Background: ERK phosphorylation is enhanced by heparanase, an enzyme associated with aggressive behavior of multiple myeloma.
Results: Heparanase activates ERK by up-regulating insulin receptor phosphorylation and insulin receptor substrate-1.
Conclusion: Heparanase activates ERK by enhancing the insulin signaling pathway.
Significance: Targeting the insulin receptor signaling pathway may block the tumor-promoting effects of heparanase.
Keywords: Cancer, ERK, Glycobiology, Growth Hormone, Insulin, Heparanase, Insulin Receptor, Insulin Receptor Substrate-1, Myeloma
Abstract
ERK signaling regulates proliferation, survival, drug resistance, and angiogenesis in cancer. Although the mechanisms regulating ERK activation are not fully understood, we previously demonstrated that ERK phosphorylation is elevated by heparanase, an enzyme associated with aggressive behavior of many cancers. In the present study, myeloma cell lines expressing either high or low levels of heparanase were utilized to determine how heparanase stimulates ERK signaling. We discovered that the insulin receptor was abundant on cells expressing either high or low levels of heparanase, but the receptor was highly phosphorylated in heparanase-high cells compared with heparanase-low cells. In addition, protein kinase C activity was elevated in heparanase-high cells, and this enhanced expression of insulin receptor substrate-1 (IRS-1), the principle intracellular substrate for phosphorylation by the insulin receptor. Blocking insulin receptor function with antibody or a small molecule inhibitor or knockdown of IRS-1 expression using shRNA diminished heparanase-mediated ERK activation in the tumor cells. In addition, up-regulation of the insulin signaling pathway by heparanase and the resulting ERK activation were dependent on heparanase retaining its enzyme activity. These results reveal a novel mechanism whereby heparanase enhances activation of the insulin receptor signaling pathway leading to ERK activation and modulation of myeloma behavior.
Introduction
Heparanase, the only known mammalian endoglycosidase that cleaves heparan sulfate, is up-regulated in most human tumors (1–4). Clinically, increased heparanase levels are associated with increased tumor metastasis, high microvessel density, and reduced postoperative survival time of cancer patients (3, 5, 6). Although some of the tumor-promoting effects of heparanase can be attributed to its ability to remodel the extracellular matrix barrier by cleaving heparan sulfate chains, heparanase is also known to regulate cell signaling and gene transcription (3, 7, 8). For example, heparanase induces endothelial cell migration via protein kinase B/Akt activation (9) and VEGF expression via Src signaling (10). Heparanase enhances syndecan-1 shedding (11, 12), and we previously demonstrated that this occurs due to heparanase-driven activation of ERK, which increases expression of MMP-9,2 a syndecan-1 sheddase (13). Shed syndecan-1 in turn acts within the tumor microenvironment to stimulate further tumor growth, angiogenesis, and dissemination (14, 15). Other studies have shown that activation of the ERK signaling pathway plays an important role in the pathogenesis of myeloma by mediating cell proliferation, survival, drug resistance, and angiogenesis (16, 17). Moreover, inhibitors of the MAPK pathway inhibit myeloma cell growth and osteoclast differentiation (18, 19). Thus, activation of ERK by heparanase may promote myeloma progression via multiple mechanisms.
The primary mediators of signal transduction are receptor tyrosine kinases (RTKs) present on cell surfaces. RTKs such as the insulin receptor couple ligand binding to downstream intracellular signaling cascades and gene transcription. The insulin receptor is a plasma cell marker that is absent on memory B cells but induced upon differentiation into plasma cells (20). It is highly expressed in normal plasma cells and multiple myeloma cells and can associate with insulin-like growth factor 1 receptor (IGF-1R) and exist as an insulin/IGF-1 hybrid receptor on XG myeloma cell lines (20). The insulin receptor is not associated with myeloma disease progression, but the interaction of insulin receptor with its ligand insulin can induce growth of myeloma cells (20). Insulin is as potent as IGF-1 in inducing growth of myeloma cells and by binding to insulin receptor, insulin can trigger the phosphorylation of insulin receptor, AKT, and MAPK in myeloma cells (20). Recent studies support the role of insulin as an important growth factor acting through the tyrosine kinase growth factor cascade to enhance tumor cell proliferation (21). Even hyperinsulinemia linked to obesity is shown to be associated with an increased risk of multiple myeloma (22).
The insulin receptor substrate 1 (IRS-1) protein is the principal intracellular substrate of insulin receptor tyrosine kinase activity and is the most upstream molecule in the signal transduction cascade mediated by insulin, interleukin 4, and IGF-1 stimulation (23, 24). IRS-1 docks with both the IGF-1R and the insulin receptor (25). IRS-1 expression and phosphorylation is often increased in human cancers and over expression of IRS-1 can induce cellular transformation. Although little is known about the role of IRS-1 in myeloma, a study looking at the variation in genes related to the IGF-1 signaling pathway identified IRS-1 as a major candidate associated with the risk of myeloma (26).
Because heparanase stimulates ERK signaling and because this signaling is known to enhance myeloma progression, we sought to determine the mechanism whereby heparanase was activating ERK. We discovered that heparanase expression leads to stimulation of insulin receptor phosphorylation and protein kinase C (PKC) activity. PKC up-regulates the expression of IRS-1, which is then phosphorylated by the kinase activity of the insulin receptor. High levels of phospho-IRS-1 in turn activate ERK signaling. These findings suggest a prominent role for heparanase in initiating the insulin signaling cascade and subsequent activation of ERK signaling in multiple myeloma.
EXPERIMENTAL PROCEDURES
Cell Lines
RPMI-8226, U266, and CAG myeloma cells were cultured in RPMI 1640 growth medium supplemented with 10% fetal bovine serum. CAG cells were transfected as previously described with empty vector or vector containing the cDNA for human heparanase to generate heparanase-low and heparanase-high cells, respectively (12). CAG cells were also transfected with a vector containing a mutated cDNA (mutated at amino acid 225) that codes for enzymatically inactive heparanase (12).
Western Blot
Western blotting was performed as described previously (13). Membranes were incubated with primary antibodies that recognize phospho-ERK (Santa Cruz Biotechnology), total ERK (Santa Cruz Biotechnology), phospho-insulin receptor (which detects the phosphorylation of tyrosine at 1162 and 1163 of β-subunit) (R&D Systems), insulin receptor (which detects the α-subunit) (Abcam), phospho-IRS-1 (Millipore), IRS-1 (Millipore), and actin (Sigma). Secondary antibody conjugated with horseradish peroxidase (HRP) (Vector Laboratories, Inc.) was used at 1:2000 dilution to detect primary antibodies, and enzymatic signals were visualized by chemiluminescence. For some experiments, cells were treated with recombinant human heparanase (250 ng/ml; provided by Dr. Israel Vlodavsky) and incubated at 37 °C for 2 h. To examine the potential role of insulin in activating ERK signaling, serum-starved CAG cells were treated with different doses of insulin (Roche Applied Science) for 15 min. For some experiments, CAG heparanase-high cells grown in complete medium were treated with anti-insulin antibody (which prevents insulin binding to the α-subunit of insulin receptor) (Abcam) or an isotype-matched control antibody. In some experiments, cells were treated with 10 μm of AG1024 (Santa Cruz Biotechnology) (an inhibitor of insulin receptor tyrosine kinase activity) (27) or 10 μm of picropodophyllin (Santa Cruz Biotechnology) (an IGF-1R inhibitor). For experiments using recombinant enzyme, serum-starved RPMI-8226 or CAG cells were pretreated with or without 30 μm AG1024 for 2 h followed by exposure to recombinant heparanase (250 ng/ml) for another 2 h. Cells were then lysed and analyzed for ERK signaling. Band densities of Western blots were quantified using NIH ImageJ software.
Immunohistochemistry
Sections from formalin-fixed tumor tissues formed from heparanase-low or heparanase-high cells were stained for phospho-ERK (Cell Signaling), phospho-IRS-1 (Millipore), and total-IRS-1 (Millipore), as described previously (13).
Phospho-RTK Array
Arrays were purchased from R&D Systems. Array membranes were incubated with cell lysates and processed as recommended by the manufacturer's instructions using a phospho-tyrosine-specific antibody conjugated to HRP. Approximately 107 CAG heparanase-high or heparanase-low cells were maintained in full serum overnight and lysed with a buffer containing 1% Nonidet P-40, 20 mm Tris-HCl, pH 8.0, 137 mm NaCl, 10% glycerol, 2 mm EDTA, 1 mm sodium orthovanadate, 10 μg/ml aprotinin, and 10 μg/ml leupeptin for 30 min. Protein assay (Pierce) was performed before incubating the lysates with the RTK membranes, and signals were visualized using chemiluminescence.
