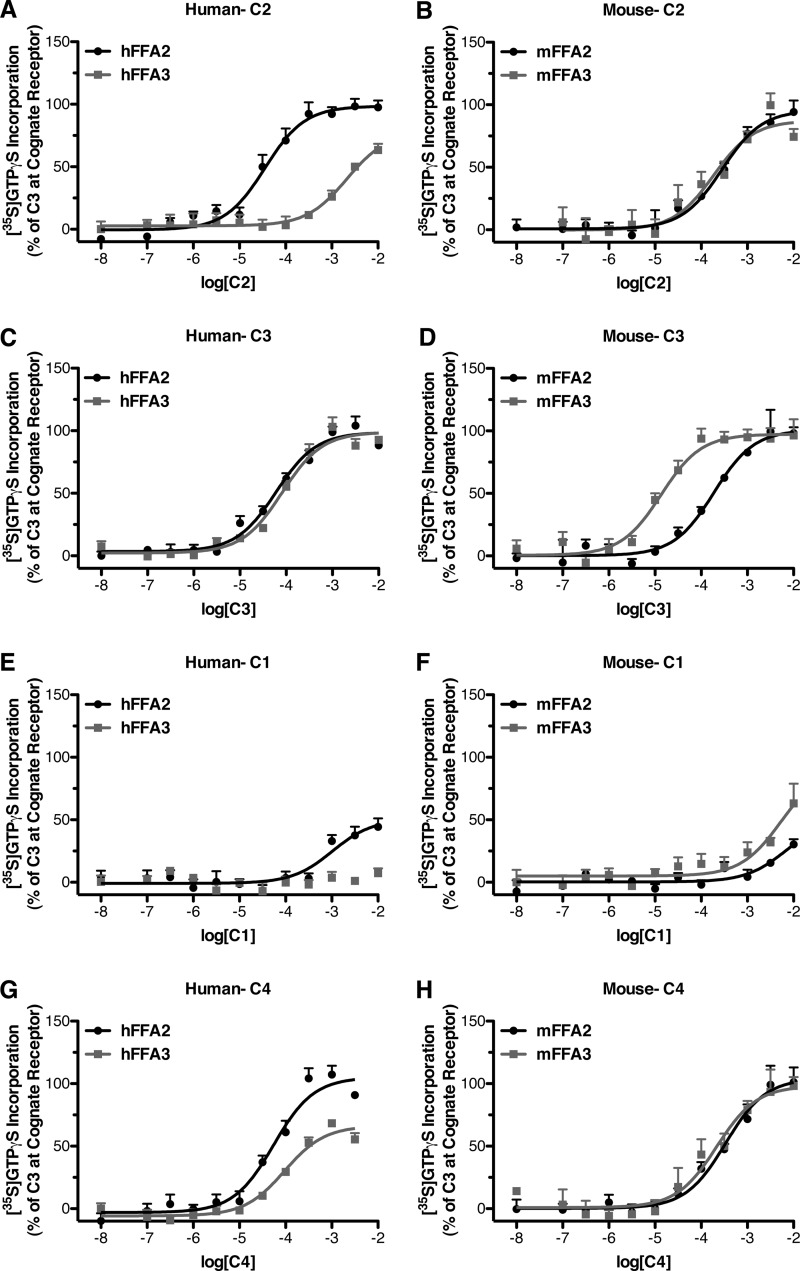
We first set out to explore whether the reported differences in the potency and resulting selectivity of the SCFA acetate (C2) to activate human FFA2 over FFA3 (14) are maintained across other species and to define, therefore, whether C2 can be employed usefully as a selective and discriminating ligand in non-human cells and tissues. Because both FFA2 and FFA3 are able to interact with and activate pertussis toxin-sensitive G proteins (7, 8) a [35S]GTPγS incorporation assay was used to study the activation of such Gi-family G proteins by human (h) and mouse (m) orthologs of FFA2 and FFA3 expressed in Flp-InTM T-RExTM 293 cells. These cells allow for Dox-mediated inducible expression of the receptor of interest. Therefore, for this assay cell membranes were harvested from cells after 24 h of treatment with Dox (500 ng/ml) to induce expression of the desired FFA2 or FFA3 ortholog. When tested in the [35S]GTPγS assay, we confirmed that as published previously (14), C2 was significantly more potent (p < 0.05) with hFFA2 than hFFA3 (Fig. 1A; Table 1). By contrast, C2 was equipotent with mFFA2 and mFFA3 (Fig. 1B; Table 1). We extended these studies to determine if other endogenously generated SCFAs display differences in selectivity between the human and mouse orthologs. As previously reported, propionate (C3) was essentially equipotent and non-selective at hFFA2 and hFFA3 (Fig. 1C; Table 1). However, when C3 was assessed at the murine orthologs, it was found to be significantly more potent (p < 0.05), and as a result ∼12-fold selective for mFFA3 over mFFA2 (Fig. 1D; Table 1). As anticipated, formate (C1) displayed only weak activity at hFFA2 and was essentially inactive at hFFA3 (Fig. 1E; Table 1). In contrast, at the mouse orthologs C1 showed weak activity at both mFFA2 and mFFA3 (Fig. 1F; Table 1). Finally, butyrate (C4) was approximately equipotent and, therefore, non-selective at both the human (Fig. 1G; Table 1) and mouse (Fig. 1H; Table 1) orthologs of FFA2 and FFA3.
FIGURE 1.
The selectivity of SCFAs for human FFA2 versus FFA3 is not preserved at the murine orthologs. Flp-InTM T-RExTM 293 cells harboring C-terminally eYFP-tagged forms of human (A, C, E, and G) or murine (B, D, F, and H) FFA2 or FFA3 were treated with Dox (500 ng/ml, 24 h) to induce expression of the appropriate receptor. Membranes generated from these cells were used to measure [35S]GTPγS incorporation in response to various concentrations of the SCFAs: C2 (A and B), C3 (C and D), C1 (E and F), or C4 (G and H). Data are presented as a percentage of the response to a maximally effective concentration of C3.
TABLE 1.
Potency and selectivity of endogenous SCFAs ligands at human and mouse orthologs of FFA2 and FFA3
NR, no response.
| Human |
Mouse |
Ortholog selectivitya |
||||||
|---|---|---|---|---|---|---|---|---|
| hFFA2b | hFFA3b | Selectc | mFFA2b | mFFA3b | Selectc | FFA2 | FFA3 | |
| C1 | 2.99 ± 0.23 | NR | 2.17 ± 0.43 | 2.28 ± 0.34 | −0.11 | 0.82 | ||
| (50 ± 7) | (50 ± 24) | (91 ± 30) | ||||||
| C2 | 4.45 ± 0.09 | 2.68 ± 0.18 | 1.77 | 3.55 ± 0.11 | 3.73 ± 0.20 | −0.18 | 0.90 | −1.05 |
| (99 ± 3) | (78 ± 10) | (96 ± 5) | (87 ± 8) | |||||
| C3 | 4.22 ± 0.08 | 4.09 ± 0.08 | 0.13 | 3.73 ± 0.09 | 4.87 ± 0.12 | −1.14 | 0.49 | −0.78 |
| (100) | (100) | (100) | (100) | |||||
| C4 | 4.26 ± 0.10 | 4.02 ± 0.10 | 0.24 | 3.47 ± 0.12 | 3.67 ± 0.17 | −0.2 | 0.79 | 0.35 |
| (105 ± 6) | (66 ± 3) | (104 ± 6) | (98 ± 8) | |||||
a Ortholog selectivity represents: human pEC50 − mouse pEC50.
b pEC50 values are reported with Emax expressed as a percentage of the C3 response in parenthesis.
c Selectivity represents comparisons within species of: FFA2 pEC50 − FFA3 pEC50.
Analysis of the potency and selectivity results (Table 1) indicated that the shortest chain length SCFAs, C1, C2, and C3 were all distinctly more potent at mFFA3 compared with hFFA3. In contrast, all four SCFAs were more potent at hFFA2 compared with mFFA2. Importantly, these results indicate that although C2 may be useful to selectively probe hFFA2 function versus hFFA3, this is not true for mFFA2. In addition, although C3 is the most selective ligand at the mouse receptors, none of the SCFAs possess sufficient selectivity to realistically be useful for specifically interrogating the function of FFA2 or FFA3 in murine systems.
