Background: The pathological mechanism of the potent modifier of TDP-43 toxicity, ataxin-2, is unknown.
Result: Ataxin-2 modified the subcellular distributions of truncated TDP-43 and mutant FUS.
Conclusion: Increased ataxin-2 leads to a mislocation of TDP-43 and FUS, leading the RNA dysregulation.
Significance: An aberrant distribution of TDP-43 and FUS mediated by ataxin-2 may be a key therapeutic target against ALS.
Keywords: Neurobiology, Neurodegeneration, RNA Binding Protein, RNA Metabolism, Stress Granule, Amyotrophic Lateral Sclerosis, FUS, TDP-43, TLS, Ataxin-2
Abstract
The RNA-binding proteins TDP-43 and Fused in Sarcoma (FUS) play central roles in neurodegeneration associated with amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Both proteins are components of messenger ribonucleoprotein (mRNP) granules and show cytoplasmic mislocalization in affected tissues. Recently, ataxin-2 was identified as a potent modifier of TDP-43 toxicity in an RNA-dependent manner. This study investigated to clarify how ataxin-2 modifies the TDP-43 and FUS pathological pathway. The expression of cytoplasmic TDP-43, the 35-kDa C-terminal fragment (TDP-p35f), and mutant FUS recruited ataxin-2 to mRNP granules, whereas increased ataxin-2 inhibited the mRNP granule formation of the 35-kDa C-terminal fragment and mutant FUS. A subcellular compartment analysis showed that the overexpressed ataxin-2 increased the cytoplasmic concentrations of both proteins, whereas it decreased their nuclear distributions. These data indicate that increased ataxin-2 impairs the assembly of TDP-43 and FUS into mRNP granules, leading to an aberrant distribution of RNA-binding proteins. Consequently, these sequences may exacerbate the impairment of the RNA-quality control system mediated by amyotrophic lateral sclerosis/frontotemporal lobar degeneration-associated RNA-binding proteins, which forms the core of the degenerative cascade.
Introduction
Advanced pathological and genetic approaches have successively identified TDP-43 and FUS2 as critical molecules in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with ubiquitin-positive inclusion bodies (FTLD-U) (1–5), furthering our understanding of the pathogenesis of ALS/FTLD-U. Because both proteins have common biochemical characteristics such as RNA-binding domains, cytoplasmic mislocalization in affected tissues, and components of messenger ribonucleoprotein (mRNP) granules, or so-called stress granules (SGs), they are conceivably part of a cooperative pathological process during the development of ALS/FTLD-U (6–8). Recently, comprehensive analyses aimed at identifying TDP-43-binding RNA targets demonstrated that TDP-43 is crucial for maintaining normal levels and splicing patterns of various mRNAs, including pre-mRNAs with very long introns and noncoding RNAs (9, 10). The nuclear RNA-binding protein FUS/TLS is also thought to have an important role in the translation, stability, and metabolism of various mRNAs. Therefore, both TDP-43 and FUS are thought to be involved in a similar pathological process via the RNA quality control system.
We previously reported the expression of a truncated TDP-43 protein, a 35-kDa C-terminal fragment (TDP-p35f), and its isoform, which lacks a nuclear localization signal and is redistributed to the cytoplasm, leading to the formation of mRNP granules that exhibit some properties of SGs, which package mRNA and RNA-binding proteins during cell stress (7). Furthermore, we also demonstrated previously that mutant FUS, which is missing the nuclear traffic activity of the C terminus, is mislocated to the cytoplasm and assembled into similar SG-like mRNP granules (6). Therefore, excessive cytoplasmic RNA binding proteins such as TDP-43 and FUS induce a conjoint pathological cascade of neurodegeneration that leads to ALS/FTLD-U (8). However, the pathological consequences of mRNP granules in neurodegenerative diseases remain unclear.