Flow Cytometry
The expression of insulin receptor was evaluated by incubating cells with anti-human insulin receptor antibody (Abcam) in PBS on ice for 1 h. The cells were then washed and incubated with secondary antibody conjugated to phycoerythrin (BD Biosciences) on ice for 30 min. Following incubation, the cells were washed, resuspended in PBS, and subjected to flow cytometry analysis using a Becton Dickinson FACSCalibur.
Quantification of IRS-1
Endogenous levels of IRS-1 in cells were measured using a commercially available IRS-1 sandwich ELISA kit (Cell Signaling), according to the manufacturer's instructions. The magnitude of absorbance for the color developed is proportional to the quantity of total IRS-1.
Knockdown of IRS-1 by shRNA
IRS-1 knockdown was performed using MISSION lentiviral transduction particles from Sigma. Lentiviral transduction particles are produced from a lentiviral plasmid vector containing the following two different shRNA sequences for the human IRS-1 gene: IRS-1 shRNA 1, CCGGGCTAAGCAACTATATCTGCATCTCGAGATGCAGATATAGTTGCTTAGCTTTTTG; IRS-1 shRNA 2, CCGGCCTACTACTCATTGCCAAGATCTCGAGATCTTGGCAATGAGTAGTAGGTTTTTG. The non-target shRNA control transduction particles containing the sequence CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT does not target any human gene but will activate the RNAi pathway. Briefly, to 105 CAG heparanase-high cells, 25 μl of lentiviral particles were added and incubated for 18–20 h at 37 °C in a humidified incubator. The next day, the medium containing the lentiviral particles was removed, and fresh complete RPMI medium was added to each well. The cells were then selected with puromycin (5 μg/ml) and assessed for IRS-1 knockdown by ELISA and Western blotting.
Gelatin Zymography
After cells were incubated with serum-free medium for 48 h, supernatants were collected and concentrated in Centriplus columns with a 30-kDa cut-off value (Millipore). Protein in the concentrated media was quantified using the BCA protein assay reagent kit (Pierce), and an equal amount of protein (50 μg) was mixed with non-reducing sample buffer (62.5 mm Tris-HCl, pH 6.8, 25% glycerol, 4% SDS, and 0.01% bromphenol blue) and analyzed by SDS-PAGE using 10% polyacrylamide gels co-polymerized with gelatin (Bio-Rad). Electrophoresis was carried out at 10 mA for 2 h. The SDS in the acrylamide gel was extracted by incubation with 2.5% Triton X-100 solution for 2 h at room temperature, and gelatinolytic activities were developed in a buffer containing 50 mm Tris-HCl, pH 7.5, 200 mm NaCl, 5 mm CaCl2, and 0.02% Brij 35 at 37 °C overnight. The gel was then stained with Coomassie Blue. Following destaining, sites of proteolytic activity were visualized as clear bands against the blue background of stained gelatin.
Quantification of Syndecan-1
Medium conditioned for 48 h by CAG cells (106 cells/ml) was collected, and the level of shed syndecan-1 present in the conditioned medium was determined by ELISA as described (12). In some experiments, the cells were incubated with either the PKC inhibitor staurosporine (100 nm) (Sigma) or dimethyl sulfoxide control for 24 h, the cell culture medium was collected, and levels of shed syndecan-1 were assessed.
Protein Kinase C Assay
PKC activity was measured using a commercially available, nonradioactive protein kinase assay kit (Calbiochem). The kit utilizes a peptide pseudosubstrate precoated on a 96-well plate. Following exposure of wells to whole cells extracts, the extent of reaction is measured using a biotinylated monoclonal antibody that recognizes the phosphorylated form of the peptide pseudosubstrate. The biotinylated monoclonal antibody is detected using HRP-conjugated streptavidin. The absorbance was read at 492 nm. The PKC activity is directly proportional to the color intensity.
Real Time PCR
Approximately 106 cells were treated with the PKC inhibitor staurosporine (100 nm) for 12 h. RNA was extracted (RNeasy mini kit, Qiagen), and cDNA was synthesized (Clontech, Mountain View, CA). Real-time PCR was conducted using the following primers: MMP-9 (F), TGACAGCGACAAGAAGTG; MMP-9 (R), CAGTGAAGCGGTACATAGG; IRS-1 (F), CCACTCGGAAAACTTCTTCTTCAT; IRS-1 (R),AGAGTCATCCACCTGCATCCA; and SYBR Green Supermix (Bio-Rad). Expression was determined relative to 28 S rRNA.
Statistical Analysis
All results were representative of at least three independent experiments. Except where noted, comparisons between two groups were analyzed by Student's t test, and a p value ≤ 0.05 was considered statistically significant. Data are means ± S.D.
RESULTS
Heparanase Induces ERK1/2 Activation in Myeloma
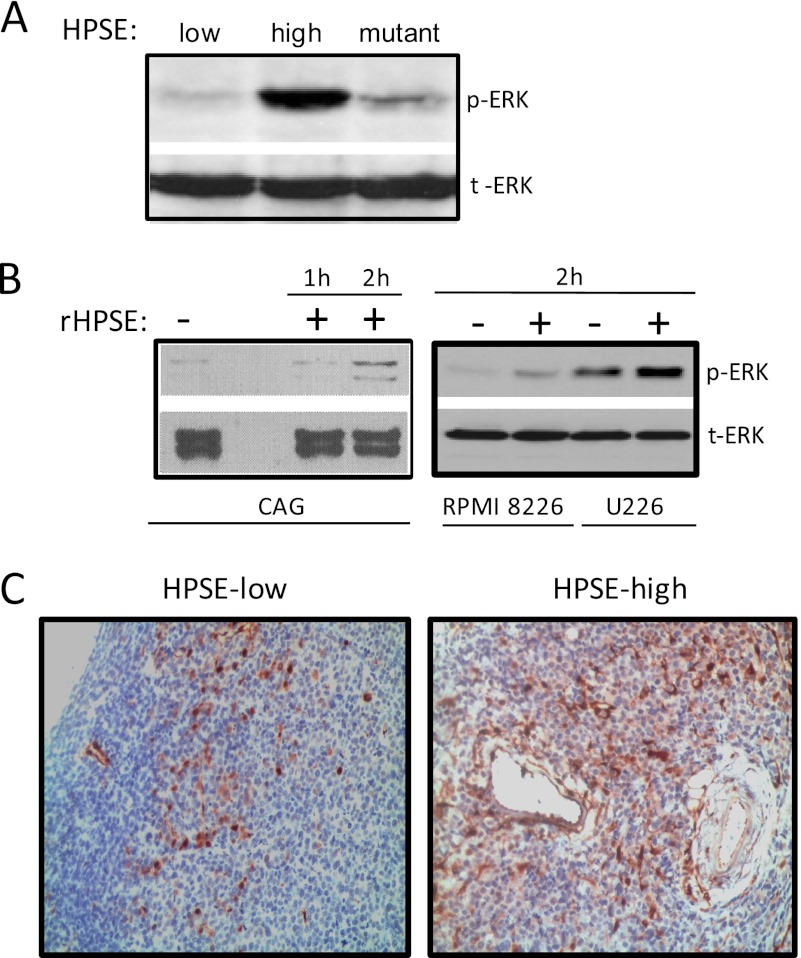
Activation of the ERK1/2 signaling cascade mediates human multiple myeloma growth, drug resistance, and survival (18, 28, 29). Here, using three different models, we examined the effect of heparanase on ERK activation. In the first model, CAG myeloma cells engineered to express low or high levels of heparanase or a mutated form of heparanase that lacks heparan sulfate-degrading enzyme activity were utilized. Western blot analysis demonstrates that heparanase-high cells have significantly higher levels of phospho-ERK1/2 compared with heparanase-low or mutant cells lacking enzyme activity (Fig. 1A). It is important to note that the level of heparanase expression and activity in the heparanase-high CAG cells is similar to that found in some myeloma patient tumors (6, 30). Thus, the increase in ERK activation is not due to an enhancement of heparanase expression beyond levels that are likely to be present in the human cancer microenvironment. In addition, a role for heparanase in ERK activation is supported by our previous finding that treatment of heparanase-high cells with SST0001, an inhibitor of heparanase, lowers ERK activation and blocks the aggressive tumor growth of heparanase-high cells (31).
FIGURE 1.
