Background: SAMHD1 is a novel antiviral factor counteracted by the viral protein Vpx
Results:Several residues in Vpx affect its ability to increase infection and degrade SAMHD1.
Conclusion:Vpx functionality correlates with SAMHD1 degradation, but not with Vpx stability and Vpx-Vpx association.
Significance:Several mutants provide further insights into the molecular mechanism of Vpx-induced protection.
Keywords: HIV, Protein Degradation, Retrovirus, Viral Protein, Virulence Factors, Dendritic Cells, HIV-1, Macrophages, SAMHD1, Vpx
Abstract
SAMHD1 is a newly identified restriction factor that targets lentiviruses in myeloid cells and is countered by the SIVSM/HIV-2 Vpx protein. By analyzing a large panel of Vpx mutants, we identify several residues throughout the 3-helix bundle predicted for Vpx that impair both its functionality and its ability to degrade SAMHD1. We determine that SAMHD1 is a strictly non-shuttling nuclear protein and that as expected WT Vpx localizes with it in the nucleus. However, we also identify a functional Vpx mutant with predominant cytoplasmic distribution that colocalizes with SAMHD1 in this location, suggesting that Vpx may also retain SAMHD1 in the cell cytoplasm, prior to its entry into the nucleus. Several mutations in Vpx were shown to affect the stability of Vpx, as well as Vpx:Vpx interactions. However, no strict correlation was observed between these parameters and the functionality of Vpx, implying that neither properties is absolutely required for this function and indicating that even unstable Vpx mutants may be very efficient in inducing SAMHD1 degradation. Overall, our analysis identifies several Vpx residues required for SAMHD1 degradation and points to a very efficient and plastic mechanism through which Vpx depletes this restriction factor.
Introduction
Myeloid cells play numerous roles in the regulation and homeostasis of the immune system and are central players in antiviral responses. Despite being infected themselves, although to an extent related to their differentiation/activation status, circulating monocytes, resident macrophages, and dendritic cells (DCs)6 display a strong resistance toward primate lentiviruses, a phenomenon particularly acute during the early phases of infection (1, 2). This hostile environment is likely the result of numerous layers of restrictions that dynamically affect viral replication at multiple stages and that are only now starting to be understood from a molecular point of view.
So far, only Vpx, a protein packaged into virion particles and coded by members of the HIV-2/SIVSM lineage, has been described to counter this restrictive environment, at least under certain conditions, and to exert a strong positive effect during the early phases of infection of myeloid cells with both cognate and non-cognate lentiviruses (Refs. 3–7, and for a review, Ref. 8). Through this viral protein, two cellular restriction factors whose antiviral activity seems mostly specific to human myeloid cells have been described so far: the apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3A member (APOBEC3A or A3A) and the sterile α motif-hydroxylase domain 1 protein (SAMHD1) (9–13). More specifically, SAMHD1 has been recently shown to restrict lentiviral infection by diminishing the pool of intracellular dNTPs (14, 15), providing a molecular explanation for previous observations indicating that myeloid cells infection was inefficient because of low dNTPs concentrations (16–18).
Here, we characterize a large panel of Vpx mutants to determine the relationship existing between the functionality of Vpx proteins during primate lentiviral infection of primary myeloid cells and their ability to induce SAMHD1 degradation. Using the same collection of mutants, we explore the intracellular localization at which the interaction between these two proteins may occur. Our results define a number of mutations spread over the 3-helix bundle structure predicted for Vpx that impair both Vpx functionality and its ability to deplete SAMHD1 in target DCs. Certain specific mutations alter the stability of Vpx and its ability to undergo Vpx:Vpx interactions. However, no strict correlation exists between these parameters and Vpx functionality, indicating that they are not limiting factors during the early phases of infection. We define here that SAMHD1 is a non-shuttling nuclear protein that therefore accumulates in the nucleus and is unable to leave it. If WT Vpx partially colocalize with SAMHD1 in the nucleus, we identify a specific functional Vpx mutant that displays an exquisite cytoplasmic distribution and that is able to induce the partial retention of SAMHD1 in the cell cytoplasm. In light of our findings that SAMHD1 does not leave the nucleus, we believe that this mutant retains SAMHD1 in the cytoplasm before this protein enters the nucleus, probably after its translation. Overall, these results point out to an efficient and plastic manner in which Vpx counters SAMHD1.
EXPERIMENTAL PROCEDURES
Cell Culture, Cytokines, and Antibodies
293T, HeLa and NIH3T3 cells were cultured in Dulbecco's Eagle Modified Medium (DMEM) plus 10% of fetal calf serum (FCS) and 100 units of penicillin/streptomycin. Primary blood human monocytes were purified from the blood of healthy donors obtained at the blood bank of Lyon (EFS-Lyon), as described before (7, 19). Monocytes were differentiated into macrophages or immature DCs upon incubation for 4 to 5 days in RPMI1640 complete medium supplemented with 100 ng/ml of GM-CSF alone or in combination with IL-4 (AbCys). Anti-Flag, anti-VSVg, and anti-tubulin monoclonal antibodies were purchased from Sigma, anti-SAMHD1 antibody was purchased from AbCam and the anti-CA antibody was provided by the AIDS Reagents and Reference Program of the NIH.
DNA Constructs and Viral Production
A codon optimized and VSVg-epitope tagged version of SAMHD1 was synthesized and purchased by GenScript. Flag-tagged Vpx/Vpr coding plasmids were described before (20) and novel ones were generated using standard mutagenesis techniques. Nomenclature: when two identical residues are mutated within the same Vpx mutant only the identity of the first amino acid is specified (ex: K84/85A indicates that two lysines at position 84 and 85 have been mutated to alanine).