FFA2 and FFA3 Selectivity of Small Carboxylic Acids and an Allosteric Agonist Are Maintained across the Human and Mouse Orthologs
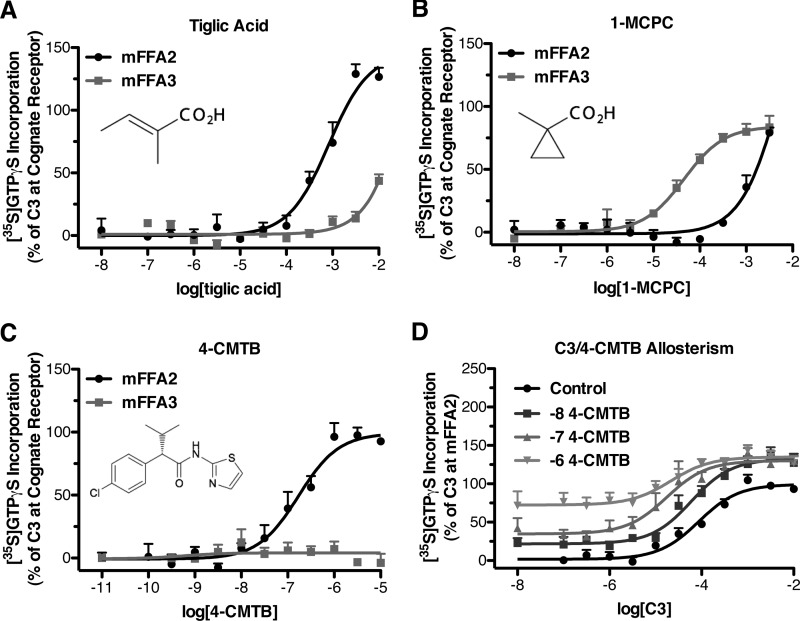
We next set out to define the molecular basis of the species differences we observed in potency and selectivity between FFA2 and FFA3. For this it was first considered whether there may be fundamental differences between the orthosteric binding sites of the human and mouse orthologs of these receptors. We recently described a series of SCAs that are selective orthosteric activators of either hFFA2 or hFFA3 (14). The described hFFA2 selective ligand, trans-2-methylcrotonic acid (tiglic acid) was also markedly selective (∼40-fold) for mFFA2 over mFFA3 (Fig. 2A), with a pEC50 of 3.08 ± 0.10 at mFFA2 compared with <1.5 at mFFA3. A second SCA 1-MCPC, previously shown to be somewhat selective for hFFA3 over hFFA2 (14), also retained marked selectivity for the mFFA3 ortholog (Fig. 2B; pEC50 of 4.34 ± 0.11 at mFFA3 compared with 2.22 ± 0.37 at mFFA2).
FIGURE 2.
Selective orthosteric and an allosteric ligand for human FFA2 and FFA3 retain selectivity at the murine orthologs. Flp-InTM T-RExTM 293 cells harboring C-terminally eYFP-tagged forms of mFFA2 or mFFA3 were treated with Dox (500 ng/ml) to induce receptor expression. Membranes produced from these cells were used to measure [35S]GTPγS incorporation in response to various ligands shown previously to be selective for hFFA2 (A and C) or hFFA3 (B). The ago-allosteric behavior of 4-CMTB was preserved at murine FFA2 (D).
In addition to these orthosteric FFA2- and FFA3-selective SCAs, a hFFA2-selective allosteric agonist, 4-CMTB, has also been reported (18, 20). Having observed differences in potency and selectivity of the orthosteric SCFAs, we considered whether differences might be observed with this compound, as it is often suggested that allosteric binding sites are less subject to evolutionary pressures than the orthosteric site and, therefore, may be less likely to be conserved. Despite this, we demonstrated that 4-CMTB was also a highly selective agonist for mFFA2 (pEC50 = 6.77 ± 0.12) while showing no measurable activity at mFFA3 (Fig. 2C). At hFFA2, 4-CMTB is reported to be an ago-allosteric ligand (18, 20), whereby not only does it act as a direct FFA2 agonist, but also its co-addition allosterically enhances the potency of C3. We confirmed the allosteric properties of 4-CMTB at mFFA2 by conducting C3 concentration-response curves in the presence of a set of increasing fixed concentrations of 4-CMTB (Fig. 2D). The measured pEC50 values for C3 were 4.07 ± 0.09, 4.22 ± 0.12, 4.76 ± 0.19, and 4.67 ± 0.24 when in the presence of 0, 0.01, 0.1, and 1 μm 4-CMTB, respectively. Together with our findings using the SCAs tiglic acid and 1-MCPC, these results with 4-CMTB suggest that each of these compounds could potentially be useful to selectively probe mFFA2 or mFFA3 function.
Human and Mouse Orthologs of FFA2 and FFA3 Display Different Patterns of Constitutive Activity in the [35S]GTPγS Assay
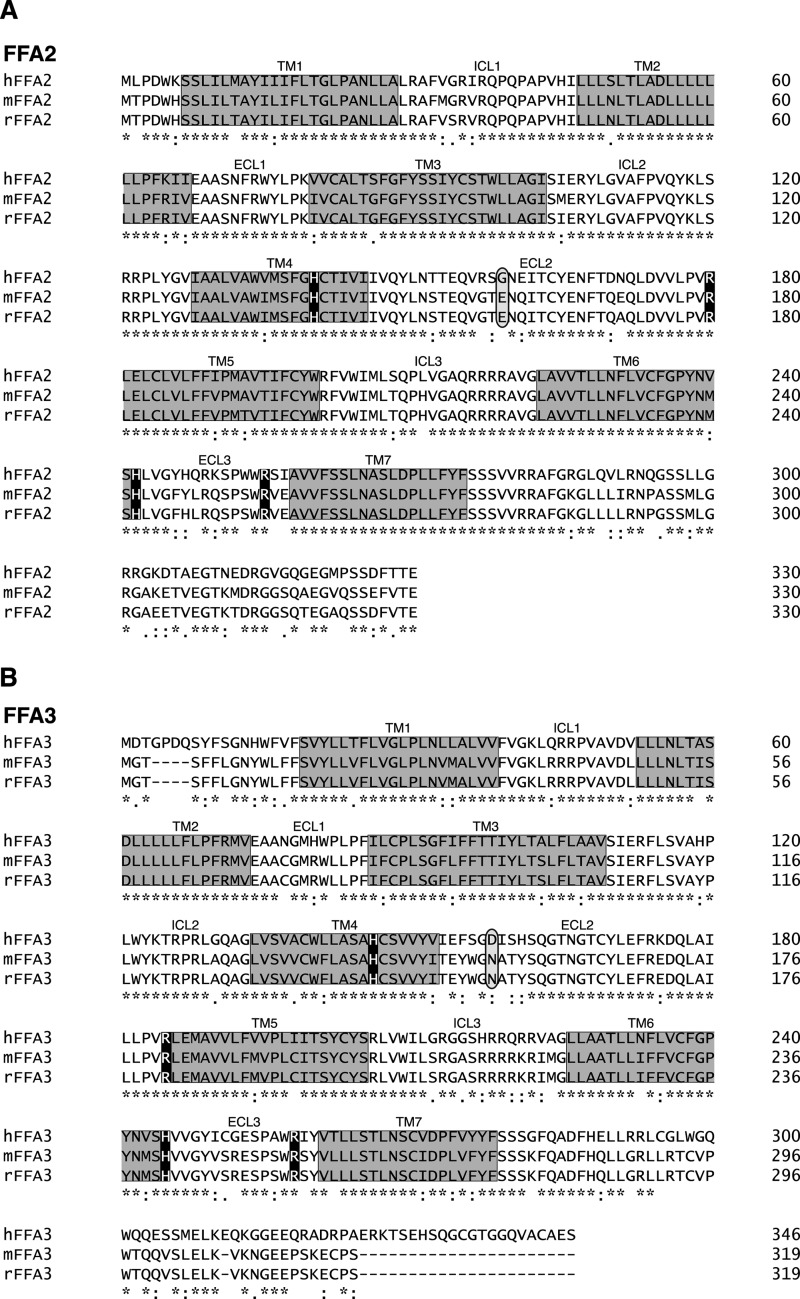
To further examine the molecular basis for the higher potency of the SCFAs at hFFA2 and mFFA3 relative to their mFFA2 and hFFA3 counterparts, sequence alignments of the human, mouse, and rat orthologs were constructed for FFA2 (Fig. 3A) and FFA3 (Fig. 3B). These revealed a high degree of sequence identity between human and mouse orthologs of FFA2 (84%) and FFA3 (75%). Importantly, each of the four key positively charged residues previously reported to facilitate SCFA binding to hFFA2 and hFFA3 (19) were fully conserved across all three species. This, in combination with the fact that the selectivity of the SCAs tiglic acid and 1-MCPC is maintained in the mouse orthologs likely indicates that a fundamental difference in the binding site is not responsible for the observed species differences. We, therefore, next examined whether the species differences in SCFA potency might relate to differing levels of constitutive activity in the species orthologs of these receptors. We recently demonstrated using a [35S]GTPγS assay that hFFA2 is a constitutively active receptor (21), and although nothing was known about the constitutive activity of hFFA3, mFFA2, or mFFA3 given that agonists bind preferentially to the active state of a receptor, we hypothesized that altered constitutive activity might account for the potency differences we observed between the human and mouse orthologs of FFA2 and FFA3.
FIGURE 3.