Heparanase promotes ERK phosphorylation in myeloma. A, protein lysates from CAG human myeloma cells expressing either low or high levels of heparanase (HPSE-low or HPSE-high) or mutant heparanase that lacks enzyme activity were subjected to Western blot analysis. Membranes were probed with anti-phospho-ERK (p-ERK) antibody or total ERK (t-ERK) antibody. B, serum-starved wild-type CAG, RPMI 8266, or U226 cells were treated with or without recombinant heparanase (rHPSE) for the indicated time points, and cell lysates were immunoblotted for p-ERK and total ERK. C, heparanase enhances ERK activation in myeloma tumors growing in vivo. Subcutaneous tumors in SCID mice formed by heparanase-low or heparanase-high cells were removed and immunostained with antibody to p-ERK. Original magnification, 1300×.
To confirm and extend these findings, we used recombinant human heparanase, which when introduced to cells is taken up by cells and remains biologically active (12, 13, 32). 2 h after addition of recombinant heparanase, levels of phosphorylated ERK were elevated in three different myeloma cell lines compared with cells not receiving exogenous heparanase (Fig. 1B). This indicates that exogenous heparanase can enhance a relatively rapid up-regulation of ERK activation and that the effect on ERK activation seen in heparanase-high myeloma cells is not simply an artifact related to their transfection.
Because heparanase promotes tumor progression (6) and because MEK/ERK signaling plays a central role in the pathogenesis of myeloma (18), we investigated whether the heparanase mediated up-regulation of ERK activation seen in vitro also occurs within tumors growing in vivo. Immunohistochemistry revealed that tumors formed in mice by heparanase-high cells have high levels of phospho-ERK compared with cells within tumors formed by heparanase-low cells (Fig. 1C).
Heparanase Promotes Insulin Receptor Activation
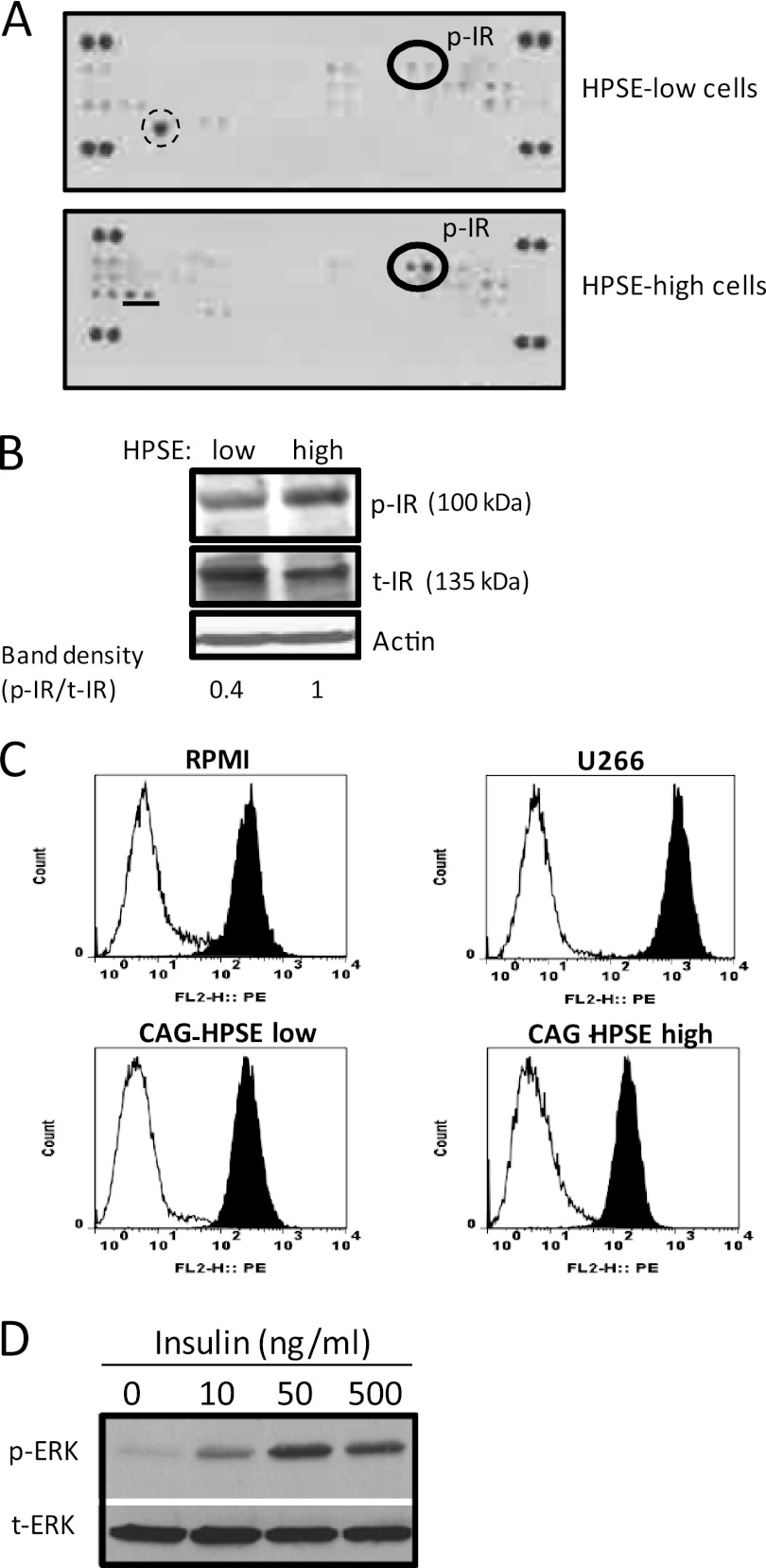
We used an antibody array system that simultaneously examines the relative tyrosine phosphorylation level of 42 different RTKs (supplemental Table 1) to identify the upstream signaling events driving ERK phosphorylation. Results demonstrate that heparanase evoked an increase in phosphorylation of the insulin receptor in the heparanase-high cells compared with heparanase-low cells (Fig. 2A). Western blotting confirmed that there was more phosphorylated insulin receptor present in the heparanase-high cells than in the heparanase-low cells (Fig. 2B).
FIGURE 2.
Insulin receptor (IR) is the prominent RTK activated by heparanase in myeloma cells. A, an antibody array system that simultaneously examines the relative tyrosine phosphorylation level of 42 different RTKs was utilized to analyze cell lysates from CAG cells expressing either low or high levels of heparanase. Membranes were probed using a phospho-tyrosine-specific antibody, dots containing phosphorylated insulin receptor (p-IR) are circled. The large single spot circled by dashed lines in heparanase (HSPE)-low cells is a nonspecific spot and does not correspond to an RTK present on the membrane. The spots represented by an underline in HPSE-high cells correspond to neurotrophic tyrosine kinase receptor type 1 (TrkA). This RTK, along with two other RTKs (c-Ret and FGFR2), is also activated in wild-type CAG myeloma cells, suggesting that these RTKs are not regulated by heparanase expression.3 The duplicate dots at each corner represent phospho-tyrosine positive controls. B, whole cell extracts were subjected to Western blot analysis and probed with antibodies against the total IR (t-IR) or phosphorylated IR (p-IR). The band density was determined by analysis of scanned images using ImageJ software. C, cell surface levels of IR were analyzed on myeloma cells by flow cytometry using an antibody specific for the α-subunit of IR. X-axis values represent arbitrary logarithmic fluorescent units of the phycoerythrin (PE)-tagged mAb. D, insulin stimulates ERK activation in myeloma cells. CAG cells were serum-starved overnight and then stimulated with insulin at the indicated concentration for 15 min. Aliquots of cell extracts that contained equivalent amounts of total protein were resolved by SDS gel electrophoresis and then immunoblotted using antibody specific for p-ERK or total ERK.