HIV-1 GFP-coding lentiviral vectors were produced by co-transfection of 293T cells with 3 plasmids coding: the packaging proteins Gag-Pro-Pol and viral non structural proteins, a mini viral genome bearing a CMV-driven eGFP reporter, and the vesicular stomatitis virus G envelope (VSVg) for ample cellular tropism (7). Virions were purified through a 25% sucrose cushion, resuspended and normalized by infectious titers as determined onto HeLa cells or by protein content against standards of known infectivity (exo-RT activity). Non-infectious virion-like particles (VLPs) were similarly produced using the Gag-Pol of SIVMAC, VSVg and the indicated Vpx proteins in the absence of viral genome. Virions were purified as above and normalized by exo-RT.
Infections and Transfections
Infections were carried out for 2 h on 105 cells prior to flow cytometry analysis 3 days afterward. DCs were challenged with the indicated virus in a final volume of 100 μl in flat bottom 96-well plates. Two hours postchallenge, cells were directly spun on the plate and the supernatant replaced with fresh media. The result of the infection was assessed by flow cytometry 3 days later. Macrophages were similarly treated except that 24 well plates were used and infections were carried out in a final volume of 400 μl. To obtain sufficient amounts of protein for WB analysis of SAMHD1 degradation, 5 × 105 DCs were challenged with an MOI equivalent of 2 of VLPs-Vpx and cells were analyzed 24 h post challenge. Transfections were carried out with calcium phosphate of HeLa, 293T or murine NIH3T3 cells, as previously described (20).
Confocal Microscopy Analysis
Cells were grown on coverslips, then fixed in 4% formalin (Sigma). Free aldehydes were quenched with 50 mm NH4Cl-PBS and cells were then permeabilized with 0.2% or 0.5% Triton X-100 (Macrophages/DCs and HeLa/NIH3T3, respectively). After immunostaining, images were acquired using a spectral Leica sp5 or LSM 710 microscopes. Primary antibodies are described above, rhodamine-labeled Phallodin was purchased from Sigma, while the secondary antibodies were purchased from Vector laboratories. For heterokaryons, HeLa and NIH 3T3 were cocultured on coverslips and fused at a ratio of 1:1 using PEG8000 (50%, Sigma) in the presence of cyclohexamide (CHX at 100 μg/ml, Sigma). Cells were analyzed by confocal microscopy 2 h afterward. The degree of colocalization between SAMHD1 and Vpx was measured on 20 cells using the Pearson's correlation coefficient (JACoP tool: where a value of 1 indicates perfect colocalization, while a value of 0 indicates random distribution). The fraction of cytoplasmic SAMHD1 was analyzed by quantifying the Manders overlap coefficient (Fuji image software).
Pulse/Chase Analysis
Transfected HeLa cells were pulsed for 30 min with 30 μCi of [35S]Met/Cys, prior to extensive cell washing. Cells were then chased for different time points, prior to cell lysis and immunoprecipitation, as described (21). Vpx was immunoprecipitated using anti-Flag coated beads (Sigma). Samples were run on an SDS-PAGE gel, prior to phosphorimager quantification.
Vpx Binding Assays
The ability of the different Vpx mutants to associate to WT Vpx was assessed after co-transfection of 293T cells with a GST-FLAG-WT SIVMAC Vpx construct along with FLAG-Vpx mutants prior to lysis and precipitation with glutathione-coated agarose beads. Precipitates were then loaded on an SDS-PAGE gel and analyzed by WB.
RESULTS
The Analysis of a Large Panel of Vpx Mutants Strictly Correlates the Functionality of Vpx during the Early Phases of Infection of DCs with Its Ability to Induce SAMHD1 Depletion
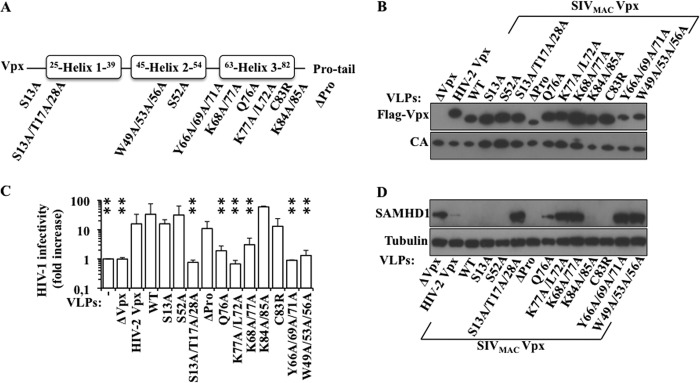
To identify residues of Vpx required for SAMHD1 depletion in DCs, we took advantage of a large panel of Vpx mutants that we had previously characterized with respect to their ability to be incorporated into virion particles and to increase the infectivity of HIV-1 vectors in DCs (20). Some of these mutations had also been previously characterized for their ability to affect HIV-2 or SIV replication (22, 23). The different mutations in Vpx span the 3 helix-bundle core predicted for this protein based on its close homology with Vpr (Fig. 1A). Two novel mutants in conserved residues of helix 3 were added to this collection (K77A/L72A and C83R). The various Vpx mutant proteins were incorporated into non-infectious virion-like particles derived from SIVMAC (VLPs) upon co-transfection of 293T cells with an SIVMAC-derived Gag-Pro-Pol construct. VLPs were then harvested, normalized by protein content by exo-RT, examined for their Vpx content by WB (Fig. 1B), and used on DCs to vehicle Vpx during infection. The ability of Vpx-VLPs to mediate an increase in HIV-1 infectivity was assessed by flow cytometry 3 days after infection, while their ability to deplete SAMHD1 in target DCs was assessed by WB. In line with what we had previously reported, all the Vpx mutants were readily incorporated into VLPs upon co-transfection with Gag-Pol expression plasmids, although 3 mutants displayed lower incorporation rates (ΔPro, Y66A/69A/71A, and W49A/53A/56A, Fig. 1B). Then, exo-RT normalized Vpx-VLPs were provided in trans during the infection of DCs with a constant amount of GFP-coding HIV-1 LVs and their effect on infectivity measured by flow cytometry 3 days afterward (Fig. 1C). As we and others have previously reported (20), a number of mutations in Vpx affect its ability to relieve the restrictive phenotype of DCs to incoming HIV-1 particles (S13A/T17A/28A, K68/77A, Y66A/69A/71A, W49A/53A/56A, and Q76A). Of the novel mutants tested here, one was functional, while the second was not (C83R and K77A/L72A, respectively). Next, the ability of these mutants to degrade endogenous SAMHD1 during the early phases of infection of DCs was assessed by WB (Fig. 1D). All non-functional Vpx mutants were unable to deplete SAMHD1 in DCs (S13A/T17A/28A, K77A/L72A, K68/77A, Y66A/69A/71A, and the W49A/53A/56A, while Q76A only modestly and non-reproductively affected SAMHD1 levels). On the contrary, all functional mutants did degrade SAMHD1 in DCs. In the case of the Y66A/69A/71A and W49A/53A/56A Vpx mutants, the impairment in inducing SAMHD1 depletion cannot be ascribed to their lower incorporation into VLPs, because the ΔPro Vpx mutant is incorporated to similar levels, yet it is able to induce SAMHD1 degradation.