Sequence alignments of human and rodent orthologs of FFA2 and FFA3. Sequences for human, mouse, and rat FFA2 (A) and FFA3 (B) were aligned. Predicted transmembrane domains are boxed in gray. Key positively charged amino acids known to define the orthosteric binding site for SCFAs are in black boxes, whereas non-conserved negatively charged amino acids in extracellular loop 2 that were selected for detailed study are circled.
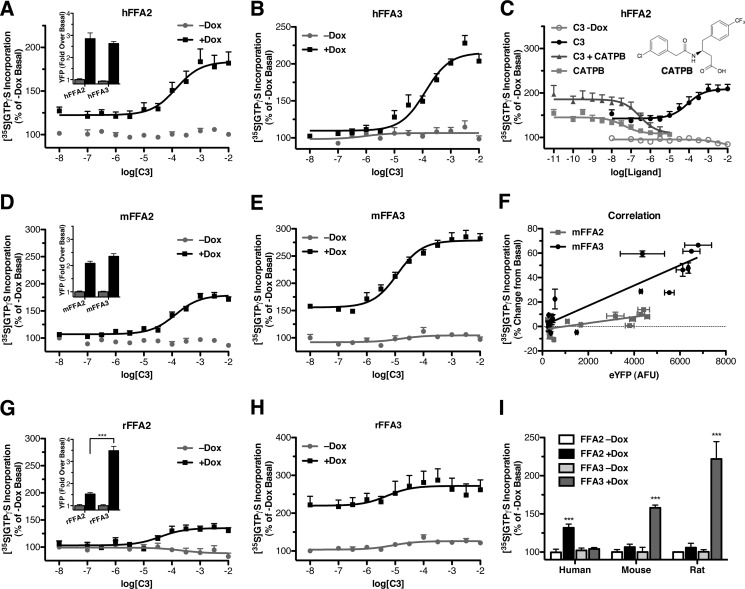
To examine constitutive activity in these receptors, [35S]GTPγS incorporation was measured in membranes produced from Flp-InTM T-RExTM 293 cells induced with Dox to express the receptor of interest and compared with membranes from uninduced cells that, therefore, do not express the receptor but are otherwise equivalent. Initially, we aimed to compare the levels of constitutive activity in hFFA2 with those of hFFA3; however, it was noted that using a maximum (500 ng/ml) concentration of Dox to induce receptor expression resulted in substantially greater levels of hFFA2 than hFFA3.3 Because expression levels may greatly affect observed constitutive activity, the Dox concentration was, therefore, reduced for hFFA2 down to 4 ng/ml so as to generate hFFA2 expression that was not significantly different from that of hFFA3 (Fig. 4A, inset). When examining [35S]GTPγS incorporation in hFFA2 expressing cell membranes (Fig. 4A), C3 produced a concentration-dependent increase in [35S]GTPγS incorporation only in the Dox (4 ng/ml)-induced membranes, consistent with an effect via hFFA2. More importantly, the ligand-independent, basal level of [35S]GTPγS incorporation was also significantly greater (32 ± 4%; p < 0.001) after hFFA2 induction, consistent with hFFA2 displaying marked constitutive activity. In contrast, when similar experiments were carried out using membranes generated from cells able to induce expression of hFFA3, Dox (500 ng/ml) induction of the receptor had no effect on basal [35S]GTPγS incorporation (p > 0.05) (Fig. 4B). This did not reflect poor expression after Dox induction given that, as measured by eYFP, hFFA3 expression was equivalent to that of hFFA2 (Fig. 1A), nor did it reflect a functional defect in hFFA3, as C3 did generate a robust concentration-dependent increase. These data suggest that hFFA2 but not hFFA3 displays marked constitutive activity in the [35S]GTPγS binding assay. To further confirm that the higher level of basal [35S]GTPγS incorporation after hFFA2 induction did in fact reflect constitutive activity of the receptor, we explored the effect of a previously described FFA2 antagonist (22) compound CATPB (Fig. 4C). In the [35S]GTPγS assay CATPB acted as an antagonist inhibiting the response to an EC80 concentration of C3 in a concentration-dependent manner if cells were induced with Dox (500 ng/ml; pIC50 = 6.54 ± 0.24). Interestingly, in these experiments CATPB reduced [35S]GTPγS incorporation to below the +Dox basal level, approaching the levels observed in receptor uninduced −Dox membranes. Such an observation is consistent with CATPB acting as an inverse agonist at hFFA2, and therefore, we also examined the effects of CATPB on +Dox (500 ng/ml) membranes expressing hFFA2 but without the addition of C3. Once again CATPB reduced basal [35S]GTPγS incorporation to levels approaching those from −Dox membranes in a concentration-dependent manner (pIC50 = 7.38 ± 0.26) and with potency higher than when competing with an EC80 concentration of C3 (p < 0.05). Together these observations indicate that hFFA2 displays substantial constitutive activity and that CATPB is an inverse agonist at this receptor.
FIGURE 4.
Variation in constitutive activity between human and rodent orthologs of FFA2 and FFA3. Flp-InTM T-RExTM 293 cells harboring C-terminally eYFP-tagged forms of human (A and C), mouse (D and F), or rat (G and H) FFA2 or FFA3 were maintained with or without Dox. Relative expression levels for the human, mouse, and rat orthologs of FFA2 and FFA3 without (gray bars) or with (black bars) Dox induction was assessed by eYFP fluorescence and is shown as insets to A, D, and G, respectively. Membranes from these cells were used to measure [35S]GTPγS incorporation in response to various concentrations of C3. In A, B, D, E, G, and H, direct comparisons are made between membranes of uninduced cells and those induced to express FFA2 or FFA3; the point on each curve corresponding to a log[C3] of −8 represents data from basal unstimulated membranes. In C the ability of compound CATPB (structure inserted) to inhibit either basal (squares) or C3-stimulated (triangles) incorporation of [35S]GTPγS in membranes expressing hFFA2 is shown. In F, the extent of expression of mFFA2 (light squares) or mFFA3 (dark circles) was controlled by induction of cells with varying concentrations of Dox. In I, the relative extent of constitutive activity of human, mouse, and rat orthologs of FFA2 and FFA3 is shown.
Levels of constitutive activity were next examined for the mouse orthologs of FFA2 and FFA3. Unlike the human receptor cell lines where different Dox concentrations were required to obtain equivalent expression levels, maximum Dox concentrations (500 ng/ml) yielded similar levels of mFFA2 and mFFA3 expression (Fig. 4D, inset). When [35S]GTPγS incorporation assays were conducted on membranes of mFFA2 cells grown in the absence or presence of Dox (500 ng/ml), C3 again produced a concentration-dependent increase only after Dox-induced mFFA2 expression (Fig. 4D). However, unlike hFFA2, membranes induced to express mFFA2 displayed no significant ligand-independent [35S]GTPγS incorporation compared with their −Dox controls. Unfortunately, it was not possible to explore this further using CATPB, as this ligand is a species ortholog-selective antagonist for hFFA2.3 Similar experiments were then conducted on membranes derived from cells harboring mFFA3 and once again C3 increased [35S]GTPγS incorporation only in membranes of cells that had been pretreated with Dox (500 ng/ml) (Fig. 4E). Interestingly, unlike mFFA2, mFFA3 did display significant ligand-independent activity, increasing [35S]GTPγS incorporation by 58 ± 4% from the uninduced basal level (p < 0.001), contrasting with the lack of significant constitutive activity noted for the hFFA3 ortholog. No antagonists of FFA3 have yet been reported that could be tested as inverse agonists; thus, to confirm the greater constitutive activity of mFFA3 relative to mFFA2, we induced cells harboring mFFA2 or mFFA3 with varying concentrations of Dox, collected membranes, and then either indirectly measured receptor expression via levels of eYFP fluorescence or assessed ligand-independent incorporation of [35S]GTPγS (Fig. 4F). Correlation of these two parameters resulted in linear increases in [35S]GTPγS binding with eYFP levels, but the slope of this correlation was significantly greater for mFFA3 than mFFA2 (p < 0.001), consistent with mFFA3 displaying higher levels of constitutive activity.
We extended our studies to determine if similar differences in constitutive activity were apparent in the rat orthologs of FFA2 and FFA3, which share 96 and 92% sequence identity with mFFA2 and mFFA3, respectively. However, this was complicated somewhat by the observation that Dox induction of rFFA2 resulted in significantly lower (p < 0.001) expression than that of rFFA3 (Fig. 4G, inset) When examining [35S]GTPγS incorporation into membranes derived from cells engineered to harbor rFFA2 or rFFA3 (Fig. 4, G and H), similar results were obtained as for the mouse orthologs. Specifically, no significant ligand-independent [35S]GTPγS incorporation was observed with rFFA2, whereas a significant (p < 0.001) 122 ± 22% increase over basal was observed with rFFA3. In addition, as noted for the mouse orthologs, C3 was selective for rFFA3 over rFFA2, yielding pEC50 values of 5.28 ± 0.69 and 4.41 ± 0.29, respectively. Despite broadly similar results between the rat and mouse orthologs, further experiments were carried out only on the mouse receptors due to the expression level difference observed between rFFA2 and rFFA3. Taken together, our basal [35S]GTPγS data across all three species orthologs (Fig. 4I) are consistent with higher constitutive activity of hFFA2, mFFA3, and rFFA3.