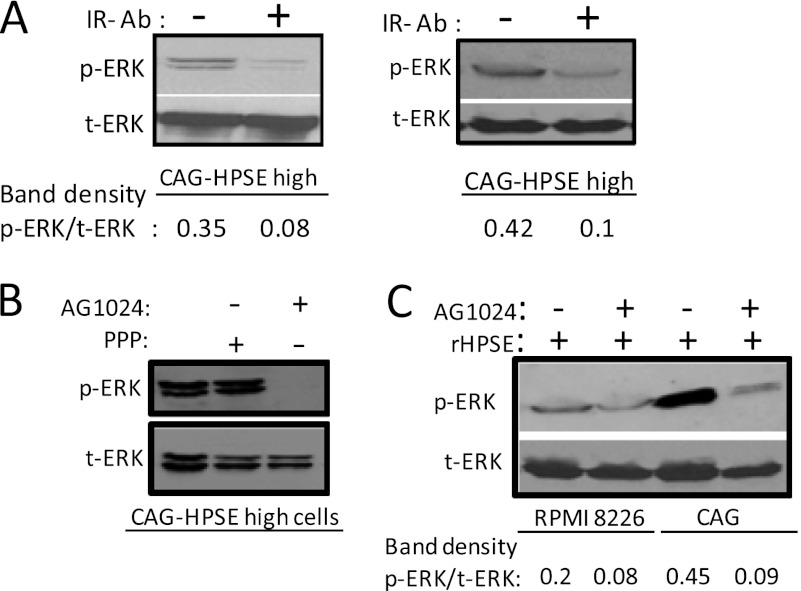
It has been demonstrated that the insulin receptor is a plasma cell marker, and its expression is increased throughout normal differentiation of plasma cells (20). FACS analysis using an antibody specific for the α-subunit of insulin receptor demonstrated that in all three multiple myeloma cell lines tested (CAG, RPMI 8226, and U266), there were high levels of cell surface insulin receptor (Fig. 2C). Interestingly, insulin receptor expression goes down slightly in heparanase-high CAG cells compared with heparanase-low CAG cells. However, there does not appear to be a consistent correlation between levels of insulin receptor expression and heparanase expression because U266 cells, which express high levels of heparanase,3 have high levels of insulin receptor (Fig. 2C). Three different approaches were then utilized to investigate whether triggering the insulin receptor can induce ERK signaling in myeloma cells. In the first approach, we added varying doses of insulin to serum-starved CAG cells. Western blot analysis revealed that insulin stimulates ERK activation and the levels of phospho-ERK increased with increasing doses of insulin (Fig. 2D). In the second approach, to heparanase-high cells growing in complete medium, we added either an anti-insulin receptor antibody (which binds to the α-subunit of insulin receptor and blocks insulin binding) or AG1024 (a specific inhibitor of IGF-1 and insulin receptor tyrosine kinase activity). After an overnight culture, the cells were harvested and analyzed for phospho-ERK by Western blot. Results demonstrate that both anti-insulin receptor antibody and AG1024 significantly reduced ERK phosphorylation (Fig. 3, A and B). Treatment of heparanase-high cells with picropodophyllin (a specific inhibitor for IGF-1R) did not affect ERK activation (Fig. 3B), confirming that the ERK activation is occurring via insulin receptor signaling. In a third approach, serum-starved CAG or RPMI 8226 wild-type cells were pretreated with or without AG1024 (30 μm) for 2 h followed by treatment with recombinant heparanase for additional 2 h. Western blot analysis revealed that activation of ERK by recombinant heparanase is inhibited in the presence of AG1024 (Fig. 3C). These results support the previously published studies demonstrating that insulin is a potent multiple myeloma cell growth factor (20) and that the MEK/ERK signaling pathway mediates myeloma cell survival and growth (18).
FIGURE 3.
Blocking insulin receptor signaling inhibits ERK activation by heparanase. A, blocking IR decreases ERK activation in heparanase (HPSE)-high CAG cells. IR function blocking antibody (clone 47-9) or an isotype-matched control antibody was added to heparanase-high CAG cells. After overnight incubation at 37 °C, whole-cell lysates were prepared and subjected to immunoblotting for p-ERK and total (t) ERK. The two bands represent p44 and p42 MAPK (Erk1 and Erk2), respectively. The two blots represent replicates of the same experiment. B, HPSE-high CAG cells were treated overnight with an IGF-1R inhibitor picropodophyllin (PPP) or an insulin receptor tyrosine kinase activity inhibitor AG1024 (10 μm). After overnight incubation at 37 °C, whole-cell lysates were prepared and subjected to immunoblotting for p-ERK and total ERK. C, serum-starved wild-type CAG cells or RPMI 8226 cells were pretreated with or without AG1024 (30 μm) for 2 h followed by addition of recombinant HPSE (rHPSE) (250 ng/ml). After 2 h of recombinant HPSE treatment, cell lysates were prepared and were immunoblotted for p-ERK and total ERK.
Heparanase Enhances the Expression of IRS-1 in Myeloma Cells
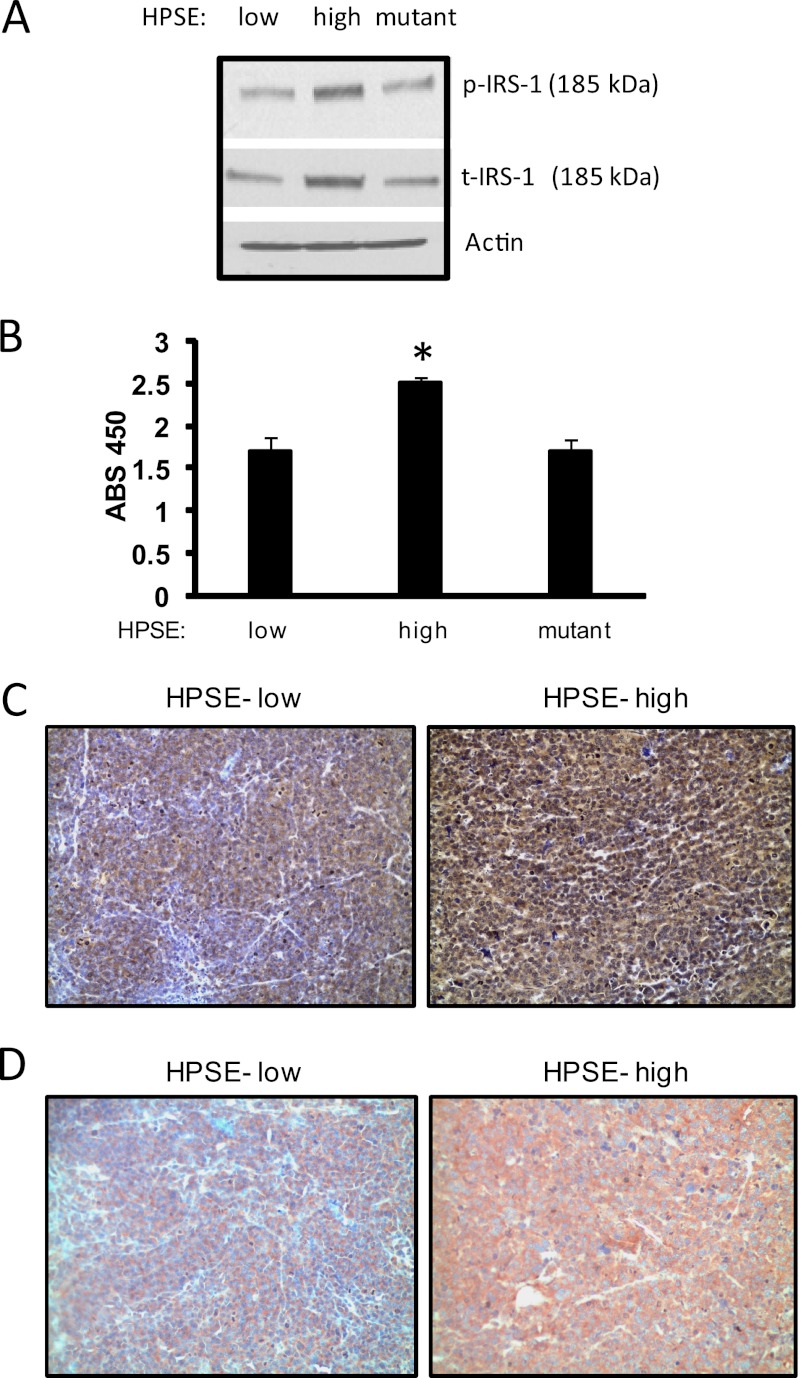
IRS-1 is the principle intracellular substrate of insulin receptor tyrosine kinase activity and is the most upstream molecule in the signal transduction cascade mediated by insulin and IGF (23, 24). It has also been proposed that IRS-1 is essential for insulin-induced mitogenic effects (33, 34). During insulin signaling, IRS-1 functions as an insulin receptor-specific docking protein to engage multiple downstream signaling molecules, including ERK (35). Because tyrosine phosphorylation of IRS-1 is involved in transmitting signals downstream to occupation of IGF-1 and insulin receptors, we sought to determine whether the enhanced insulin receptor tyrosine kinase activity mediated by heparanase is coupled with enhanced phosphorylation of IRS-1. By Western blot analysis, we found that phopho-IRS-1 levels were significantly elevated in heparanase-high cells compared with heparanase-low cells (Fig. 4A). To examine whether the difference in phosphorylated IRS-1 levels between these cells was secondary to an increase of IRS-1 content in heparanase-high cells, Western blot analysis of IRS-1 was performed. Results demonstrate that the amount of total IRS-1 was also up-regulated by heparanase expression in these cells (Fig. 4A). This was further confirmed by ELISA measuring the amount of total IRS-1 in whole cell extracts (Fig. 4B). Interestingly, levels of phospho-IRS-1 and total-IRS-1 were not elevated in cells expressing the mutated form of heparanase that lacks enzymatic activity, suggesting that elevation in IRS-1 levels is dependent on heparanase-mediated degradation of heparan sulfate chains. Together, these results indicate that heparanase stimulates the insulin signaling cascade in myeloma cells by both triggering insulin receptor phosphorylation and by up-regulating IRS-1 levels.