Elden et al. (11) demonstrated recently that ataxin-2, a polyglutamine protein that contains tracts of expanded glutamine residues, results in hereditary spinocerebellar ataxia-2 and is a potent modifier of TDP-43 toxicity. They showed that the overexpression or reduced expression of ataxin-2 enhances or attenuates TDP-43 toxicity, respectively, in the retinas of flies and that ataxin-2 and TDP-43 form a complex in a longer polyglutamine expansion-dependent manner. Furthermore, in a genomic analysis of ALS patients, they also found that intermediate-length ataxin-2 polyglutamine expansions (27–33 glutamine residues) are associated with an increased risk for ALS and that this is accompanied by an earlier age of onset. Notably, ataxin-2 is also a cytoplasmic RNA binding protein with a Like Sm (Lsm) domain that binds to RNA oligonucleotides (12). Recent studies have shown that ataxin-2 is a constituent protein of SGs and that the cellular ataxin-2 concentration is important for the assembly of SG and also processing bodies (P-bodies) (13).
Here, we investigated the associations among ataxin-2, TDP-43, and FUS in cells expressing these proteins to clarify how ataxin-2 is involved in the TDP-43 and FUS pathological pathway. We demonstrated that increased ataxin-2 levels modulate the subcellular distributions of truncated TDP-43 and mutant P525L FUS via the mRNP granule machinery. Our findings indicated that ataxin-2 leads to an aberrant distribution of RNA-binding proteins, such as TDP-43 and FUS, leading to the enhancement of RNA dysregulation, which forms the core of the ALS/FTLD-U degenerative cascade (8, 14).
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
HeLa human carcinoma cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen) containing 10% fetal bovine serum, as described previously (6, 7, 15, 16). Human neuroblastoma SK-N-SH cells were cultured in αMEM (Invitrogen) supplemented with 10% fetal bovine serum. Arsenite was purchased from Sigma. Transfection was performed using Lipofectamine 2000 (Invitrogen), according to the instructions of the manufacturer. The transfection efficiency in HeLa cells was ∼30% for single plasmids and ∼10% for double plasmids.
cDNA
pcDNA-HA-Ataxin-2 Gln-22, Gln-33, and Gln-79 were generated from pcDNA4HisMax-Sca2CAG-Q22 and pcDNA4HisMax-Sca2CAG-Q79, a generous gift from Dr. Manfred Schwab (German Cancer Research Center, Heidelberg, Germany) using PCR and the following primer pairs: sense, 5′-CGCTCAGCGGCCGCAGCTCCTCGGAGTCCCGCGGT and antisense, 5′-AGCGTAATCTGGAACATCGTATGGGTAAGAACCCCCCATGGTTTCGG-3′. Deletion mutants were generated using KOD-Plus-site-directed mutagenesis (Toyobo, Osaka, Japan), according to the instructions of the manufacturer. pGFP-ataxin-2-(Q22) and -(Q58) were kindly provided by Dr. Stefan M. Pulst (Department of Neurology, University of Utah, Salt Lake City, UT). The plasmids used to express human TDP-43 (wild-type and TDP-p35f) and FUS (wild-type and P525L) were described previously (6, 7). The plasmid containing the gene for RFP-TIA1 was kindly provided by Dr. John Goodier (Department of Genetics, University of Pennsylvania School of Medicine).
Antibodies
Mouse monoclonal anti-ataxin-2 was purchased from BD Biosciences. Mouse monoclonal anti-V5 antibodies were obtained from Invitrogen. Rabbit polyclonal anti-V5 (C464.6) was obtained from Bethyl Labs (Montgomery, TX). Rabbit polyclonal anti-G3BP was obtained from Proteintech (Chicago, IL). Anti-HA monoclonal antibody (16B12) was obtained from Covance Research Products, Inc. (Princeton, NJ). Anti-DDX6 polyclonal antibody was obtained from Novus Biologicals (Littleton, CO).
Immunofluorescence
Cultured cells on glass coverslips coated with poly-l-lysine were transfected with various expression plasmids. After 48 h, the cells were fixed with 4% paraformaldehyde for 10 min and then permeabilized in 0.2% Triton X-100 for 10 min. After blocking for nonspecific binding, the cells were incubated with primary antibodies diluted in PBS containing 0.2% Tween 20 and 3% bovine serum albumin. After 3 washes, the cells were incubated with Alexa Fluor 488-conjugated and Alexa Fluor 633-conjugated anti-rabbit secondary antibodies, or Alexa Fluor 586-conjugated anti-mouse secondary antibodies (Invitrogen) (1:200). Immunofluorescent images were acquired using a Leica TCS SP5 confocal microscope (Leica, Wetzlar, Germany).