[35S]GTPγS Constitutive Activity and SCFA Potency Can Be Switched between hFFA2 and mFFA2 by Mutation of a Single Residue in Extracellular Loop 2
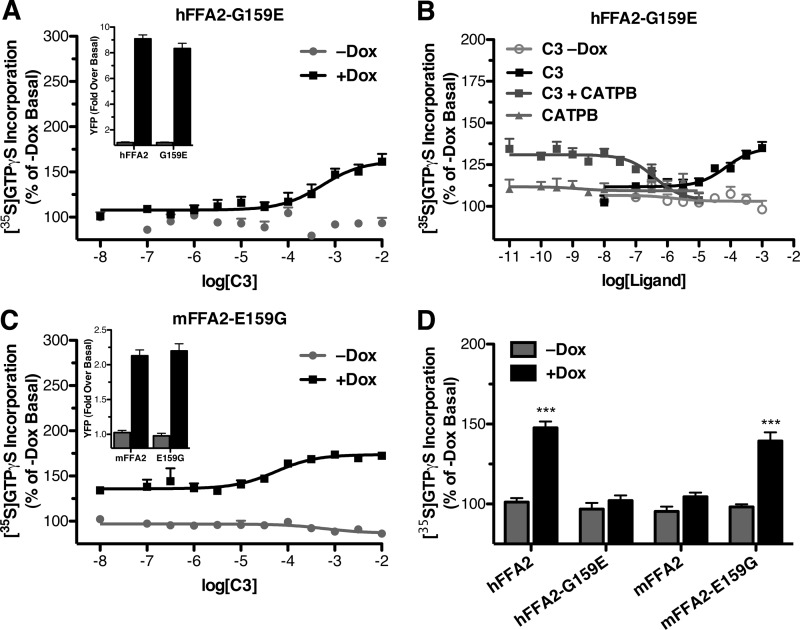
Having identified different patterns of constitutive activity in the human and rodent orthologs of FFA2 and FFA3, we next aimed to define the molecular basis for these differences and determine whether this contributes to the increased potency of SCFAs observed at hFFA2 relative to mFFA2. In the related long chain fatty acid receptor FFA1, previous studies suggested that a pair of ionic locks between arginine residues in transmembrane domains V and VII and glutamate residues in extracellular loop 2 (ECL2) constrain constitutive activity of the receptor (23). We, therefore, examined the aligned sequences of human, murine, and rat FFA2 (Fig. 3A) in an attempt to identify negatively charged residues in ECL2 not conserved between the human and rodent orthologs that would be consistent with such an ionic-lock mechanism accounting for the species differences in constitutive activity. From the alignment of FFA2, one residue was potentially consistent with such a model; Gly-159 in human corresponds to Glu-159 in both mouse and rat FFA2. To test whether Glu-159 might be forming an ionic lock in rodent FFA2, which is absent in hFFA2, mutagenesis was carried out to generate reciprocal hFFA2-G159E and mFFA2-E159G mutants that were then incorporated into Flp-InTM T-RExTM 293-inducible cell lines. Unlike wild type hFFA2, induced expression of hFFA2-G159E did not result in increased basal incorporation of [35S]GTPγS relative to the levels in uninduced membranes, although C3 did still produce a concentration-dependent response when the receptor was expressed (Fig. 5A). This difference could not be explained by decreased expression levels of the G159E mutant, as there was no difference in eYFP levels in membranes from cells induced to express hFFA2-G159E-eYFP compared with those expressing wild type hFFA2-eYFP (Fig. 5A, inset). To confirm the lack of constitutive activity of hFFA2-G159E, we took advantage of the inverse agonism properties of CATPB at hFFA2 and examined the effect of this compound to inhibit both an EC80 concentration of C3 and basal [35S]GTPγS incorporation (Fig. 5B). As was observed with wild type hFFA2, CATPB also inhibited the C3-dependent [35S]GTPγS incorporation at hFFA2-G159E (pIC50 = 6.40 ± 0.23). However, unlike with wild type, CATPB had no effect on basal [35S]GTPγS incorporation in membranes expressing hFFA2-G159E, further suggesting that this mutant had lost constitutive activity.
FIGURE 5.
Alterations of an extracellular loop 2 glutamate residue reciprocally modulates constitutive activity in human and murine FFA2. Reciprocal hFFA2-G159E and mFFA2-E159G mutants were generated, and Flp-InTM T-RExTM 293 cells harboring C-terminally eYFP-tagged forms of these were produced. Membranes were prepared from both uninduced and Dox-induced cells, and [35S]GTPγS binding assays were performed (A and B, human G159E; C, murine E159G FFA2). In A and C, the relative expression levels of wild type and G159E hFFA2-eYFP and of wild type and E159G mFFA2-eYFP are shown as insets (−Dox in gray bars, +Dox in black bars). In B, effects of compound CATPB on G159E human FFA2-eYFP are displayed. D shows mean constitutive activity measured from wild type and mutant forms of hFFA2 and mFFA2.
We next examined the reciprocal E159G mutant of mFFA2 to determine if it displayed a gain of constitutive activity (Fig. 5C). Consistent with the hypothesis, membranes from Dox-induced mFFA2-E159G cells not only responded to C3 in a concentration-dependent manner but also showed a clear, statistically significant, 41 ± 5% (p < 0.001) increase in basal [35S]GTPγS incorporation compared with membranes from uninduced cells. Again, this could not be attributed to differences in receptor expression levels, because as measured by eYFP fluorescence, mFFA2-E159G did not differ from wild type mFFA2 in expression (Fig. 5C, inset). It is, therefore, apparent that residue 159 in ECL2 dictates ligand-independent constitutive activity at these species orthologs of FFA2 (Fig. 5D).
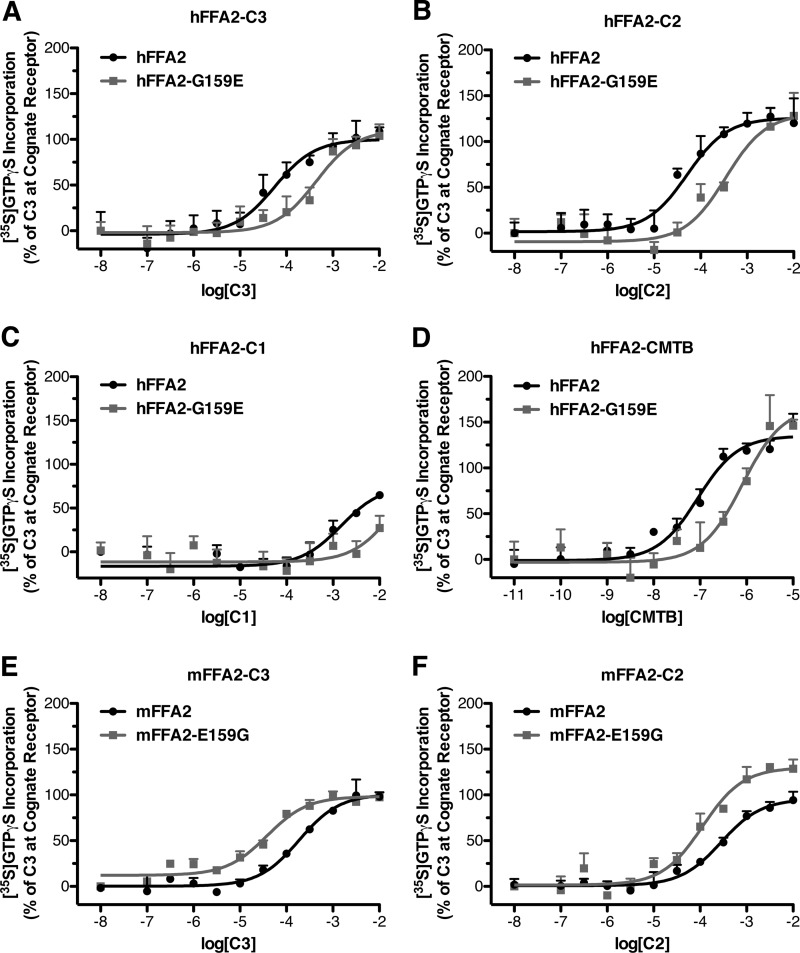
To test the possibility that the increased potency of SCFAs at hFFA2 relative to mFFA2 results from the noted constitutive activity differences, we compared the potency of FFA2 ligands at the wild type and non-constitutively active G159E hFFA2 mutant. In these experiments C3 displayed significantly reduced potency (p < 0.05) at the G159E mutant compared with wild type (pEC50 values = 4.25 ± 0.22 with wild type and 3.36 ± 0.19 with G159E) (Fig. 6A). Similarly, C2 was nearly 7 times more potent with wild type hFFA2 than with G159E hFFA2 (pEC50 values = 4.29 ± 0.19 and 3.46 ± 0.17, respectively) (Fig. 6B), whereas C1 was at least 10-fold more potent with wild type (pEC50 values = 2.83 ± 0.33 and <1.7) (Fig. 6C). The allosteric agonist 4-CMTB, although by definition binding to a different site than the SCFAs, also displayed significantly (p < 0.05) reduced potency (pEC50 values of 7.05 ± 0.12 with wild type hFFA2 and 6.09 ± 0.19 with the mutant) at the G159E mutant (Fig. 6E). The potencies of C3 and C2 were also assessed with the reciprocal mFFA2-E159G mutant, and in this case the constitutively active E159G mutant displayed significantly increased potency (p < 0.05) to both C3 (pEC50 values = 3.73 ± 0.09 with wild type and 4.42 ± 0.10 with E159G), and C2 (pEC50 values = 3.55 ± 0.11 with wild type and 3.95 ± 0.13 with E159G).