FIGURE 4.
Heparanase up-regulates IRS-1 levels in myeloma cells. An equal number of heparanase (HPSE)-low or heparanase-high or heparanase-mutant CAG cells were harvested, lysed, and subjected to Western blot analysis for phosphorylated IRS-1 (p-IRS-1) or total IRS-1 (t-IRS-1) (A) or quantified for IRS-1 protein level by ELISA (B). *, p ≤ 0.05 versus heparanase-low cells. Data are representative of three independent experiments. ABS, absorbance. C and D, heparanase enhances phosphorylation and total IRS-1 levels in myeloma tumors growing in vivo. Subcutaneous tumors formed by heparanase-low or heparanase-high cells were removed and immunostained for p-IRS-1 (C) and total IRS-1 (D) and counterstained with hematoxylin. Heparanase-high cells show intense staining for both p-IRS-1 and total IRS-1. Original magnification, 1300×.
Previously, we demonstrated that elevation of heparanase expression in CAG myeloma cells enhances their growth and metastasis in vivo as compared with control cells (6). Because IRS-1 is up-regulated in many cancers and plays an important role in tumor progression, we investigated whether the heparanase-mediated up-regulation of IRS-1 expression also occurs in tumors growing in mice. Immunostaining of myeloma tumors formed from heparanase-high CAG cells revealed that they have high levels of phosphorylated IRS-1 (Fig. 4 C) and total IRS-1 (Fig. 4D). In contrast, tumors formed from heparanase-low CAG cells contained low levels of the phosphorylated and total IRS-1. This dramatic increase in active IRS-1 correlates with the aggressive phenotype seen in these tumors formed from cells expressing high levels of heparanase (6).
IRS-1 Induces ERK Activation in Heparanase-high Cells
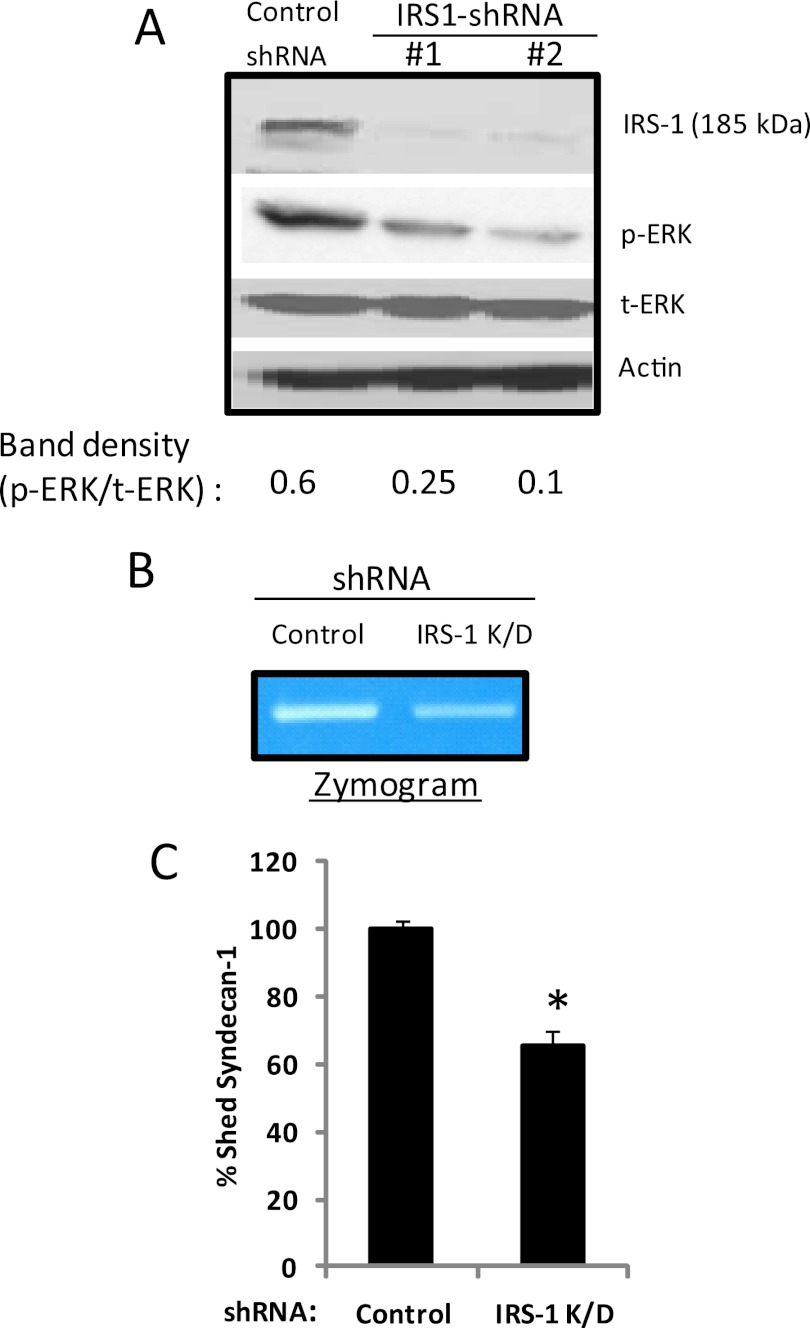
We have demonstrated that elevation of heparanase expression causes both enhanced ERK activation (Fig. 1) and increased IRS-1 levels (Fig. 4). It has been shown that IRS-1 can enhance ERK activation. Thus, we determined whether the enhanced IRS-1 levels seen in heparanase-high cells contribute to up-regulation of ERK activation. We used two distinct non-overlapping shRNA sequences in lentiviral vectors to knock down IRS-1 expression in heparanase-high cells. Vector containing a shRNA sequence that does not target any human gene served as a control. Following transduction with lentiviral vectors containing IRS-1 shRNA, as determined by Western blotting, IRS-1 expression was knocked down significantly (approaching 100%) by both the shRNA sequences (IRS-1 shRNA 1 and IRS-1 shRNA 2) (Fig. 5A). ELISA analysis for IRS-1 correlated with the Western blot data and confirmed that there was a near 100% knockdown of IRS-1 levels by both the shRNAs.3 To determine whether knockdown of IRS-1 altered ERK activation, we quantified phospho-ERK levels by Western blotting. Phospho-ERK was significantly decreased in IRS-1 knockdown cells compared with control (Fig. 5A). A low level of phosphorylated ERK in IRS-1 knockdown cells suggests that there may be additional pathways involved in regulating ERK signaling in heparanase-high cells.
FIGURE 5.
Knockdown of IRS-1 decreases ERK activation, MMP-9 activity, and shedding of syndecan-1 in heparanase-high cells. A, heparanase (HPSE)-high CAG cells were infected with lentiviral vectors coding for control or IRS-1 knockdown shRNAs. Western blotting of cell extracts of stably infected cells demonstrates effective knockdown of IRS-1 expression and concomitant decrease in ERK activation. B, zymogram showing levels of MMP-9 in conditioned medium of control or IRS-1 knockdown (K/D) cells indicates that MMP-9 activity levels are reduced when IRS-1 is knocked down as compared with controls. C, knockdown of IRS-1 decreases shedding of syndecan-1 in heparanase-high cells. Control or IRS-1 knockdown cells were plated at equal density for 24 h, conditioned media were harvested, and the level of syndecan-1 was quantified by ELISA. Values represent means of triplicate determination ± S.D. *, p ≤ 0.05 versus control shRNA.
We have demonstrated above that elevation of heparanase expression causes both activation of the insulin receptor-ERK signaling cascade and increased IRS-1 expression, which together induce ERK activation, thereby promoting aggressive behavior of myeloma cells (13, 15). As a biological readout of ERK activity, we monitored ERK-mediated up-regulation of MMP-9 expression and subsequent MMP-9 mediated shedding of syndecan-1 (13). To determine whether IRS-1 levels influence MMP-9 expression, serum-free conditioned media from IRS-1 knockdown and control cells were subjected to zymography. IRS-1 knockdown cells exhibited low gelatinolytic activity corresponding to pro-MMP-9 (92-kDa gelatinase) as compared with control cells (Fig. 5B). To determine whether this low MMP-9 in IRS-1 knockdown cells corresponds to decreased shedding of syndecan-1, we quantified the levels of syndecan-1 by ELISA. Fig. 5C shows that the level of shed syndecan-1 in IRS-1 knockdown cells is significantly lower compared with control cells. This confirms that heparanase mediated up-regulation of IRS-1 regulates ERK activation, leading to enhanced levels of activated MMP-9 and syndecan-1 shedding.