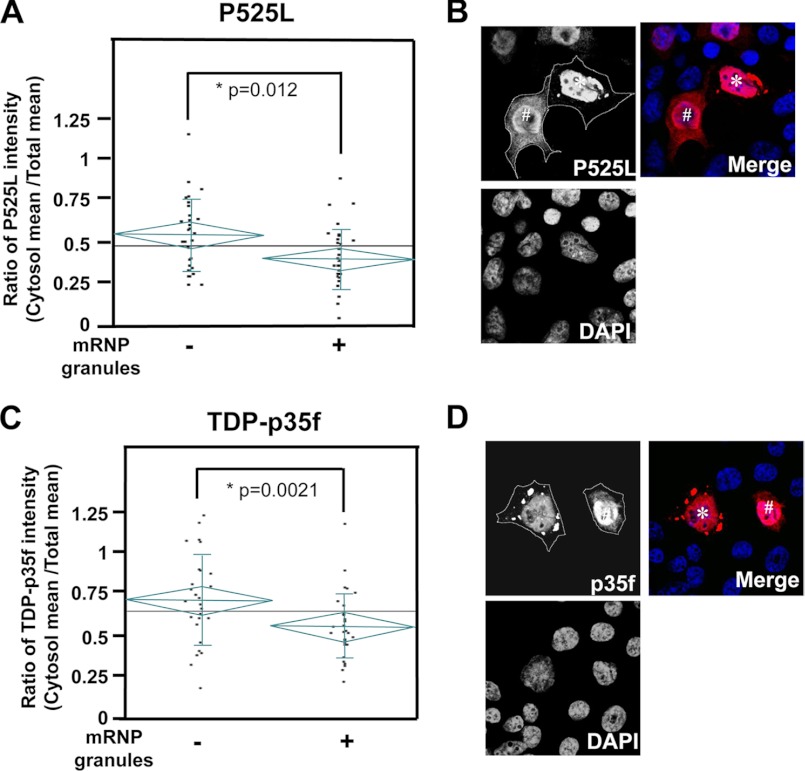
For image quantification, single confocal images were taken in the plane of the largest cytosolic area. Total fluorescence intensities of mutant FUS and p35f were quantified as “pixelsum (gray level)” using Leica Application Suite Advanced Fluorescence software (Leica). The mean total, cytosolic, or nuclear intensities were calculated from pixelsum/pixelcount. Cells were defined as mRNP granule-positive when they had cytosolic foci with a size of 1–5 μm. For Fig. 7, HeLa cells were transfected with p525L FUS-V5 or TDP-p35f-V 5 and also with GFP to define the borders of the cells.
FIGURE 7.
Distribution of mutant FUS and TDP-p35f in cells with/without mRNP granules. HeLa cells were transfected with p525L FUS-V5 (A and B) or TDP-p35f-V 5 (C and D), and pEGFP-N1 were stained with anti-V5 antibody (red). A and C, total cellular or cytoplasmic (with the exception of mRNP granules) fluorescence intensities were quantified using confocal microscopy. The graphs indicate the ratio of the mean intensity of the cytosol/whole cell. The cytosolic fluorescence intensities of cells with mRNP granules of both mutant FUS (A) and TDP-p35f (C) were significantly low. The diamonds and vertical lines indicate the 95% prediction interval and S.D., respectively. The mean values for all the cells or the cells with/without mRNP granules are shown with long horizontal lines on the graph and short horizontal lines in a diamond, respectively. Representative images are shown in B and D. Note that the diffuse cytoplasmic fluorescent distributions in cells without mRNP granules (indicated as #) are higher than those in mRNP granule+ cells (indicated as an asterisk). The cytoplasm is outlined by dotted lines on the basis of the cotransfected GFP signal.