FIGURE 6.
A reciprocal gain/loss in potency of SCFAs at hFFA2 and mFFA2 is associated with their respective G159E/E159G mutations. Membranes from cells induced to express either wild type hFFA2 (dark circles) or hFFA2-G159E (light squares) were assessed for the potency of SCFAs; C3 (A), C2 (B), and C1 (C) as well as the FFA2 ago-allosteric ligand 4-CMTB (D) to stimulate [35S]GTPγS binding. Membranes from cells induced to express either wild type mFFA2 (dark circles) or mFFA2-E159G (light squares) were assessed in the [35S]GTPγS assay for the potency of SCFAs: C3 (E) and C2 (F).
Constitutive Activity in Human and Mouse FFA3 Is Also Modulated by Mutation of a Single Amino Acid Residue in ECL2
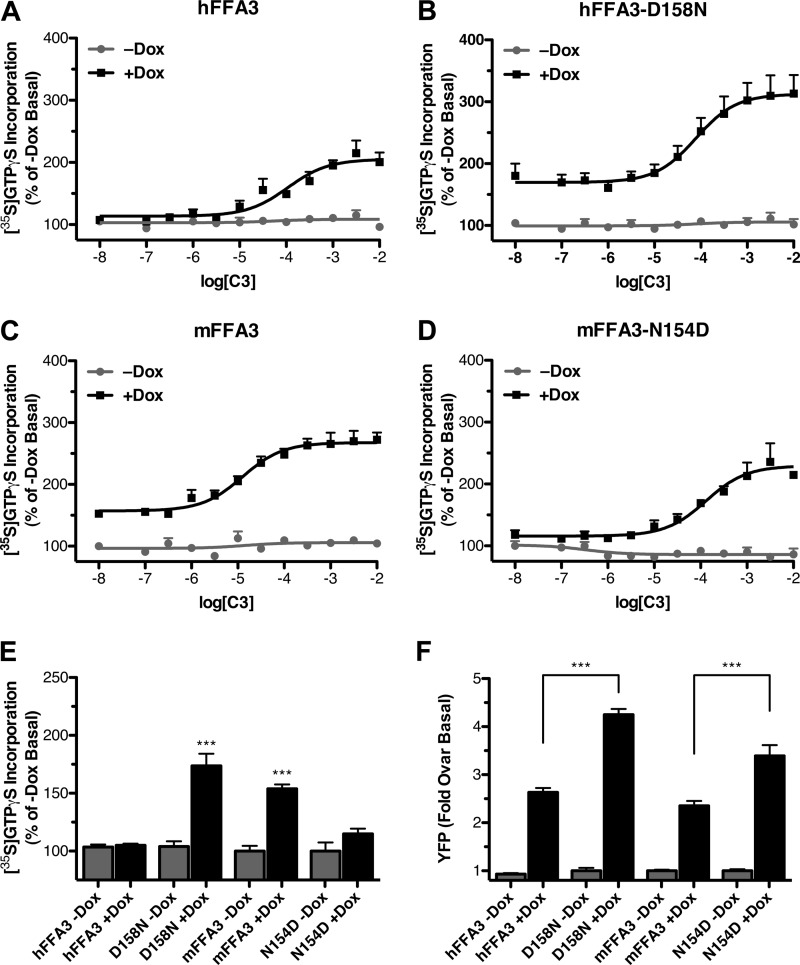
Examination of the sequence alignments for FFA3 (Fig. 3B) also identified a single negatively charged residue in ECL2 that differed between human and rodent orthologs and was in a similar context to the residue in the FFA2 orthologs. Asp-158 in hFFA3 is equivalent to Asn-154 in both of the rodent orthologs. The human and mouse orthologs were, therefore, subjected to reciprocal mutagenesis to determine whether these residues influenced constitutive activity and ligand potency. After generating a Flp-InTM T-RExTM 293-inducible cell line harboring hFFA3-D158N, we demonstrated that compared with wild type (Fig. 7A) this mutant displayed a 69 ± 15% increase in the basal [35S]GTPγS incorporation after expression was induced by Dox (Fig. 7B). Similar experiments were then performed to compare the constitutive activity of wild type mFFA3 (Fig. 7C) with that of the mFFA3-N154D mutant (Fig. 7D). In this case, although mFFA3 displayed clear ligand-independent basal [35S]GTPγS incorporation when expression was induced (54 ± 6%), the N154D mutant did not produce significantly altered basal [35S]GTPγS incorporation (Fig. 7E). To confirm that these observations were not simply the result of altered receptor expression, eYFP measurements were again taken from membranes expressing wild type and mutant hFFA3 and mFFA3 constructs (Fig. 7F). From these experiments it was clear that the hFFA3-D158N mutant did have significantly higher expression than wild type hFFA3 (p < 0.001), and the mFFA3-N154D mutant also showed significantly greater expression than did wild type mFFA3. Thus, although the enhanced expression of hFFA3-D158N relative to wild type may contribute to the observed increased constitutive activity of the mutant, because mFFA3-N154D displayed reduced constitutive activity despite its increased expression relative to wild type mFFA3, it is clear that the effect of this mutation on constitutive activity cannot be explained by altered receptor expression.
FIGURE 7.
Alterations of an extracellular loop 2 aspartate residue reciprocally modulate constitutive activity in human and murine FFA3. Reciprocal hFFA3-D158N and mFFA3-N154D mutants were generated and Flp-InTM T-RExTM 293 cells harboring C-terminally eYFP-tagged forms of these produced. Membranes were prepared from both uninduced and Dox-induced cells as well as from the corresponding wild type forms, and [35S]GTPγS binding assays were performed with varying concentrations of C3 with hFFA3 (A), hFFA3-D158N (B), mFFA3 (C), and mFFA3-N154D (D). The point on each of these curves corresponding to a log[C3] of −8 represents the data from basal unstimulated membranes. In E the relative level of constitutive activity of each receptor is displayed (***, p < 0.001 compared with appropriate −Dox control), whereas F shows the relative expression level of each receptor as measured by eYFP fluorescence.
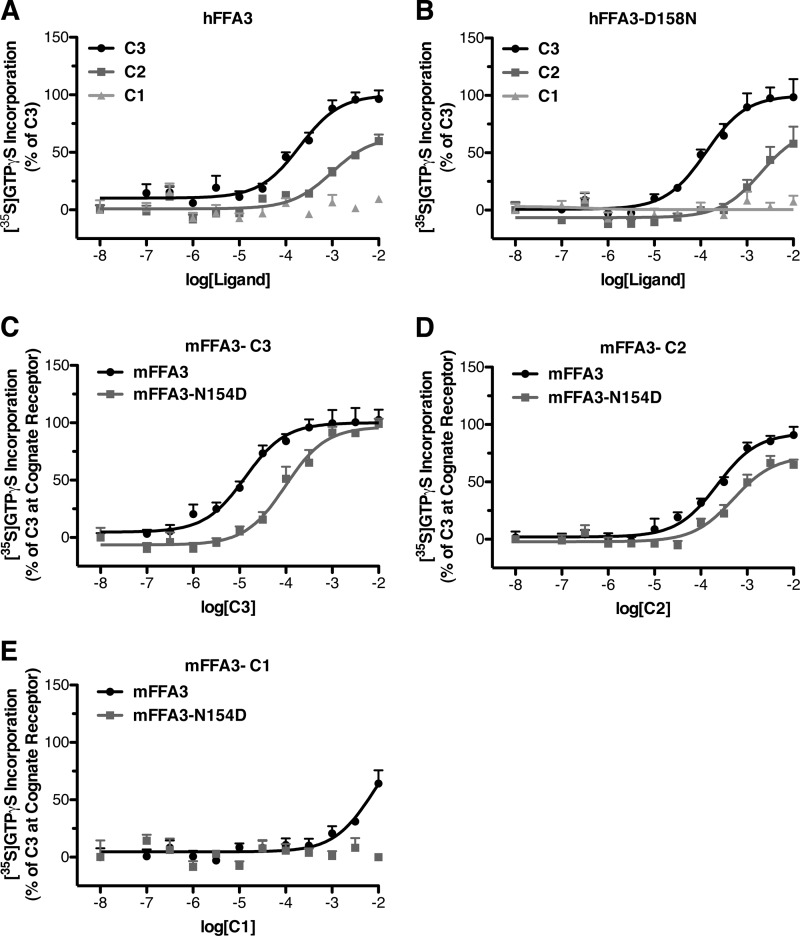
Finally, we explored if altering the level of constitutive activity of FFA3 also affected the potency of SCFAs and thus might explain the increased potency of C1, C2, and C3 at mFFA3 compared with hFFA3. In comparisons between wild type hFFA3 (Fig. 8A) and the constitutively active hFFA3-D158N mutant (Fig. 8B) there were no significant differences observed in ligand potencies. We also performed similar experiments using wild type mFFA3 and the N154D mutant. In this case C3 was found to have significantly (p < 0.05) lower potency at the N154D mutant (Fig. 8C) with pEC50 values of 4.88 ± 0.11 with wild type mFFA3 and 4.01 ± 0.11 with the N154D mutant. Similarly, C2 (pEC50 values = 3.65 ± 0.09 with wild type and 3.30 ± 0.12 with N154D) and C1 (pEC50 = 2.10 ± 0.31 with wild type, whereas no response was observed with N154D) lost potency with the D154N mutant compared with wild type mFFA3 (Fig. 8, D and E).