Protein Kinase C Enhances the Expression of IRS-1 in Heparanase-high Cells
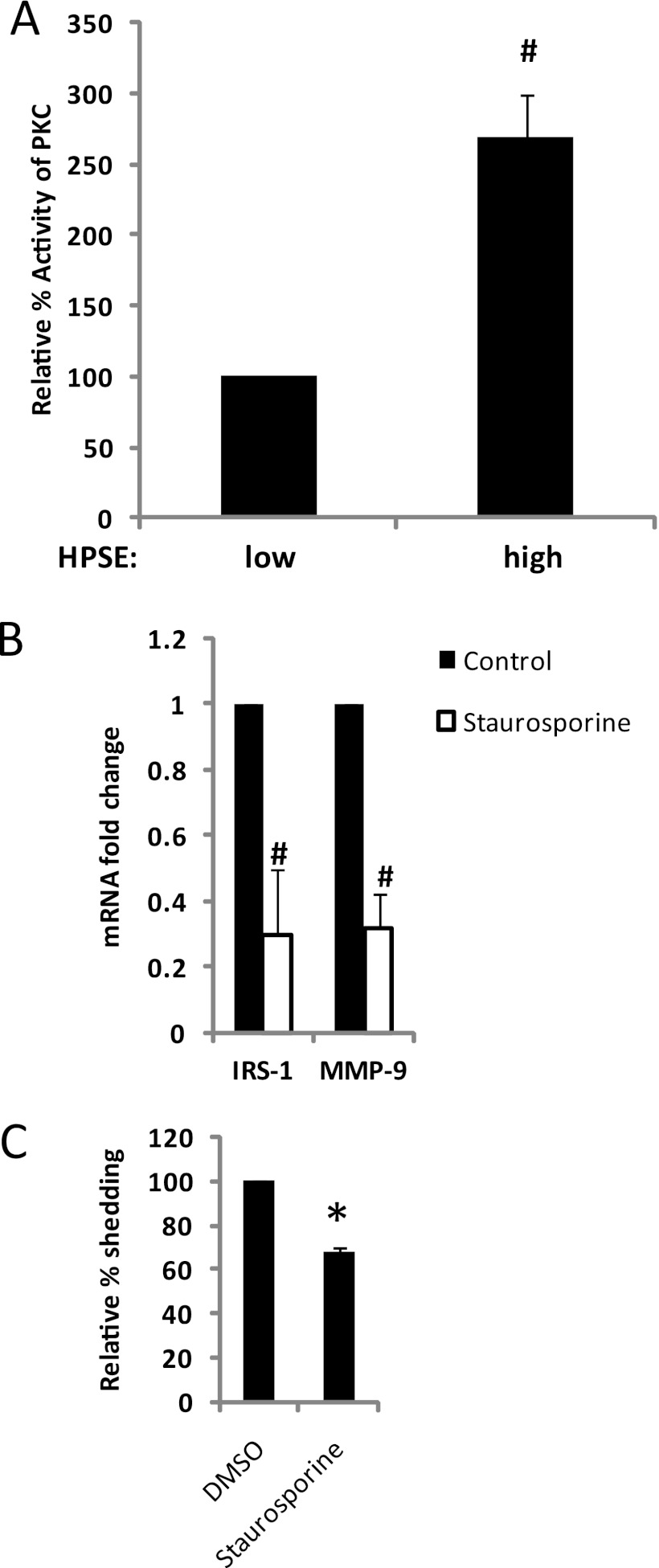
Studies have also shown that the PKC signaling pathway can regulate IRS-1 expression (36). For example, inhibition of PKC activity in breast cancer cells decreases IRS-1 levels (36). Thus, we sought to determine whether myeloma cells with high heparanase and high IRS-1 levels have high PKC activity. PKC activity was assayed in heparanase-high and low cells using an ELISA-based detection method. Results demonstrate that heparanase-high cells had significantly elevated levels of PKC activity compared with heparanase-low cells (Fig. 6A).
FIGURE 6.
Heparanase-mediated up-regulation of PKC activity is responsible for enhanced IRS-1 expression in myeloma cells. A, whole-cell extracts isolated from CAG human myeloma cells expressing either low or high levels of heparanase (HPSE) were assayed for their level of PKC activity by an ELISA kit, which utilizes a synthetic PKC pseudosubstrate and a monoclonal antibody that recognizes the phosphorylated form of that peptide (#, p ≤ 0.001). Data are expressed as relative % activity ± S.D. B, inhibition of PKC activity suppresses IRS-1 and MMP-9 expression in heparanase-high cells. CAG heparanase-high cells were treated with 100 nm of PKC inhibitor staurosporine and the expression of IRS-1 and MMP-9 was assessed by real time PCR and normalized to 28 S rRNA levels. Data are from three separate experiments ± S.D. #, p ≤ 0.001 versus heparanase-high cells without staurosporine. C, suppression of PKC activity decreases syndecan-1 shedding. CAG cells expressing high levels of heparanase were plated at equal density, and treated with or without 100 nm of PKC inhibitor staurosporine. After 24 h, conditioned media were harvested, and the level of shed syndecan-1 was quantified by ELISA. Values represents means of triplicate determination ±S.D. *, p ≤ 0.05 versus HPSE-high cells without staurosporine. DMSO, dimethyl sulfoxide.
Next, we determined whether the enhanced PKC activity seen in heparanase-high cells contributes to up-regulation of expression of IRS-1. CAG heparanase-high cells were grown in the presence or absence of PKC inhibitor staurosporine (37). Because PKC is known to regulate IRS-1 expression at the gene transcriptional level, we examined IRS-1 expression at the mRNA level. Following a 12-h exposure, staurosporine significantly reduced IRS-1 mRNA levels in heparanase-high cells (Fig. 6B). Staurosporine treatment did not reduce CAG cell viability. These results indicate that maintenance of the PKC pathway is necessary to maintain the IRS-1 levels contained in CAG heparanase-high cells. We do not know which isoform or isoforms of PKC are involved in the regulation of IRS-1 expression, but a previous study has shown that PKC-δ isoform regulates the mRNA expression of IRS-1 in breast cancer cell lines (36).
Because IRS-1 induces ERK activation (Fig. 5A) and because heparanase enhances MMP-9 expression by regulating ERK phosphorylation (13), we explored whether inhibiting PKC activity inhibits MMP-9 expression. Treatment of cells with staurosporine significantly decreased the mRNA levels of MMP-9 in heparanase-high cells (Fig. 6B), indicating that their high level of expression is dependent on PKC activity.
Because MMP-9 expression is regulated by PKC activity (Fig. 6B), we determined whether inhibiting PKC activity would decrease shedding of syndecan-1. The accumulation of the syndecan-1 ectodomain in cell culture media from heparanase-high cells in the presence or absence of staurosporine was quantified by ELISA. Staurosporine significantly blocked shedding of syndecan-1 by the heparanase-high cells (Fig. 6C).
DISCUSSION
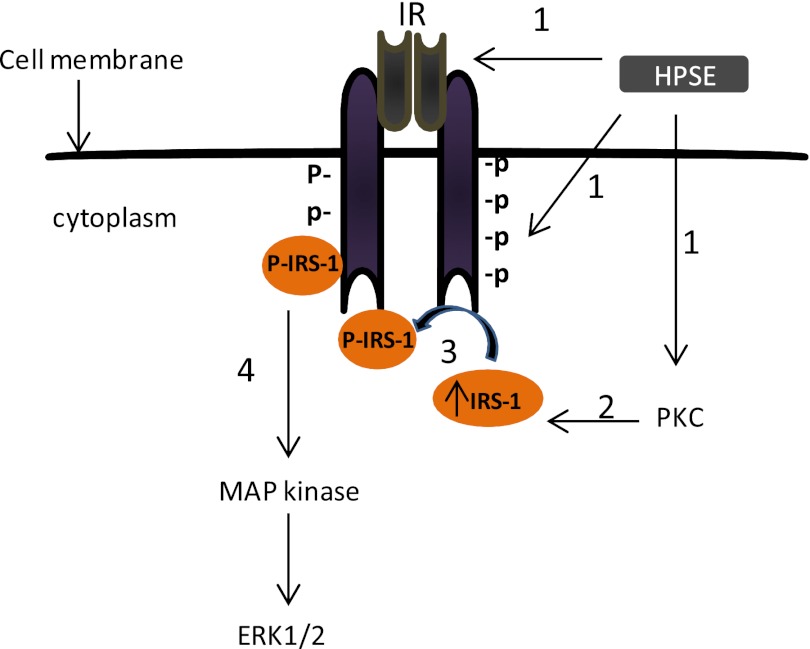
Our previous work has implicated heparanase as a master regulator of the aggressive phenotype in myeloma, which is due, at least in part, to heparanase up-regulation of ERK signaling (1, 13). In the present work, we have demonstrated that heparanase-mediated ERK activation occurs via the insulin receptor signaling pathway. The data support a model as shown in Fig. 7, whereby 1) heparanase triggers the phosphorylation/kinase activity of the insulin receptor and also up-regulates PKC activity; 2) high PKC activity enhances expression of IRS-1, the primary substrate for insulin receptor tyrosine kinase; 3) IRS-1 docks with the insulin receptor and undergoes phosphorylation; and 4) phospho-IRS-1 engages multiple downstream signaling molecules, resulting in ERK phosphorylation. This new insight into the mechanism of heparanase-enhanced ERK activation provides further understanding of how heparanase is able to wield its powerful impact on cancer progression. This is particularly relevant given the known effect of ERK signaling on promoting myeloma proliferation, survival, drug resistance, and angiogenesis (16, 17).