Immunoblotting
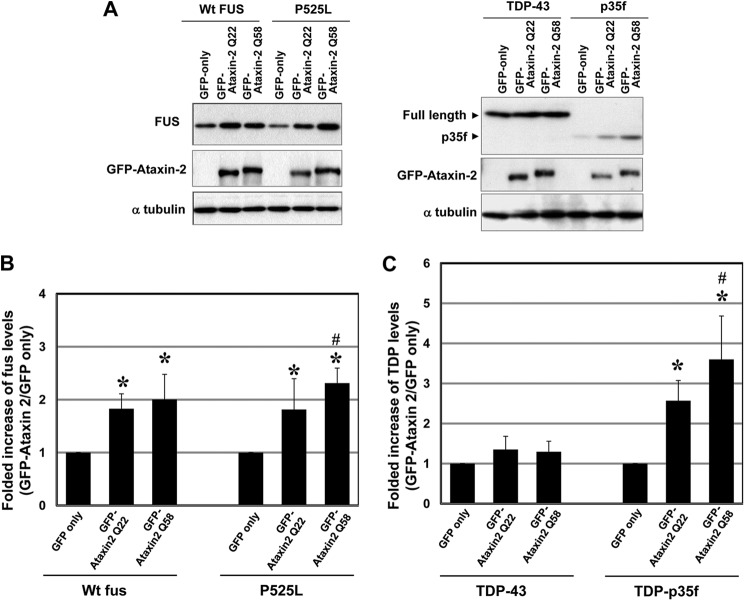
Cells were briefly sonicated in cold lysis buffer (50 mm Tris-HCl (pH7.4), 150 mm NaCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 0.25% sodium dodecyl sulfate, 5 mm EDTA, and protease inhibitor mixture (Sigma)). Cell lysates were then separated via a reducing SDS-PAGE on a 4–20% Tris-glycine gradient gel (Invitrogen), after which the proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). The membrane was then incubated with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents (PerkinElmer Life Sciences). The proteins were then visualized using Amersham Biosciences HyperfilmTM film (GE Healthcare). Protein levels in Fig. 9 were determined by densitometry using an Epson ES-2000 scanner and ImageJ (National Institutes of Health, Bethesda, MD).
FIGURE 9.
Overexpression of ataxin-2 increases the expression levels of FUS and TDP-p35f. A, lysates from HeLa cells cotransfected with GFP-ataxin-2 (Gln-22 or Gln-58) or an empty vector and wild-type FUS-V5, mutant FUS-V5TDP-43-V5, or TDP-p35f-V5 were subjected to a Western blot analysis with an anti-V5 antibody. Note that the levels of wild-type and mutant FUS and TDP-p35f were increased by ataxin-2 overexpression. Data are representative of three independent experiments. B and C, quantitation analysis of FUS and TDP protein levels. Immunoblot analyses were scanned and analyzed using densitometry. The protein levels were normalized using an internal control (α-tubulin). The graphs show the fold increases in the TDP and FUS levels in cells cotransfected with GFP-ataxin-2 (Gln-22 and Gln-58) compared with the levels in cells transfected with an empty vector. The values represent the mean ± S.D. of five independent experiments. *, p < 0.01, significant difference versus the empty vector. #, p < 0.04, significant difference between GFP-ataxin-2 (Gln-22) and Gln-58.
Statistical Analysis
A statistical analysis of the data were performed using a one-way analysis of variance with Fisher's protected least squares difference test using the Statview 5.0 system (Statview, Berkley, CA) or JMP 8 (SAS Institute, Inc.).