FIGURE 1.
Identification of residues in Vpx required for the degradation of SAMHD1. A, schematic representation of the mutants used here. Our laboratory has already characterized these Vpx mutants with respect to incorporation into virion-like particles and ability to increase HIV-1 infectivity in DCs, with the exception of the K77A/L72A and the C83R mutants. The results depicted in B and C have been therefore presented in essence in Ref. 19 apart from the mutants specified above and are presented here to clearly underline the strict correlation existing between infectivity and SAMHD1 degradation. B, non-infectious VLPs incorporating Vpx were obtained after transfection of 293T cells, purification through sucrose, and normalization by exo-RT. Normalized VLPs were analyzed by WB. C, normalized amounts of VLPs-Vpx were added to DCs along with a constant amount of GFP-coding HIV-1 vectors. The extent of infection was measured 3 days afterward by flow cytometry. D, as in C, but DCs were analyzed by WB 24 h postinfection. The panels are representative of 3 to 4 independent experiments, while the graph presents averages values obtained in four experiments.
SAMHD1 Is Degraded Rapidly and Does Not Leave the Nucleus during Infection
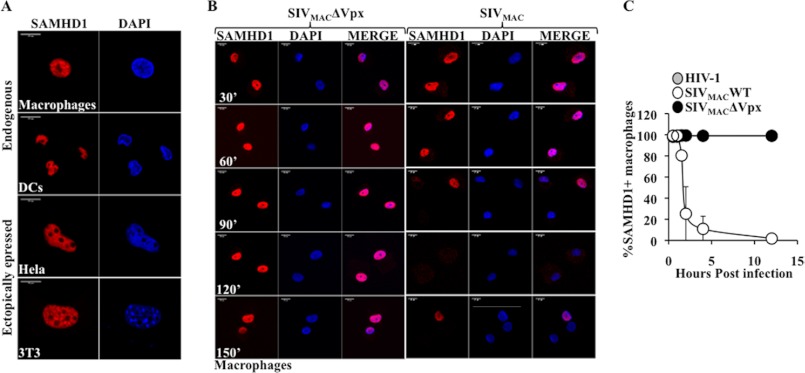
The intracellular localization of SAMHD1 during infection remains unclear, as published data support either a nuclear or a cytoplasmic relocalization (24–26). In the absence of infection, endogenous SAMHD1 presented an exclusively nuclear staining in both primary macrophages and DCs, and an identical localization was observed upon ectopic expression in HeLa cells and in murine NIH3T3 (Fig. 2A), confirming the described nuclear localization of SAMHD1 under normal conditions. To determine whether SAMHD1 relocalized during infection, primary macrophages, that also display a Vpx-dependent phenotype but that by their tight adherence and spread cytoplasm proved more amenable than DCs for extensive confocal microscopy studies, were challenged with equal amounts of HIV-1 or SIVMAC vectors containing or devoid of Vpx (at a multiplicity of infection, MOI, of 2). Cells were then examined at different time points after infection to determine the presence and the localization of SAMHD1 (Fig. 2B displays representative cells over 400 scored per virus/time point/experiment and for space constraints only results obtained up to 150 min with SIVMAC vectors are presented). Under these conditions, degradation of SAMHD1 occurred rapidly in the presence of Vpx, and the protein virtually disappeared by 12 h postinfection (t50% value ≅ 1.45 h, Fig. 2C). These values are more rapid than those reported previously upon WB analysis, but we believe these differences are solely due to the distinct techniques used (15). On the contrary, SAMHD1 was clearly detected when macrophages where challenged with an SIVMAC virus devoid of Vpx or with HIV-1 (not shown here). Of note, the Vpx protein present in incoming viral particles could not be reliably detected in transduced cells, most likely because of amounts that are below the levels of detection of the assay.
FIGURE 2.
SAMHD1 is a nuclear protein degraded rapidly during infection in the absence of cytoplasmic re-distribution. A, intracellular localization of ectopically expressed or of endogenous SAMHD1 was examined by confocal microscopy in HeLa cells, murine NIH3T3 (3T3) and in primary macrophages and DCs, respectively. B, primary macrophages were challenged with the indicated virus at an MOI of 2. Cells were then analyzed by confocal microscopy at different times points after infection. For lack of space, results obtained upon HIV-1 infection are not displayed here but only in the following graph. The pictures are representative of over 400 cells scored per time point, per virus and per experiment in 3 to 4 independent experiments. C, graph presents the averages of SAMHD1-positive cells over time quantified in B.
At no time point during infection with any of the viruses used here, SAMHD1 was observed outside the nucleus, indicating that this protein does not leave this location during viral infection (Fig. 2B for representative pictures).
Overall, these results indicate very rapid kinetics of degradation of SAMHD1, in agreement with the kinetics of the early phases of infection that we and others have measured in myeloid cells (27).