FIGURE 8.
The constitutively active hFFA3-D158N mutant does not have altered potency for SCFAs, but the non-constitutively active mFFA-N154D does show reduced potency. Membranes from cells induced to express either wild type hFFA3 (A) or hFFA3-D158N (B) were assessed for the potency to stimulate [35S]GTPγS incorporation of C1, C2, and C3. Membranes from cells induced to express either wild type mFFA3 (dark circles) or mFFA3-N154D (light squares) were assessed in the [35S]GTPγS assay for the potency of C3 (C), C2 (D), and C1 (E).
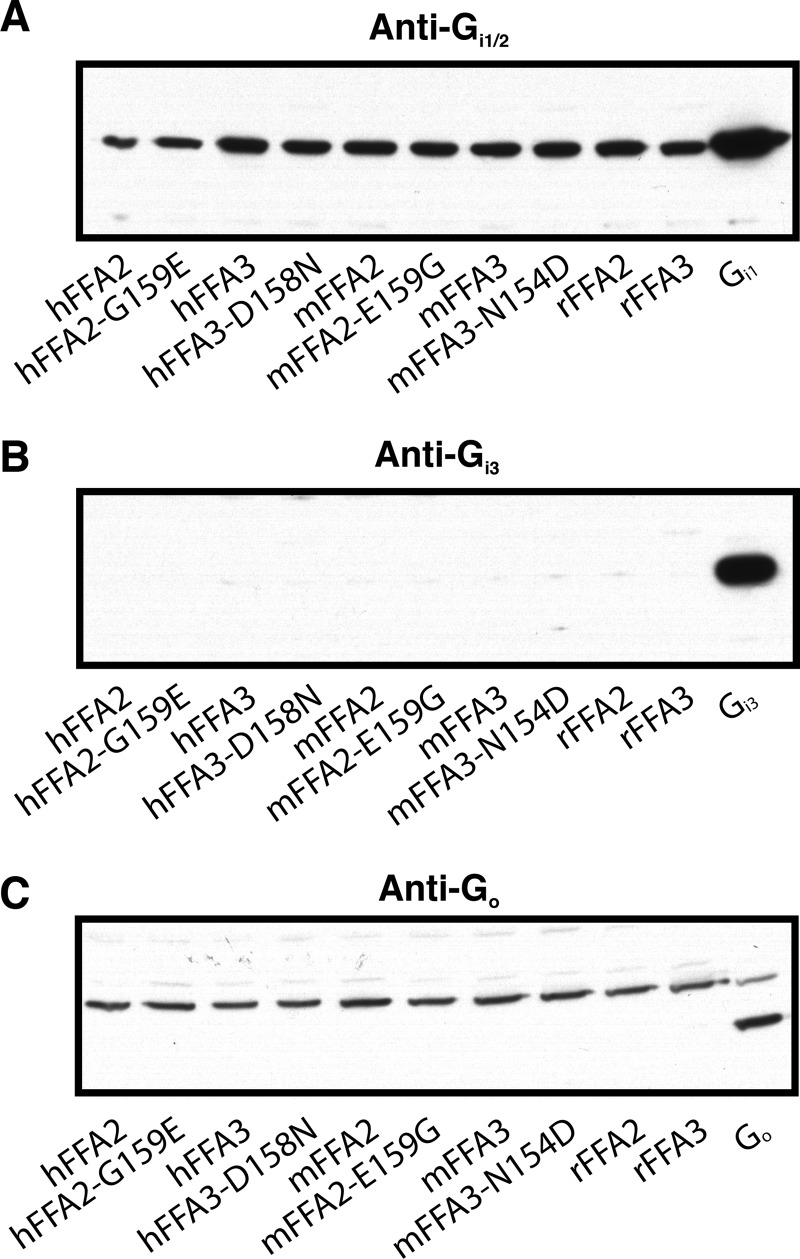
It was somewhat surprising that we did not observe potency differences between the wild type and D158N mutant of hFFA3 given that potency differences were observed between each other wild type receptor and their altered constitutive activity mutant. One possible explanation for this would be that the G protein expression levels are different between the wild type and D158N hFFA3 cell lines. To assess this, immunoblots to detect Gαi/o expression were carried out on membranes isolated from each of the cell lines used in this study. In these experiments it was found that all cell lines produced similar intensity bands detected by an anti-Gi1/2 antibody (Fig. 9A), whereas neither Gi3 nor Go could be detected in any of the cell lines (Fig. 9, B and C). These results suggest that the [35S]GTPγS signal reflects activation of Gi1 and/or Gi2 and that the potency differences observed between cell lines are not caused by differences in G protein expression levels.
FIGURE 9.
Immunoblots demonstrate similar expression of Gi1/2 across all cell lines. Representative immunoblots to assess Gi1/2 (A), Gi3 (B), or Go (C) expression in Dox-induced (500 ng/ml) membranes isolated from each of the cell lines used in this study are shown. In each case a positive control to show the specificity of the primary antibody is included using membranes isolated form HEK 293 cells transiently transfected with a plasmid encoding either Gi1, Gi3, or Go. Note, in C a nonspecific band was observed in all cell lines at a higher molecular mass than the predicted size; however, none of the cell lines produced a band of the predicted molecular mass, as demonstrated by the Go-transfected control.
DISCUSSION
Efforts to explore the physiological function of closely related GPCRs that respond to either the same or similar endogenously generated ligands generally rely on the availability of synthetic small molecule chemicals that selectively block or activate a single receptor. However, in the case of poorly characterized or recently de-orphanized receptors, such ligands are often either lacking or are not widely available. In such situations studies are often restricted to the use of the endogenous ligand(s). This is currently the case for the free fatty acid receptors FFA2 and FFA3. These are often co-expressed and are activated by the same group of SCFAs (7, 8). Although distinct structure activity relationships for the C1-C5 SCFAs have been noted at hFFA2 versus hFFA3, the SCFA C2 is the only one within this group with sufficient reported selectivity to potentially be used to discriminate actions of FFA2 from FFA3 (14). However, such selectivity has only been defined at the human orthologs of these GPCRs despite a desire and requirement to explore the function of these receptors in cells and tissues derived from other, particularly rodent, species. Our initial studies were designed, therefore, to assess whether the selectivity of hFFA2 over hFFA3 to be activated by C2 was maintained at the mouse orthologs. This proved not to be true, and instead C2 was equipotent at mFFA2 and mFFA3. As such, C2 clearly cannot be used in the absence of knock-out or knock-down studies to imply a specific role for FFA2 in mouse. By extending the range of SCFAs studied, a general pattern emerged in which each of the SCFAs was more potent at human FFA2 than at the mouse ortholog, whereas at least for C1-C3, the opposite was true for the orthologs of FFA3.
In addition to examining the selectivity of various SCFAs at mFFA2 and mFFA3, we also considered two SCAs previously shown to be selective for hFFA2 or hFFA3 (14). Interestingly, although we found the selectivity of these compounds was maintained, tiglic acid for mFFA2 and 1-MCPC for mFFA3, there were marked differences in their degree of selectivity at the mouse orthologs compared with what has been reported with the human receptors (14). Specifically, although tiglic acid was reported as nearly 1000-fold selective for hFFA2 over hFFA3 in the [35S]GTPγS assay, it was only 40-fold selective for mFFA2 in the same assay. Similarly, 1-MCPC was reported as only 18-fold selective for hFFA3, whereas it is >100-fold selective for mFFA3. Detailed analysis of these selectivity differences revealed that, like the differences observed in SCFA selectivity at the mouse orthologs, they result from decreased potency of tiglic acid at mFFA2 compared with what has been reported for hFFA2 (14) and increased potency at mFFA3 for 1-MCPC compared to the reported values for hFFA3 (14).