RESULTS
Endogenous Ataxin-2 Is Localized in mRNP Granules Mediated by the ALS-linked Molecules TDP-43 and FUS
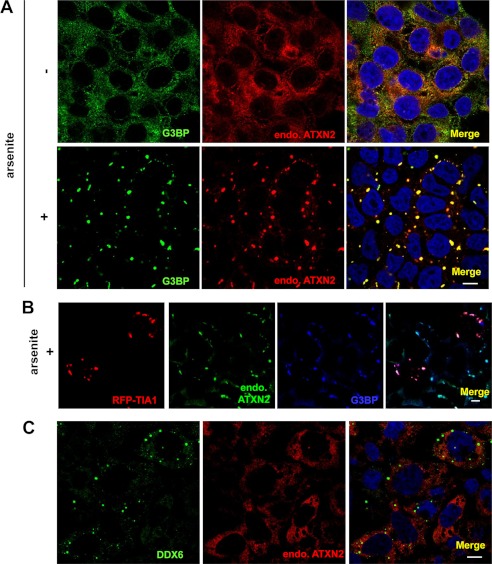
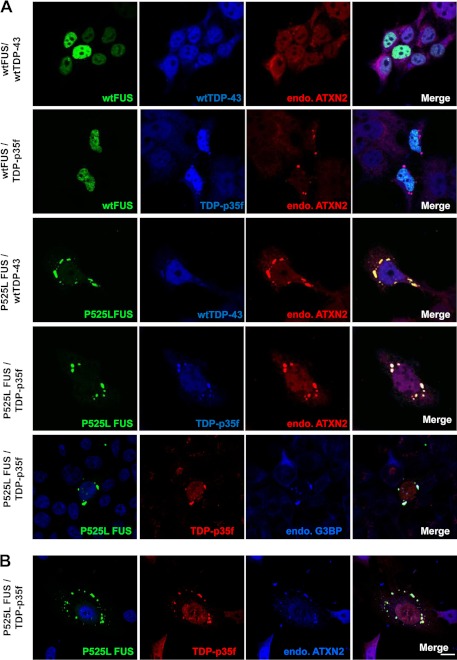
A recent study (13) and Fig. 1 show that the cytoplasmic RNA binding protein ataxin-2 is a component of cytoplasmic mRNP granules, SG, indicating that ataxin-2 is potentially involved in regulating and controlling mRNA degradation, stability, and translation via mRNP granules. We demonstrated previously that the ALS-associated RNA binding proteins TDP-43 and FUS form part of SGs, and that truncated TDP-43, termed TDP-p35f (amino acids 90–414), and mutant FUS facilitate the assembly of SG-like mRNP granules when overexpressed (6, 7). Therefore, we hypothesized that endogenous ataxin-2 may be recruited and assembled into cytoplasmic mRNP granules containing overexpressed TDP-p35f and/or mutant FUS. To examine this issue, we first performed triple immunofluorescence staining for endogenous ataxin-2 in HeLa cells expressing TDP-43 (full-length or TDP-p35f) tagged with V5 and FUS (wild-type or P525L) tagged with GFP. As shown in Fig. 2A, coexpressed TDP-p35f and P525L conjointly form mRNP granules, suggesting that the formation of TDP-p35f and mutant FUS mRNP granules possibly occurs via a similar mechanism. Immunostaining for ataxin-2 revealed that endogenous ataxin-2 is recruited to mRNP granules of TDP-p35f and/or P525L. Furthermore, in the human neuronal cell line SK-N-SH (Fig. 2B) and the motor neuronal cell line NSC-34 (data not shown), we also found that endogenous ataxin-2 was distributed substantially to mRNP granules containing TDP-p35f and/or P525L. Meanwhile, the G85R mutant Cu/Zn superoxide dismutase (SOD1) and optineurin (wild-type and glaucoma-mutant E50K, ALS mutant E478G), which are also associated with familial ALS, do not affect the distribution of ataxin-2 (data not shown). Therefore, these findings indicate a specific interaction between ataxin-2 and the ALS/FTLD-U-linked RNA binding proteins TDP-43 and FUS in mRNP granules.
FIGURE 1.
Endogenous ataxin-2 (endo.ATXN2) is recruited to SGs induced by arsenite. A, HeLa cells were treated with/without arsenite (0.5 mm) for 1 h following double-labeling with polyclonal anti-G3BP antibody (SG marker) (green) and monoclonal anti-ataxin-2 antibody (red). Cells were counterstained with DAPI (blue). B, HeLa cells transfected with RFP-TIA1 (SG marker) (red) were treated with arsenite following labeling with monoclonal anti-ataxin-2 antibody (green) and polyclonal anti-G3BP antibody (blue). C, HeLa cells were stained with anti-ataxin-2 antibody (red) and anti-DDX6 antibody (P-body marker) (green). Note that ataxin-2 is assembled with SGs but not with P-bodies. Scale bar = 10 μm.
FIGURE 2.