SAMHD1 Is a Prototypical Non-shuttling Nuclear Protein
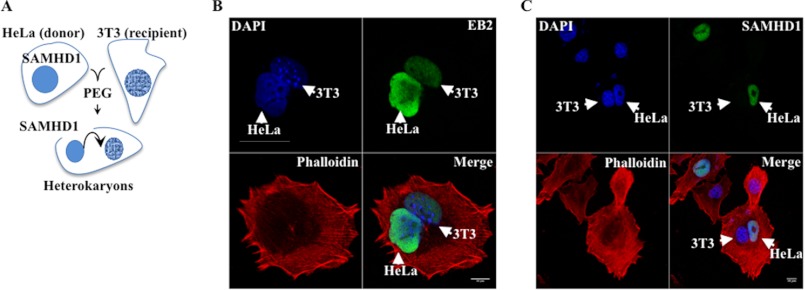
The results presented above strongly suggest that SAMHD1 may be a nuclear non-shuttling protein that is therefore unable to exit the nucleus once this destination is reached. To formally prove it, we assessed whether SAMHD1 possessed the intrinsic ability to shuttle from a donor nucleus (HeLa) to a recipient one (3T3) upon PEG-induced fusion of these two cell types in heterokaryons (according to the scheme of Fig. 3A). Cells were then examined by confocal microscopy and true heterokaryons were identified through phalloidin-mediated actin staining (Fig. 3, B and C, depicting representative pictures of over 80 heterokaryons scored per condition). This technique relies on the clear difference in DAPI pattern between murine and human species nuclei and has been largely used to define the shuttling properties of nuclear proteins (28, 29). As a positive control, the nuclear shuttling Epstein-Barr virus EB2 protein was used (30). When heterokaryons were thus examined, SAMHD1 was never found in recipient 3T3 nuclei, contrarily to EB2. Since SAMHD1 displays a nuclear staining when ectopically expressed in murine 3T3 (see Fig. 2A), this inability cannot be ascribed to species-specific differences in nuclear entry pathways. Therefore, our results define SAMHD1 as a typical non-shuttling nuclear protein that accumulates in the nucleus, but is unable to leave it.
FIGURE 3.
SAMHD1 is prototypical non-shuttling nuclear protein. A, schematic representation of the technique used here. HeLa cells expressing the control EB2 nuclear shuttling protein (B) or SAMHD1 (C) were fused to murine NIH3T3 (3T3) cells with PEG to induce heterokaryon formation, in the presence of CHX to arrest de novo protein translation. Two hours later, cells were analyzed by confocal microscopy. Arrows point to the distinct DAPI staining typically obtained in human and murine cells. Truly fused cells were identified upon phalloidin staining. The two heterokaryons shown are representative of over 80 scored per condition.
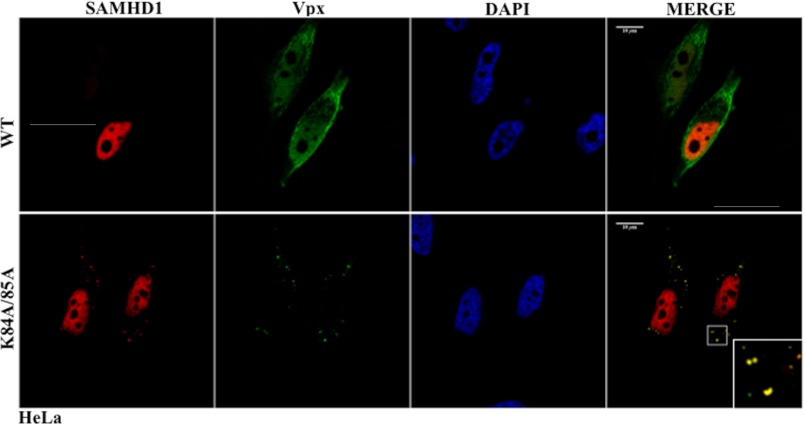
A Functional Vpx Mutant Induces the Accumulation of SAMHD1 in the Cell Cytoplasm
Because SAMHD1 is a nuclear protein and WT Vpx displays a composite intracellular distribution, in both nucleus and cytoplasm (20), WT Vpx is likely to interact with SAMHD1 directly in the nucleus, as proposed (24, 26). However, we have previously reported that the functional K84A/85A Vpx mutant displays a strict cytoplasmic distribution (20), so that a direct interaction between these two proteins in the nucleus is unlikely. To determine whether this mutant affected the localization of SAMHD1, both proteins were ectopically expressed in HeLa cells then examined by confocal microscopy (Fig. 4). While WT Vpx displayed a diffused staining that co-localized with SAMHD1 in the nucleus, the K84A/K85A Vpx mutant induced the accumulation of, and partially co-localized with, SAMHD1 in a clear punctate pattern in the cell cytoplasm. SAMHD1 and Vpx displayed elevated colocalization in the cytoplasm (Pearson coefficient of 0.96) and involved up to 5.2% ± 1.3% of the total SAMHD1 signal. Given that SAMHD1 does not leave the nucleus once it reaches it, and that the K84A/85A Vpx mutant resides in the cytoplasm, these results indicate that the K84A/85A Vpx mutant likely intercepts a fraction of SAMHD1, before it enters the nucleus.
FIGURE 4.
Intracellular distribution of SAMHD1 upon co-expression with WT and K84/85A Vpx proteins. HeLa cells were transfected with DNAs coding for SAMHD1 and the indicated Vpx proteins, prior to confocal microscopy analysis. The cells shown in the panels are representative of over 400–600 scored per condition. The inset in the figure provides a zoom of the intracytoplasmic accumulation of SAMHD1 and Vpx.