The amino acids previously shown to coordinate the carboxylate of the SCFAs within the orthosteric binding pocket (19) are fully conserved both between FFA2 and FFA3 and between the human and rodent forms. Furthermore, that selectivity of the SCAs tiglic acid and 1-MCPC was maintained at the mouse orthologs also suggests that the overall architecture of the orthosteric binding sites are likely very similar between the human and mouse orthologs of these GPCRs. We endeavored, therefore, to understand what other factors might contribute to ligand selectivity differences between species orthologs.
GPCRs must interconvert between inactive and active states spontaneously, and active states are stabilized and, therefore, enriched by the binding of an agonist ligand (24, 25). It is hence predicted that higher levels of constitutive activity and, therefore, a greater propensity to spontaneously adopt an active configuration would result in agonist ligands being able to stabilize such states more effectively than for receptors with a greater energy barrier between active and inactive states (24–26). Although conceptually true for all GPCRs, the small size and associated low binding energies of SCFAs makes such ligands and their receptors likely to display more pronounced differences than for larger ligands with high binding energies (14). We, therefore, hypothesized that the observed species differences in SCFA and SCA potency could be the result of differing levels of constitutive activity between the species orthologs of these receptors.
To explore this question, we employed cell lines based on the Flp-InTM T-RExTM 293 system. Here a GPCR harbored at the Flp-InTM T-RExTM locus is expressed only after the addition of the antibiotic Dox. Because such cells and/or membranes derived from these cells grown in the absence or presence of Dox differ only in the presence or absence of the receptor of interest, they are particularly well suited to monitor the extent of constitutive activity. As no ligands suitable for quantification of FFA2 or FFA3 receptor expression are available, we tagged each construct at the C terminus with eYFP and used the fluorescence of this auto-fluorescent protein as a surrogate marker of expression levels. Both FFA2 and FFA3 are known to couple to pertussis toxin-sensitive Gi-family G proteins (7, 8), and based on this we employed [35S]GTPγS incorporation assays, as these provided a strong quantitative end point of their activation (14, 18, 19) and were recently used to demonstrate constitutive activity of hFFA2 (21). Although the [35S]GTPγS assay was chosen for these studies, it must be noted that FFA2 and FFA3 couple to multiple other downstream signaling pathways, including in the case of FFA2 those associated with Gq/11 activation and that, therefore, the findings described here using the [35S]GTPγS assay may not necessarily directly translate to all other signaling pathways of these receptors.
In conducting these experiments, we observed marked species differences in the ligand-independent [35S]GTPγS incorporation in membranes expressing the different receptor orthologs. Specifically, the two orthologs with increased SCFA and SCA potency, hFFA2 and mFFA3, displayed much higher constitutive activity than the corresponding mFFA2 and hFFA3. The basal [35S]GTPγS binding produced in membranes expressing hFFA2 clearly reflected constitutive activity of the receptor, as it was inhibited fully and in a concentration-dependent fashion by the compound CATPB that also blocked the effect of C3 to increase [35S]GTPγS binding, and therefore, CATPB appears to be the first full “inverse agonist” described at this receptor. Although mFFA2 displayed very limited apparent constitutive activity, we could not usefully employ compound CATPB in studies on the mFFA2 ortholog, as this ligand displays marked selectivity for hFFA2, having very little, if any, activity at the mouse ortholog. This observation that hFFA2 and mFFA3 display increased constitutive activity is entirely consistent with our hypothesis that SCFAs and SCAs show higher potency at these two orthologs due to their increased constitutive activity.
Previous studies on FFA1, a closely related GPCR that responds to longer chain fatty acids than either FFA2 or FFA3, provided evidence that constitutive activity of this receptor is constrained by a pair of ionic locks formed by interactions between a pair of arginine residues, one at the top of transmembrane helix V and the other at the top of transmembrane helix VII, with a pair of glutamate residues located within the second extracellular loop that links transmembrane helices IV and V (23). Indeed, this hypothesis was supported by enhancement of constitutive activity in mutants in which these glutamates were replaced. As the two arginine residues are central to the binding of fatty acid ligands, then it was further hypothesized that binding of such fatty acids displaced these ionic locks to stabilize an active conformation of FFA1. Given that these two arginines are conserved in both human and rodent orthologs of FFA2 and FFA3 and that previous studies have demonstrated that they also coordinate the carboxylate group of SCFA ligands (19), we considered whether marked differences in the [35S]GTPγS constitutive activity between mouse and human orthologs of FFA2 and FFA3 could be the result of differential ionic locks between the species orthologs. In the case of FFA2, the high [35S]GTPγS constitutive activity of the human ortholog was largely eliminated by alteration of Gly-159 to the negatively charged residue glutamic acid, which is at the equivalent position in the murine ortholog. Importantly, the reciprocal mutation, E159G in mouse FFA2, markedly increased [35S]GTPγS constitutive activity. This mutational analysis was further consistent with the notion that higher constitutive activity promotes increased SCFA potency, as the hFFA2-G159E mutant lost potency to the SCFAs, whereas the more constitutively active mFFA2-E159G mutant gained potency to the SCFAs. Interestingly, the potency of the ago-allosteric agonist, 4-CMTB, which binds to an as yet not fully defined site on both human and mouse FFA2, also displayed reduced potency at hFFA2-G159E. This is of particular interest because 4-CMTB does not interact with the conserved arginine residues that appear to form an ionic lock in the G159E mutant (18). However, given that 4-CMTB is an ago-allosteric modulator and thus also stabilizes the active state of the receptor, the fact that it also loses potency at hFFA2-G159E would suggest that the reduced potency observed at this mutant is likely directly linked to the reduction in constitutive activity rather than to decreased access to the ligand binding pocket that might also be predicted to occur in the mutant where the ionic lock is present.
Although the extent of [35S]GTPγS constitutive activity at hFFA3 and mFFA3 was similarly regulated by reciprocal alteration of a single negatively charged residue in ECL2, the corresponding potency changes that were observed in both the human and mouse FFA2 mutants were only observed for the mFFA3-N154D mutant. In contrast, the hFFA3-D158N mutant, although gaining constitutive activity, did not display increased potency for SCFAs as might have been predicted. As this was found not to be related to altered G protein expression levels, it suggests that increased constitutive activity is necessary, but not sufficient, for the observed increase in SCFA potency at mFFA3 compared with hFFA3 and that there are likely additional, as yet unknown, factors contributing to the observed effect.
In light of our findings that increased [35S]GTPγS constitutive activity of the species orthologs of FFA2 and FFA3 generally correlates with increased potency of the SCFAs and SCAs at these receptors, it should also be considered whether the increased constitutive activity we observed for hFFA2 compared with hFFA3 may account for the differences in rank-order potency and selectivity that have been reported for these receptors previously (7, 8). Given that agonist ligands are predicted and have often been shown to have increased potency at constitutively active receptors (26, 27) and that we might expect this effect to be more pronounced for compounds with very low binding energies such as C1 and C2 (14), it could be speculated that the constitutive activity of hFFA2 might, therefore, account for the rank-order differences of SCFAs observed at this receptor compared with hFFA3. However, detailed analysis of our results reveals that in fact each species ortholog- and mutant-tested for FFA2 maintained a rank order of C3 = C2 > C1, whereas each ortholog or mutant of FFA3 maintained the rank order of C3 > C2 > C1. Although changes in selectivity were observed, for example while C2 is selective for hFFA2 over hFFA3, it is non-selective for the mouse orthologs, and whereas C3 is non-selective for the human receptors, it shows clear selectivity for mFFA3 over mFFA2; these changes actually result from broad shifts in potency across all SCFAs at the various orthologs. Therefore, it appears that differences in constitutive activity alone cannot account for the different rank order of potencies to SCFAs at hFFA2 and hFFA3.
These studies provide support for the concept that free fatty acid receptors display limited agonist-independent activity, at least in the [35S]GTPγS assay, if an ionic lock can be present between one or other of the pair of arginine residues that help to define the orthosteric binding site for fatty acids and residues of the ECL2. The ECL2 is known to contribute substantially to the ligand selectivity and mode of activation of many GPCRs (28, 29), and interestingly is the region in which the greatest diversity has been observed in the available atomic level structures of GPCRs that have been solved to date (29, 30). Given rapid progress in technical approaches that have enhanced the ability to produced such structural information (30–32) and the great interest in the free fatty acid receptors as potential therapeutic targets (8, 33, 34), it may not be long before the ionic lock proposed herein to regulate constitutive activity and SCFA potency at species orthologs of FFA2 and FFA3 will be assessed directly.
This work was supported by grants from the Wellcome Trust 089600/Z/09/Z (to G. M.), the Danish Council for Strategic Research 11-116196 (to T. U. and G. M.), and the Canadian Institutes of Health Research (fellowship to B. D. H.).