FIGURE 7.
Proposed mechanism of heparanase-mediated activation of ERK. Step 1, elevation of heparanase (HPSE) expression in multiple myeloma cells triggers the phosphorylation/kinase activity of IR and PKC activity. Step 2, elevated PKC activity up-regulates IRS-1 expression. Step 3, high levels of IRS-1 and high IR tyrosine kinase activity up-regulates the phosphorylated levels of IRS-1. Step 4, high levels of phospho-IRS-1 up-regulate ERK activation.
To our knowledge, this is the first report linking heparanase to activation of the insulin signaling cascade in cancer. A previous study reported that insulin can stimulate the growth of myeloma cells as potently as does IGF-1 (20). Our data showing that heparanase expression in myeloma cells can enhance the phosphorylation of the insulin receptor and that addition of an insulin receptor function blocking antibody can reduce ERK phosphorylation in heparanase-high cells strongly support the role of insulin receptor in regulating the ERK signaling pathway in myeloma. This is further indicated by our finding that triggering the insulin receptor in myeloma cells by addition of insulin enhances ERK activation. Although the role of IGF-R in myeloma has been studied extensively, little is known regarding the function of the insulin receptor in myeloma. By flow cytometry analysis, we found that the insulin receptor is expressed at high levels by myeloma cells, consistent with the previous report demonstrating the presence of the insulin receptor on myeloma cell lines and almost all myeloma cells from patients (20).
Mechanistically, we do not yet know how heparanase enhances the phosphorylation of the insulin receptor. One possibility is that heparanase, which is known to have multiple heparan sulfate-binding motifs, binds to the cell surface heparan sulfate proteoglycan syndecan-1, triggers clustering of syndecan-1 with the insulin receptor, and induces its dimerization and autophosphorylation. A previous study has shown that heparanase can induce the clustering of syndecan-1, thereby initiating signaling cascades that involve Rac1, Src, and the PKC pathways, resulting in enhanced cell adhesion and spreading (38). Similar to this finding, we also observed high PKC activity in heparanase-high cells and have found that heparanase-high cells spread much more avidly than do heparanase-low cells.3 Another study has shown that syndecan-1 clusters both IGF-1R and integrins resulting in integrin activation in carcinoma and endothelial cells (39). Similar to this finding, we recently observed the association of syndecan-1 with IGF-1R in CAG myeloma cells.4 Because insulin receptor and IGF-1R share 60% overall amino acid sequence homology and 84% homology in their tyrosine kinase domains, it is possible that heparanase promotes a syndecan-1·IGF-1R·insulin receptor complex on myeloma cells. In support of this notion, it was recently shown that insulin receptor associates with IGF-1R to form an insulin/IGF-1 hybrid receptor at the cell membrane of myeloma cells (20).
Our finding that heparanase up-regulates PKC and that this enhances IRS-1 expression is consistent with previous reports that IRS-1 expression is transcriptionally regulated by PKC activity (36). Activation of RTKs can enhance PKC activity, and we speculate that heparanase-induced insulin receptor activation initiates PKC activation, which, in turn, up-regulates the level of IRS-1. IRS-1 expression is often increased in human cancer (40, 41), and overexpression of IRS-1 and an increase in its phosphorylation has been shown to induce cellular transformation with activation of potent oncogenic signal transduction pathways such as Grb2-SOS-Ras and MAPK cascades (42, 43). Similar to this finding, we found that inhibiting IRS-1 by knocking down its expression decreases the levels of phosphorylated ERK in heparanase-high cells. Interestingly, studies have shown a strong association of several IRS-1 single nucleotide polymorphisms with increased risk of multiple myeloma, indicating that IRS-1 expression and/or activity may play an important role in this cancer (26).
Overall, our studies for the first time demonstrate that heparanase expression and insulin signaling act in tandem to play a major role in regulating ERK signaling in myeloma. Interestingly, insulin is known to stimulate heparanase secretion from cells (44), raising the possibility that insulin plays a dual role in activating ERK signaling via insulin receptor regulation and by enhancing secretion of heparanase, which further drives insulin receptor signaling and ERK activation. These findings underscore the potential of heparanase inhibitors and insulin receptor pathway inhibitors as anti-cancer drugs and the possibility that they could be used to reverse the aggressive phenotype of some myeloma tumors.
Acknowledgments
We thank Dr. Israel Vlodavsky (Technion, Haifa, Israel) for providing recombinant heparanase. We acknowledge Enid Keyser at the University of Alabama at Birmingham flow cytometry core facility for assistance with flow analysis (supported by National Institutes of Health Grant P30 AR48311). Histology services were performed by the University of Alabama at Birmingham Center for Metabolic Bone Disease's Histomorphometry and Molecular Analysis Core Laboratory (supported by National Institutes of Health Grant P30 AR46031).
This work was supported, in whole or in part, by National Institutes of Health Grants CA135075 and CA138340 (to R. D. S).

This article contains supplemental Table 1.
A. Purushothaman, and R. Sanderson, unpublished observations.
V. Trapp-Stamborski, A. Purushothaman, B. Ell, D. Beauvais, G. Thomas, Y. Yang, C. Pisano, R. Sanderson, and A. Rapraeger, unpublished observations.
- MMP-9
- matrix metalloproteinase 9
- HPSE
- heparanase
- IGF-1
- insulin like growth factor-1
- IGF-1R
- insulin like growth factor-1 receptor
- IR
- insulin receptor
- IRS-1
- insulin receptor substrate-1
- RTK
- receptor tyrosine kinase.