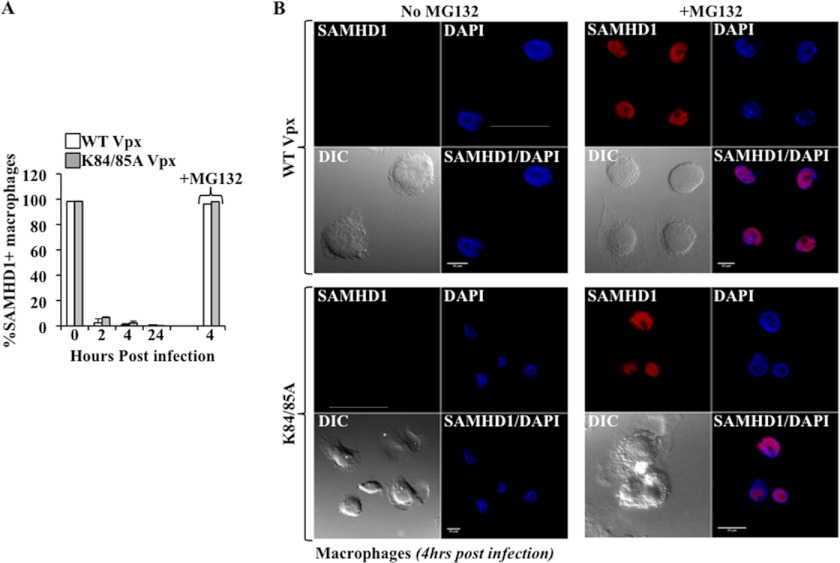
The KK84/85A Vpx Mutant Displays No Kinetic Defects over WT and Induces an MG132-dependent Degradation of SAMHD1
To determine whether functional differences existed between nuclear and cytoplasmic interactions, we determined whether the KK84/85A Vpx mutant exhibited kinetic differences in the degradation of SAMHD1 by comparing it to WT Vpx. To this end, primary macrophages were challenged as above with SIVMAC-derived VLPs incorporating either WT or mutated Vpx and the percentage of SAMHD1+ cells was scored over time (Fig. 5A, for a cumulative analysis, and B for representative cells over more than 400 scored per virus/condition/experiment). Under these conditions, no differences were observed in the kinetics of SAMHD1 degradation between WT and KK84/85A Vpx mutant, indicating that both were able to induce the rapid and efficient degradation of SAMHD1. The addition of the proteasome inhibitor MG132 during infection prevented SAMHD1 degradation in both cases, indicating that both Vpx proteins depleted SAMHD1 in a proteasome-dependent manner.
FIGURE 5.
The KK84/85A Vpx mutant displays no kinetic difference over the WT in the induction of an MG132-dependent SAMHD1 degradation. Primary macrophages were challenged as above with normalized amounts of VLPs-Vpx and the proportion of SAMHD1-positive cells was scored at different times post infection. A, graph presents averages obtained after the scoring of over 400 cells per condition and experiment in two independent experiments. B, representative pictures obtained at 4 h postinfection in the presence or absence of the proteasome inhibitor MG132.
The Ability to Degrade SAMHD1 Is Largely Independent from the Stability of Vpx
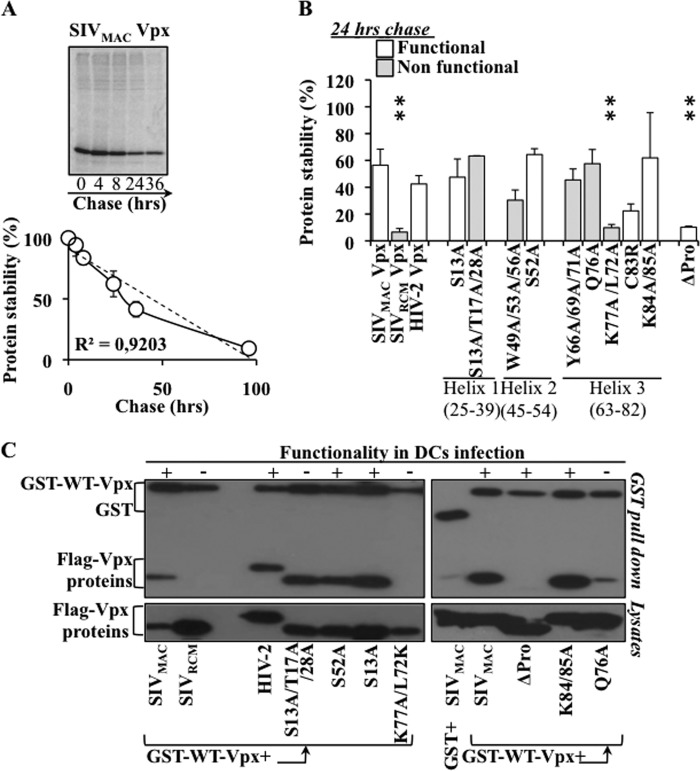
Contrarily to the pool of SAMHD1 that is supposed to be constantly renewed in the cell through protein translation, only a given amount of Vpx is present during the early phases of infection (the one present in incoming virion particles). Thus, it is possible that the efficiency with which SAMHD1 is degraded is influenced by the stability of Vpx. To determine whether this was the case, we measured the half-life of several Vpx mutants by [35S]Met/Cys metabolic labeling and immunoprecipitation in transfected HeLa cells. In agreement with a previous study (31), WT Vpx displays a t50% value of 30 h ± 2 h (panel of Fig. 6A for an example and graph for a compilation of independent experiments over a longer time frame). The stability of the different Vpx mutants was then analyzed at 24 h postlabeling with respect to the amount of protein labeled at t0 (Fig. 6B). Under these conditions, WT HIV-2 and SIVMAC Vpx displayed similar stabilities, while the distantly related, and non-functional, SIVRCM Vpx was highly unstable (8-fold less protein remaining at this time point compared with WT). Several mutants displayed wild type stability (S13A, S13A/T17A/28A, S52A, Y66A/69A/71A, K84/85A, and Q76A), two mutants displayed only a slight decrease in their stability (W49A/53A/56A, C83A, 1.8 and 2.5-fold lower than WT), while two were more drastically reduced (K77A/72A and ΔPro, 5.6 and 5.7-fold). As at least two mutants with reduced stability exhibited WT functionality (C83A and ΔPro), these results indicate that Vpx stability is not a limiting factor in the degradation of SAMHD1, at least within the variations that we have measured in the mutants described here.