B. D. Hudson, I. G. Tikhonova, S. K. Pandey, T. Ulven, and G. Milligan, unpublished observation.
- GPCR
- G protein-coupled receptor
- 1-MCPC
- 1-methylcyclopropanecarboxylic acid
- 4-CMTB
- 4-chloro-α-(1-methylethyl)-N-2-thiazolylbenzeneacetamide
- CATPB
- (S)-3-(2-(3-chlorophenyl)-acetamido)-4-(4-(trifluoromethyl)phenyl)butanoic acid
- Dox
- doxycycline
- ECL2
- extracellular loop 2
- SCA
- small carboxylic acid
- SCFA
- short chain fatty acid
- eYFP
- enhanced yellow fluorescent protein
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- FFA
- free fatty acid
- hFFA
- human FFA
- m
- mouse
- r
- rat.
REFERENCES
- 1. Fredriksson R., Schiöth H. B. (2005) The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol. Pharmacol. 67, 1414–1425 [DOI] [PubMed] [Google Scholar]
- 2. Hopkins A. L., Groom C. R. (2002) The druggable genome. Nat. Rev. Drug Discov. 1, 727–730 [DOI] [PubMed] [Google Scholar]
- 3. Williams C., Hill S. J. (2009) GPCR signaling. Understanding the pathway to successful drug discovery. Methods Mol. Biol. 552, 39–50 [DOI] [PubMed] [Google Scholar]
- 4. Schlyer S., Horuk R. (2006) I want a new drug. G-protein-coupled receptors in drug development. Drug. Discov. Today 11, 481–493 [DOI] [PubMed] [Google Scholar]
- 5. Swanson R., Beasley J. R. (2010) Pathway-specific, species, and sub-type counterscreening for better GPCR hits in high throughput screening. Curr. Pharm. Biotechnol. 11, 757–763 [DOI] [PubMed] [Google Scholar]
- 6. Jenkins L., Brea J., Smith N. J., Hudson B. D., Reilly G., Bryant N. J., Castro M., Loza M. I., Milligan G. (2010) Identification of novel species-selective agonists of the G-protein-coupled receptor GPR35 that promote recruitment of β-arrestin-2 and activate Gα13. Biochem. J. 432, 451–459 [DOI] [PubMed] [Google Scholar]
- 7. Stoddart L. A., Smith N. J., Milligan G. (2008) International Union of Pharmacology. LXXI. Free fatty acid receptors FFA1, -2, and -3. Pharmacology and pathophysiological functions. Pharmacol. Rev. 60, 405–417 [DOI] [PubMed] [Google Scholar]
- 8. Hudson B. D., Smith N. J., Milligan G. (2011) Experimental challenges to targeting poorly characterized GPCRs. Uncovering the therapeutic potential for free fatty acid receptors. Adv. Pharmacol. 62, 175–218 [DOI] [PubMed] [Google Scholar]
- 9. Alquier T., Poitout V. (2009) GPR40. Good cop, bad cop? Diabetes 58, 1035–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burant C. F., Viswanathan P., Marcinak J., Cao C., Vakilynejad M., Xie B., Leifke E. (2012) TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus. A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379, 1403–1411 [DOI] [PubMed] [Google Scholar]
- 11. Milligan G., Stoddart L. A., Smith N. J. (2009) Agonism and allosterism. The pharmacology of the free fatty acid receptors FFA2 and FFA3. Br. J. Pharmacol. 158, 146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oh D. Y., Lagakos W. S. (2011) The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 14, 322–327 [DOI] [PubMed] [Google Scholar]
- 13. Le Poul E., Loison C., Struyf S., Springael J. Y., Lannoy V., Decobecq M. E., Brezillon S., Dupriez V., Vassart G., Van Damme J., Parmentier M., Detheux M. (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 [DOI] [PubMed] [Google Scholar]
- 14. Schmidt J., Smith N. J., Christiansen E., Tikhonova I. G., Grundmann M., Hudson B. D., Ward R. J., Drewke C., Milligan G., Kostenis E., Ulven T. (2011) Selective orthosteric free fatty acid receptor 2 (FFA2) agonists. Identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J. Biol. Chem. 286, 10628–10640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zaibi M. S., Stocker C. J., O'Dowd J., Davies A., Bellahcene M., Cawthorne M. A., Brown A. J., Smith D. M., Arch J. R. (2010) Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 584, 2381–2386 [DOI] [PubMed] [Google Scholar]
- 16. Ge H., Li X., Weiszmann J., Wang P., Baribault H., Chen J. L., Tian H., Li Y. (2008) Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149, 4519–4526 [DOI] [PubMed] [Google Scholar]
- 17. Brown A. J., Goldsworthy S. M., Barnes A. A., Eilert M. M., Tcheang L., Daniels D., Muir A. I., Wigglesworth M. J., Kinghorn I., Fraser N. J., Pike N. B., Strum J. C., Steplewski K. M., Murdock P. R., Holder J. C., Marshall F. H., Szekeres P. G., Wilson S., Ignar D. M., Foord S. M., Wise A., Dowell S. J. (2003) The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 [DOI] [PubMed] [Google Scholar]
- 18. Smith N. J., Ward R. J., Stoddart L. A., Hudson B. D., Kostenis E., Ulven T., Morris J. C., Tränkle C., Tikhonova I. G., Adams D. R., Milligan G. (2011) Extracellular loop 2 of the free fatty acid receptor 2 mediates allosterism of a phenylacetamide ago-allosteric modulator. Mol. Pharmacol. 80, 163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stoddart L. A., Smith N. J., Jenkins L., Brown A. J., Milligan G. (2008) Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J. Biol. Chem. 283, 32913–32924 [DOI] [PubMed] [Google Scholar]
- 20. Lee T., Schwandner R., Swaminath G., Weiszmann J., Cardozo M., Greenberg J., Jaeckel P., Ge H., Wang Y., Jiao X., Liu J., Kayser F., Tian H., Li Y. (2008) Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol. Pharmacol. 74, 1599–1609 [DOI] [PubMed] [Google Scholar]
- 21. Hudson B. D., Christiansen E., Tikhonova I. G., Grundmann M., Kostenis E., Adams D. R., Ulven T., Milligan G. (2012) Chemically engineering ligand selectivity at the free fatty acid receptor 2 based on pharmacological variation between species orthologs. FASEB J. doi: 10.1096/fj.12-213314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brantis C. E., Ooms F., Bernard F. (2011) Novel Amino acid derivatives and their use as GPR43 receptor modulators. WO2011092284 [Google Scholar]
- 23. Sum C. S., Tikhonova I. G., Costanzi S., Gershengorn M. C. (2009) Two arginine-glutamate ionic locks near the extracellular surface of FFAR1 gate receptor activation. J. Biol. Chem. 284, 3529–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kenakin T. (2001) Inverse, protean, and ligand-selective agonism. Matters of receptor conformation. FASEB J. 15, 598–611 [DOI] [PubMed] [Google Scholar]
- 25. Kenakin T. (2004) Principles. Receptor theory in pharmacology. Trends Pharmacol. Sci. 25, 186–192 [DOI] [PubMed] [Google Scholar]
- 26. Cotecchia S. (2007) Constitutive activity and inverse agonism at the α1 adrenoceptors. Biochem. Pharmacol. 73, 1076–1083 [DOI] [PubMed] [Google Scholar]
- 27. Samama P., Cotecchia S., Costa T., Lefkowitz R. J. (1993) A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 268, 4625–4636 [PubMed] [Google Scholar]
- 28. Peeters M. C., van Westen G. J., Li Q., IJzerman A. P. (2011) Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol. Sci. 32, 35–42 [DOI] [PubMed] [Google Scholar]
- 29. Wheatley M., Wootten D., Conner M. T., Simms J., Kendrick R., Logan R. T., Poyner D. R., Barwell J. (2012) Lifting the lid on GPCRs. The role of extracellular loops. Br. J. Pharmacol. 165, 1688–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenbaum D. M., Rasmussen S. G., Kobilka B. K. (2009) The structure and function of G-protein-coupled receptors. Nature 459, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Topiol S., Sabio M. (2009) X-ray structure breakthroughs in the GPCR transmembrane region. Biochem. Pharmacol. 78, 11–20 [DOI] [PubMed] [Google Scholar]
- 32. Congreve M., Langmead C., Marshall F. H. (2011) The uses of GPCR structures in drug design. Adv. Pharmacol. 62, 1–36 [DOI] [PubMed] [Google Scholar]
- 33. Rayasam G. V., Tulasi V. K., Davis J. A., Bansal V. S. (2007) Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin. Ther. Targets 11, 661–671 [DOI] [PubMed] [Google Scholar]
- 34. Talukdar S., Olefsky J. M., Osborn O. (2011) Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol. Sci. 32, 543–550 [DOI] [PMC free article] [PubMed] [Google Scholar]