Endogenous ataxin-2 (endo.ATXN2) is recruited to mRNP granules formed by TDP-p35f and mutant FUS. A, 48 h after cotransfection with wild-type or P525L mutant FUS with GFP at the N terminus and full-length TDP-43 or TDP-p35f with the V5 tag at the C terminus, the cells were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and labeled with anti-V5 and ataxin-2 or G3BP antibodies. A, HeLa cells. B, human neuronal cell line SK-N-SH cell. Scale bar = 10 μm. Note that mRNP granules formed by TDP-p35f and mutant FUS exhibit ataxin-2 immunoreactivity.
The Lsm Domain and PAM2 Motif Are Important for Ataxin-2 Assembly with mRNP Granules Induced by Arsenite
Next, we examined whether ataxin-2 expression leads to the formation of mRNP granules, similar to overexpressed mutant FUS and TDP-p35f. HeLa cells were transfected with HA-tagged ataxin-2 with 22 (normal repeat), 30 (intermediate-length), or 79 (SCA2 pathogenic repeat) consecutive glutamines and stained with anti-HA antibody and the SG marker G3BP 2 days after transfection. As shown in supplemental Fig. 1A, overexpressed ataxin-2, even the pathogenic polyglutamine repeats, does not form G3BP-positive mRNP granules. On the other hand, overexpressed ataxin-2 was colocalized with endogenous TDP-43 or FUS in G3BP-positive granules after treatment with arsenite, a well established SG inducer (supplemental Fig. 1B), indicating that the three ALS/FTLD-U-linking molecules could assemble in the same SG cellular compartment.
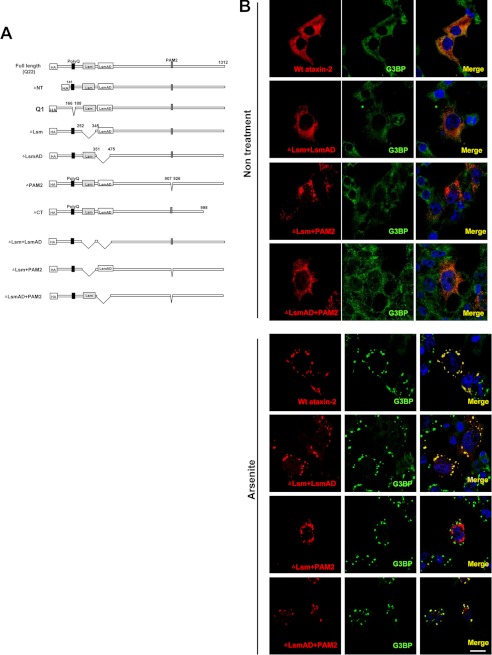
We next sought to determine what region(s) of ataxin-2 are required for assembly with SG induced by arsenite (17). To do this, we constructed a series of mutant constructs and assessed the SG of mutant FUS in HeLa cells, as described in Fig. 3A. The cells were transiently transfected with single or expanded polyglutamine constructs (Gln-1 and Gln-79) and deletion series constructs in which the NH2 terminus Lsm, which is known to contribute to self-assembly into ring formations and to bind to RNA oligonucleotides (12); the Lsm-associated domain (LsmAD), which contains a trans-Golgi signal; the poly(A)-binding protein interacting motif (PAM2), which is a known the poly(A)-binding protein (PABP)-interacting motif; or the COOH terminus had been deleted (ΔNT, ΔLsm, ΔLsmAD, ΔPAM2, and ΔCT, respectively) (Fig. 3A). The results of confocal microscopy showed that only the ΔPAM2 construct showed a punctate distribution in the cytoplasm under normal conditions and partially impaired the recruitment to the SG induced by arsenite, whereas SG assembly was retained in the other mutants (supplemental Fig. 2). Next, because a recent study revealed that both the Lsm/LsmAD domain and the PAM2 motif independently mediate the assembly of ataxin-2 with polyribosomes (18), we further focused on the Lsm, LsmAD, and the PAM2 motif and generated double deletion constructs for each. As shown in Fig. 3B, the Lsm and PAM2 double-deleted forms of ataxin-2 (ΔLsm + PAM2) drastically affected recruitment to the SG. Therefore, this data indicates that the PAM2 motif and also the Lsm domain mediate ataxin-2 assembly with SG induced by arsenite.