FIGURE 6.
Study of the correlation between Vpx protein stability, ability to undergo Vpx:Vpx interactions and functionality. HeLa cells were transfected with the indicated Vpx coding construct, then labeled with [35S]Met/Cys for 30 min. After extensive cell washing, cells were lysed at the indicated times points and Vpx was immunoprecipitated using M2-Flag-coated agarose beads. After migration on SDS-PAGE gels, gels were dried and exposed for phosphorimager quantification. A, representative example of a typical pulse/chase analysis of WT Vpx and graph presenting the stability of WT Vpx obtained in four different experiments. B, stability of the different Vpx mutant proteins as quantified after a 24 h chase with respect to the amount labeled at t0. The graph presents averages obtained in 3 to 6 different experiments. C, 293Tcells were transfected with DNA coding the indicated Flag-tagged Vpx mutants along with DNA coding for GST or for a WT Flag-tagged-SIVMAC Vpx GST-fusion protein (GST-WT-Vpx). Cells were lysed 2 days post-transfection and complexes isolated with glutathione-beads prior to WB analysis. The panels present representative data obtained from 3–5 independent experiments.
The Decreased Stability of Vpx May Relate to a Decreased Ability to Undergo Vpx:Vpx Interactions
To determine whether the Vpx mutations affected Vpx:Vpx interactions, a set of Vpx mutants was assayed for its ability to associate to WT-Vpx-GST upon coexpression in 293T cells (Fig. 6C). WT SIVMAC and HIV-2 Vpxproteins, but not the distantly related SIVRCM, associated to WT-Vpx-GST, as most of the mutants examined here. The sole exceptions were the mutant Q76A that bound to a lower extent and the ΔPro and K77A/L72K mutants that had lost their ability to associate to WT-Vpx-GST. As shown above, the latter two Vpx mutants displayed decreased stability over wild type, indicating that the ability to undergo Vpx:Vpx interactions may stabilize Vpx. However, this property does not seem to be associated to the functionality of Vpx mutants during the early phases of infection of DCs.
DISCUSSION
SAMHD1 has been recently identified as a prominent factor counteracted by Vpx, and to better characterize this interaction, we have re-examined a large panel of Vpx mutants that we had previously characterized for their ability to increase lentiviral infectivity in DCs. Our results indicate that a strict correlation exists between the functionality of Vpx in DCs infection and its ability to mediate SAMHD1 depletion, pointing to the fact that the removal of this factor is a key event for the efficient lentiviral infection of these cells. Our analysis identifies several residues distributed throughout Vpx that are important for this function. Some of them have been reported, albeit controversially, to be sites of post translational modification of Vpx, as phosphorylation (23, 32). These modifications may finely regulate the ability of Vpx to interact with SAMHD1 and with other cellular factors that concur in the restrictive environment of myeloid cells against lentiviruses, a hypothesis that we are currently exploring.
Given that only a definite amount of Vpx is present during the early phases of infection (the one incorporated into virions), we assessed whether Vpx protein stability could ultimately affect its functionality. Our results indicate that Vpx stability is not a determining factor in Vpx functionality, as at least two Vpx mutants with decreased stability retain wild type functionality during the infection of DCs (C83R and ΔPro). Similarly, the ability to undergo Vpx:Vpx interactions does not seem a major factor in the functionality of Vpx, as two Vpx mutants fail to associate to WT Vpx, yet behave as wild type (K77A/L72A and ΔPro). Thus, although the stability of Vpx seems to be influenced by Vpx:Vpx interactions, neither parameters are limiting in the functionality of Vpx, suggesting that Vpx may remove SAMHD1 as a monomer and in an highly efficient manner, through the Cul4A-DDB1-DCAF1 E3 ubiquitin ligase complex, (3, 4, 33, 34), although a DCAF1-independent mechanism of action of Vpx has been also proposed (20, 35).
The intracellular localization in which SAMHD1 and Vpx interact during infection remains unclear. The localization of Vpx does not seem to be a limiting factor, given that Vpx exhibits both nuclear and cytoplasmic distributions. In contrast, the localization of SAMHD1 in the presence or absence of viral challenge is less clear. If most reports indicate SAMHD1 as essentially nuclear (24, 26, 36, 37), a recent study indicated that SAMHD1 could be also found in the cytoplasm, at least in quiescent lymphocytes (38). Similarly, SAMHD1 degradation had been suggested to occur entirely in the nucleus based on the observations that NLS mutants of SAMHD1 (i.e. cytoplasmic) could resist Vpx-mediated degradation (24, 26). However, this is in contrast with the fact that Vpx removes both nuclear and cytoplasmic fractions of SAMHD1 during the infection of quiescent lymphocytes. Lastly, Vpx has been proposed to induce the relocalization of SAMHD1 in the cell cytoplasm (25).
Our analysis of the intracellular distribution of SAMHD1 in primary macrophages during viral infection indicates that endogenous SAMHD1 does not relocalize in the cell cytoplasm during infection. This observation is supported by heterokaryon experiments that indicate that SAMHD1 does not possess a nuclear export signal and that therefore once it enters the nucleus, lacks the ability to leave it. Given that Vpx reaches the nucleus, the simplest explanation is that WT Vpx degrades SAMHD1 directly in this location as proposed (24, 26). However, the functionality of a Vpx mutant that displays an exquisite cytoplasmic distribution suggests a more complex and perhaps dynamic mechanism of degradation. Before reaching its final destination in the nucleus, SAMHD1 has to transit through the cell cytoplasm after its translation. The results obtained with our K84/85A Vpx mutant suggest that during this time SAMHD1 is also targeted for degradation by Vpx. Since WT Vpx is also distributed in the cell cytoplasm, this mechanism of degradation may therefore apply also to WT Vpx. The fact that these cytoplasmic foci of SAMHD1 and Vpx are detectable only upon co-expression with the K84A/K85A and not WT Vpx may indicate that the mutant is more efficient at retaining SAMHD1 in the cytoplasm, so that its accumulation becomes detectable only in this case with the technique used here.