REFERENCES
- 1. Barash U., Cohen-Kaplan V., Dowek I., Sanderson R. D., Ilan N., Vlodavsky I. (2010) Proteoglycans in health and disease: new concepts for heparanase function in tumor progression and metastasis. FEBS J. 277, 3890–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levy-Adam F., Ilan N., Vlodavsky I. (2010) Tumorigenic and adhesive properties of heparanase. Semin. Cancer Biol. 20, 153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ilan N., Elkin M., Vlodavsky I. (2006) Regulation, function, and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol. 38, 2018–2039 [DOI] [PubMed] [Google Scholar]
- 4. Sanderson R. D., Yang Y., Suva L. J., Kelly T. (2004) Heparan sulfate proteoglycans and heparanase–partners in osteolytic tumor growth and metastasis. Matrix Biol. 23, 341–352 [DOI] [PubMed] [Google Scholar]
- 5. Doweck I., Kaplan-Cohen V., Naroditsky I., Sabo E., Ilan N., Vlodavsky I. (2006) Heparanase localization and expression by head and neck cancer: correlation with tumor progression and patient survival. Neoplasia 8, 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Y., Macleod V., Bendre M., Huang Y., Theus A. M., Miao H. Q., Kussie P., Yaccoby S., Epstein J., Suva L. J., Kelly T., Sanderson R. D. (2005) Heparanase promotes the spontaneous metastasis of myeloma cells to bone. Blood 105, 1303–1309 [DOI] [PubMed] [Google Scholar]
- 7. Fux L., Ilan N., Sanderson R. D., Vlodavsky I. (2009) Heparanase: busy at the cell surface. Trends Biochem. Sci. 34, 511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Purushothaman A., Hurst D. R., Pisano C., Mizumoto S., Sugahara K., Sanderson R. D. (2011) Heparanase-mediated loss of nuclear syndecan-1 enhances histone acetyltransferase (HAT) activity to promote expression of genes that drive an aggressive tumor phenotype. J. Biol. Chem. 286, 30377–30383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gingis-Velitski S., Zetser A., Flugelman M. Y., Vlodavsky I., Ilan N. (2004) Heparanase induces endothelial cell migration via protein kinase B/Akt activation. J. Biol. Chem. 279, 23536–23541 [DOI] [PubMed] [Google Scholar]
- 10. Zetser A., Bashenko Y., Edovitsky E., Levy-Adam F., Vlodavsky I., Ilan N. (2006) Heparanase induces vascular endothelial growth factor expression: correlation with p38 phosphorylation levels and Src activation. Cancer Res. 66, 1455–1463 [DOI] [PubMed] [Google Scholar]
- 11. Mahtouk K., Hose D., Raynaud P., Hundemer M., Jourdan M., Jourdan E., Pantesco V., Baudard M., De Vos J., Larroque M., Moehler T., Rossi J. F., Rème T., Goldschmidt H., Klein B. (2007) Heparanase influences expression and shedding of syndecan-1, and its expression by the bone marrow environment is a bad prognostic factor in multiple myeloma. Blood 109, 4914–4923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Y., Macleod V., Miao H. Q., Theus A., Zhan F., Shaughnessy J. D., Jr., Sawyer J., Li J. P., Zcharia E., Vlodavsky I., Sanderson R. D. (2007) Heparanase enhances syndecan-1 shedding: a novel mechanism for stimulation of tumor growth and metastasis. J. Biol. Chem. 282, 13326–13333 [DOI] [PubMed] [Google Scholar]
- 13. Purushothaman A., Chen L., Yang Y., Sanderson R. D. (2008) Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. J. Biol. Chem. 283, 32628–32636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Y., Yaccoby S., Liu W., Langford J. K., Pumphrey C. Y., Theus A., Epstein J., Sanderson R. D. (2002) Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood 100, 610–617 [DOI] [PubMed] [Google Scholar]
- 15. Purushothaman A., Uyama T., Kobayashi F., Yamada S., Sugahara K., Rapraeger A. C., Sanderson R. D. (2010) Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood 115, 2449–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hideshima T., Bergsagel P. L., Kuehl W. M., Anderson K. C. (2004) Advances in biology of multiple myeloma: clinical applications. Blood 104, 607–618 [DOI] [PubMed] [Google Scholar]
- 17. Kim K., Kong S. Y., Fulciniti M., Li X., Song W., Nahar S., Burger P., Rumizen M. J., Podar K., Chauhan D., Hideshima T., Munshi N. C., Richardson P., Clark A., Ogden J., Goutopoulos A., Rastelli L., Anderson K. C., Tai Y. T. (2010) Blockade of the MEK/ERK signalling cascade by AS703026, a novel selective MEK1/2 inhibitor, induces pleiotropic anti-myeloma activity in vitro and in vivo. Br. J. Haematol. 149, 537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tai Y. T., Fulciniti M., Hideshima T., Song W., Leiba M., Li X. F., Rumizen M., Burger P., Morrison A., Podar K., Chauhan D., Tassone P., Richardson P., Munshi N. C., Ghobrial I. M., Anderson K. C. (2007) Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis. Blood 110, 1656–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Breitkreutz I., Raab M. S., Vallet S., Hideshima T., Raje N., Chauhan D., Munshi N. C., Richardson P. G., Anderson K. C. (2007) Targeting MEK1/2 blocks osteoclast differentiation, function, and cytokine secretion in multiple myeloma. Br. J. Haematol. 139, 55–63 [DOI] [PubMed] [Google Scholar]
- 20. Sprynski A. C., Hose D., Kassambara A., Vincent L., Jourdan M., Rossi J. F., Goldschmidt H., Klein B. (2010) Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia 24, 1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyd D. B. (2003) Insulin and cancer. Integr. Cancer Ther. 2, 315–329 [DOI] [PubMed] [Google Scholar]
- 22. Calle E. E., Rodriguez C., Walker-Thurmond K., Thun M. J. (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 348, 1625–1638 [DOI] [PubMed] [Google Scholar]
- 23. Myers M. G., Jr., Sun X. J., White M. F. (1994) The IRS-1 signaling system. Trends Biochem. Sci. 19, 289–293 [DOI] [PubMed] [Google Scholar]
- 24. White M. F., Kahn C. R. (1994) The insulin signaling system. J. Biol. Chem. 269, 1–4 [PubMed] [Google Scholar]
- 25. DeAngelis T., Morrione A., Baserga R. (2010) Mutual interaction and reciprocal down-regulation between c-met and insulin receptor substrate-1. J. Cell Physiol. 224, 658–663 [DOI] [PubMed] [Google Scholar]
- 26. Birmann B. M., Tamimi R. M., Giovannucci E., Rosner B., Hunter D. J., Kraft P., Mitsiades C., Anderson K. C., Colditz G. A. (2009) Insulin-like growth factor-1- and interleukin-6-related gene variation and risk of multiple myeloma. Cancer Epidemiol. Biomarkers Prev. 18, 282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Townsend M., Mehta T., Selkoe D. J. (2007) Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. J. Biol. Chem. 282, 33305–33312 [DOI] [PubMed] [Google Scholar]
- 28. Hideshima T., Catley L., Yasui H., Ishitsuka K., Raje N., Mitsiades C., Podar K., Munshi N. C., Chauhan D., Richardson P. G., Anderson K. C. (2006) Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood 107, 4053–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsitoura D. C., Rothman P. B. (2004) Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells. J. Clin. Invest. 113, 619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kelly T., Miao H. Q., Yang Y., Navarro E., Kussie P., Huang Y., MacLeod V., Casciano J., Joseph L., Zhan F., Zangari M., Barlogie B., Shaughnessy J., Sanderson R. D. (2003) High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 63, 8749–8756 [PubMed] [Google Scholar]
- 31. Ritchie J. P., Ramani V. C., Ren Y., Naggi A., Torri G., Casu B., Penco S., Pisano C., Carminati P., Tortoreto M., Zunino F., Vlodavsky I., Sanderson R. D., Yang Y. (2011) SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis. Clin. Cancer Res. 17, 1382–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L., Sanderson R. D. (2009) Heparanase regulates levels of syndecan-1 in the nucleus. PloS one 4, e4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rose D. W., Saltiel A. R., Majumdar M., Decker S. J., Olefsky J. M. (1994) Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. Proc. Natl. Acad. Sci. U.S.A. 91, 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waters S. B., Yamauchi K., Pessin J. E. (1993) Functional expression of insulin receptor substrate-1 is required for insulin-stimulated mitogenic signaling. J. Biol. Chem. 268, 22231–22234 [PubMed] [Google Scholar]
- 35. White M. F. (1998) The IRS-signaling system: a network of docking proteins that mediate insulin action. Mol. Cell Biochem. 182, 3–11 [PubMed] [Google Scholar]
- 36. deVente J. E., Carey J. O., Bryant W. O., Pettit G. J., Ways D. K. (1996) Transcriptional regulation of insulin receptor substrate 1 by protein kinase C. J. Biol. Chem. 271, 32276–32280 [DOI] [PubMed] [Google Scholar]
- 37. Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. (1986) Staurosporine, a potent inhibitor of phospholipid/Ca++-dependent protein kinase. Biochem. Biophys. Res. Commun. 135, 397–402 [DOI] [PubMed] [Google Scholar]
- 38. Levy-Adam F., Feld S., Suss-Toby E., Vlodavsky I., Ilan N. (2008) Heparanase facilitates cell adhesion and spreading by clustering of cell surface heparan sulfate proteoglycans. PloS One 3, e2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beauvais D. M., Rapraeger A. C. (2010) Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation. J. Cell Sci. 123, 3796–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tanaka S., Mohr L., Schmidt E. V., Sugimachi K., Wands J. R. (1997) Biological effects of human insulin receptor substrate-1 overexpression in hepatocytes. Hepatology 26, 598–604 [DOI] [PubMed] [Google Scholar]
- 41. Chang Q., Li Y., White M. F., Fletcher J. A., Xiao S. (2002) Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: therapeutic implications. Cancer Res. 62, 6035–6038 [PubMed] [Google Scholar]
- 42. Ito T., Sasaki Y., Wands J. R. (1996) Overexpression of human insulin receptor substrate 1 induces cellular transformation with activation of mitogen-activated protein kinases. Mol. Cell Biol. 16, 943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dalmizrak O., Wu A., Chen J., Sun H., Utama F. E., Zambelli D., Tran T. H., Rui H., Baserga R. (2007) Insulin receptor substrate-1 regulates the transformed phenotype of BT-20 human mammary cancer cells. Cancer Res. 67, 2124–2130 [DOI] [PubMed] [Google Scholar]
- 44. Shafat I., Ilan N., Zoabi S., Vlodavsky I., Nakhoul F. (2011) Heparanase levels are elevated in the urine and plasma of type 2 diabetes patients and associate with blood glucose levels. PloS One 6, e17312. [DOI] [PMC free article] [PubMed] [Google Scholar]