This mechanism of cytoplasmic degradation is highly efficient as no kinetic differences were observed in primary macrophages between mutant and WT Vpx protein and in both cases, degradation proceeded through a proteasome-dependent mechanism, as attested by the impairment of SAMHD1 degradation in the presence of MG132. Thus, while the nuclear pool of SAMHD1 is depleted directly in the nucleus, Vpx may also intercept newly generated SAMHD1 before it enters the nucleus, exerting the very potent degradation activity that is observed here.
Finally, the restrictive environment of myeloid cells is likely the result of several mechanisms of defense to which numerous cellular factors and among them SAMHD1, contribute more or less effectively. Multiple lines of evidence clearly indicate the presence of additional factors that are not counteracted by Vpx, as demonstrated by the drastic drop in infectivity observed upon stimulation of DCs with interferons in viruses naturally coding Vpx (HIV-2/SIVSM members) (35). Whether and how these restrictions are connected to the basal mechanism of resistance specified by SAMHD1 remains to be determined.
Acknowledgments
We thank Michelle Ainouze for technical support, Jeanine Bernaud and Dominique Rigal for help with blood sample collection, Evelyne Manet and Henri Gruffat for help with heterokaryons, the PLATIM of the UMS3444/US8 in Lyon for help in imaging, and the AIDS Reagents and Reference Program of the National Institutes of Health for providing the indicated material.
This work was supported by the ANRS, the ENS-L, the FRM, the InCa, and Sidaction.
- DC
- dendritic cell
- SAMHD1
- sterile α motif-hydroxylase domain 1 protein
- VLP
- virion-like particle
- CHX
- cyclohexamide.
REFERENCES
- 1. Piguet V., Steinman R. M. (2007) The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 28, 503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malim M. H., Emerman M. (2008) HIV-1 accessory proteins-ensuring viral survival in a hostile environment. Cell Host Microbe 3, 388–398 [DOI] [PubMed] [Google Scholar]
- 3. Srivastava S., Swanson S. K., Manel N., Florens L., Washburn M. P., Skowronski J. (2008) Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 4, e1000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergamaschi A., Ayinde D., David A., Le Rouzic E., Morel M., Collin G., Descamps D., Damond F., Brun-Vezinet F., Nisole S., Margottin-Goguet F., Pancino G., Transy C. (2009) The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection. J. Virol. 83, 4854–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharova N., Wu Y., Zhu X., Stranska R., Kaushik R., Sharkey M., Stevenson M. (2008) Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 4, e1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goujon C., Jarrosson-Wuillème L., Bernaud J., Rigal D., Darlix J. L., Cimarelli A. (2006) With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Ther. 13, 991–994 [DOI] [PubMed] [Google Scholar]
- 7. Goujon C., Rivière L., Jarrosson-Wuilleme L., Bernaud J., Rigal D., Darlix J. L., Cimarelli A. (2007) SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ayinde D., Maudet C., Transy C., Margottin-Goguet F. (2010) Limelight on two HIV/SIV accessory proteins in macrophage infection: is Vpx overshadowing Vpr? Retrovirology 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berger A., Münk C., Schweizer M., Cichutek K., Schüle S., Flory E. (2010) Interaction of Vpx and apolipoprotein B mRNA-editing catalytic polypeptide 3 family member A (APOBEC3A) correlates with efficient lentivirus infection of monocytes. J. Biol. Chem. 285, 12248–12254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berger G., Durand S., Fargier G., Nguyen X. N., Cordeil S., Bouaziz S., Muriaux D., Darlix J. L., Cimarelli A. (2011) APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 7, e1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hrecka K., Hao C., Gierszewska M., Swanson S. K., Kesik-Brodacka M., Srivastava S., Florens L., Washburn M. P., Skowronski J. (2011) Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Ségéral E., Yatim A., Emiliani S., Schwartz O., Benkirane M. (2011) SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berger A., Sommer A. F., Zwarg J., Hamdorf M., Welzel K., Esly N., Panitz S., Reuter A., Ramos I., Jatiani A., Mulder L. C., Fernandez-Sesma A., Rutsch F., Simon V., König R., Flory E. (2011) SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutières syndrome are highly susceptible to HIV-1 infection. PLoS Pathog. 7, e1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lahouassa H., Daddacha W., Hofmann H., Ayinde D., Logue E. C., Dragin L., Bloch N., Maudet C., Bertrand M., Gramberg T., Pancino G., Priet S., Canard B., Laguette N., Benkirane M., Transy C., Landau N. R., Kim B., Margottin-Goguet F. (2012) SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 13, 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim B., Nguyen L. A., Daddacha W., Hollenbaugh J. A. (2012) Tight interplay among SAMHD1 protein level, cellular dNTP levels, and HIV-1 proviral DNA synthesis kinetics in human primary monocyte-derived macrophages. J. Biol. Chem. 287, 21570–21574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diamond T. L., Roshal M., Jamburuthugoda V. K., Reynolds H. M., Merriam A. R., Lee K. Y., Balakrishnan M., Bambara R. A., Planelles V., Dewhurst S., Kim B. (2004) Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 279, 51545–51553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Brien W. A., Namazi A., Kalhor H., Mao S. H., Zack J. A., Chen I. S. (1994) Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J. Virol. 68, 1258–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Triques K., Stevenson M. (2004) Characterization of restrictions to human immunodeficiency virus type 1 infection of monocytes. J. Virol. 78, 5523–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berger G., Durand S., Goujon C., Nguyen X. N., Cordeil S., Darlix J. L., Cimarelli A. (2011) A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat. Protoc. 6, 806–816 [DOI] [PubMed] [Google Scholar]
- 20. Goujon C., Arfi V., Pertel T., Luban J., Lienard J., Rigal D., Darlix J. L., Cimarelli A. (2008) Characterization of simian immunodeficiency virus SIVSM/human immunodeficiency virus type 2 Vpx function in human myeloid cells. J. Virol. 82, 12335–12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cimarelli A., Sandin S., Höglund S., Luban J. (2000) Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J. Virol. 74, 3046–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Belshan M., Kimata J. T., Brown C., Cheng X., McCulley A., Larsen A., Thippeshappa R., Hodara V., Giavedoni L., Hirsch V., Ratner L. (2012) Vpx is critical for SIVmne infection of pigtail macaques. Retrovirology 9, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Belshan M., Mahnke L. A., Ratner L. (2006) Conserved amino acids of the human immunodeficiency virus type 2 Vpx nuclear localization signal are critical for nuclear targeting of the viral preintegration complex in non-dividing cells. Virology 346, 118–126 [DOI] [PubMed] [Google Scholar]
- 24. Hofmann H., Logue E. C., Bloch N., Daddacha W., Polsky S. B., Schultz M. L., Kim B., Landau N. R. (2012) J. Virol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laguette N., Rahm N., Sobhian B., Chable-Bessia C., Münch J., Snoeck J., Sauter D., Switzer W. M., Heneine W., Kirchhoff F., Delsuc F., Telenti A., Benkirane M. (2012) Evolutionary and functional analyses of the interaction between the myeloid restriction factor SAMHD1 and the lentiviral Vpx protein. Cell Host Microbe 11, 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brandariz-Nuñez A., Valle-Casuso J. C., White T. E., Laguette N., Benkirane M., Brojatsch J., Diaz-Griffero F. (2012) Role of SAMHD1 nuclear localization in restriction of HIV-1 and SIVmac. Retrovirology 9, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arfi V., Lienard J., Nguyen X. N., Berger G., Rigal D., Darlix J. L., Cimarelli A. (2009) Characterization of the behavior of functional viral genomes during the early steps of human immunodeficiency virus type 1 infection. J. Virol. 83, 7524–7535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borer R. A., Lehner C. F., Eppenberger H. M., Nigg E. A. (1989) Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 56, 379–390 [DOI] [PubMed] [Google Scholar]
- 29. Boyd M. T., Vlatkovic N., Rubbi C. P. (2011) The nucleolus directly regulates p53 export and degradation. J. Cell Biol. 194, 689–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hiriart E., Farjot G., Gruffat H., Nguyen M. V., Sergeant A., Manet E. (2003) A novel nuclear export signal and a REF interaction domain both promote mRNA export by the Epstein-Barr virus EB2 protein. J. Biol. Chem. 278, 335–342 [DOI] [PubMed] [Google Scholar]
- 31. Kewalramani V. N., Emerman M. (1996) Vpx association with mature core structures of HIV-2. Virology 218, 159–168 [DOI] [PubMed] [Google Scholar]
- 32. Rajendra Kumar P., Singhal P. K., Subba Rao M. R., Mahalingam S. (2005) Phosphorylation by MAPK regulates simian immunodeficiency virus Vpx protein nuclear import and virus infectivity. J. Biol. Chem. 280, 8553–8563 [DOI] [PubMed] [Google Scholar]
- 33. Lim E. S., Fregoso O. I., McCoy C. O., Matsen F. A., Malik H. S., Emerman M. (2012) The ability of primate lentiviruses to degrade the monocyte restriction factor SAMHD1 preceded the birth of the viral accessory protein Vpx. Cell Host Microbe 11, 194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahn J., Hao C., Yan J., DeLucia M., Mehrens J., Wang C., Gronenborn A. M., Skowronski J. (2012) HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1. J. Biol. Chem. 287, 12550–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pertel T., Reinhard C., Luban J. (2011) Vpx rescues HIV-1 transduction of dendritic cells from the antiviral state established by type 1 interferon. Retrovirology 8, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rice G. I., Bond J., Asipu A., Brunette R. L., Manfield I. W., Carr I. M., Fuller J. C., Jackson R. M., Lamb T., Briggs T. A., Ali M., Gornall H., Couthard L. R., Aeby A., Attard-Montalto S. P., Bertini E., Bodemer C., Brockmann K., Brueton L. A., Corry P. C., Desguerre I., Fazzi E., Cazorla A. G., Gener B., Hamel B. C., Heiberg A., Hunter M., van der Knaap M. S., Kumar R., Lagae L., Landrieu P. G., Lourenco C. M., Marom D., McDermott M. F., van der Merwe W., Orcesi S., Prendiville J. S., Rasmussen M., Shalev S. A., Soler D. M., Shinawi M., Spiegel R., Tan T. Y., Vanderver A., Wakeling E. L., Wassmer E., Whittaker E., Lebon P., Stetson D. B., Bonthron D. T., Crow Y. J. (2009) Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goncalves A., Karayel E., Rice G. I., Bennett K. L., Crow Y. J., Superti-Furga G., Bürckstümmer T. (2012) SAMHD1 is a nucleic acid-binding protein that is mislocalized due to aicardi-goutières syndrome-associated mutations. Hum. Mutat. 33, 1116–1122 [DOI] [PubMed] [Google Scholar]
- 38. Baldauf H. M., Pan X., Erikson E., Schmidt S., Daddacha W., Burggraf M., Schenkova K., Ambiel I., Wabnitz G., Gramberg T., Panitz S., Flory E., Landau N. R., Sertel S., Rutsch F., Lasitschka F., Kim B., Konig R., Fackler O. T., Keppler O. T. (2012) Nat. Med., in press [DOI] [PMC free article] [PubMed] [Google Scholar]