FIGURE 3.
Effects of deleting specific domains of ataxin-2 on recruitment to SGs induced by arsenite. A, schematic diagram showing the location of deletions introduced into ataxin-2. All the ataxin-2 constructs contained an HA tag at the N terminus. B, HeLa cells expressing ataxin-2 deletion constructs were treated with/without arsenite (0.5 mm) for 1 h after double-labeling with anti-HA antibody (red) and monoclonal anti-G3BP antibody (green). Cells were counterstained with DAPI (blue). Scale bar = 10 μm. Note that ataxin-2 lacking both Lsm and PAM2 (ΔLsm + PAM2) is not localized in SGs.
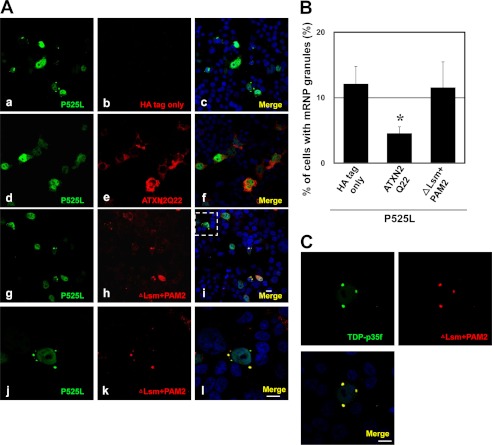
Next, we asked whether the PAM2 motif and the Lsm domain of ataxin-2 are also required for assembly during the formation of mRNP granules induced by the overexpression of mutant FUS and TDP-p35f. Surprisingly, ΔLsm+PAM2 did not affect recruitment to the mutant FUS and TDP-p35f mRNP granules (Fig. 6A, j–l, and C). We also examined the distributions of other deletion series and found that all the deletion series were recruited to mutant FUS and TDP-p35f mRNP granules (data not shown), indicating that multiple regions are involved in ataxin-2 assembly with mRNP granules induced by mutant FUS and TDP-p35f. These findings suggest that mRNP granules of cytoplasmic TDP-p35f and mutant FUS exhibit different characteristics of SG induced by arsenite, at least with regard to the assembly machinery of ataxin-2.
FIGURE 6.
PAM2 motif and the Lsm domain are critical for interference in mRNP granule formation. A, HeLa cells were transfected with plasmids for HA only, HA-Ataxin-2 (Gln-22) or HA-ΔLsm+PAM2 and P525L FUS-V5. The cells were stained with anti-HA (red) and V5 (green) antibodies. j, k, and l show high magnifications of the inset in i. Note that ΔLsm+PAM2 is recruited to mRNP granules of mutant FUS. B, in ∼800 transfected cells from four independent experiments, the percentages of cells with mRNP granules of mutant FUS were calculated. Error bars show S.D. *, p < 0.003, significant difference versus HA tag only; *, p < 0.001, HA-ΔLsm+PAM2. C, HeLa cells transfected with HA-ΔLsm+PAM2 and TDP-p35f-V5 were stained with anti-V5 (green) and HA (red) antibodies. Scale bar = 10 μm.
Overexpressed Ataxin-2 Levels Interfere with the Formation of Mutant FUS and TDP-p35f-mRNP Granules
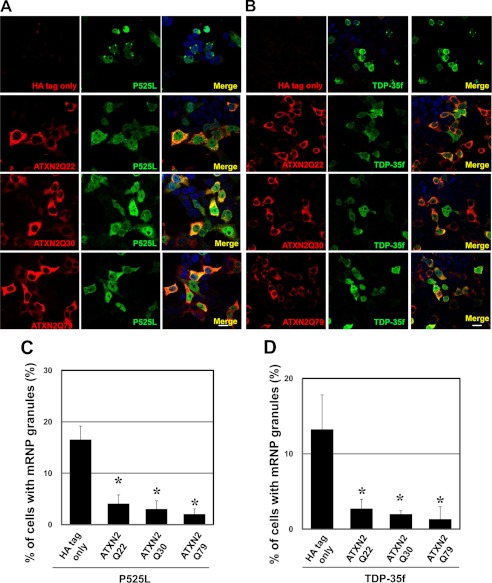
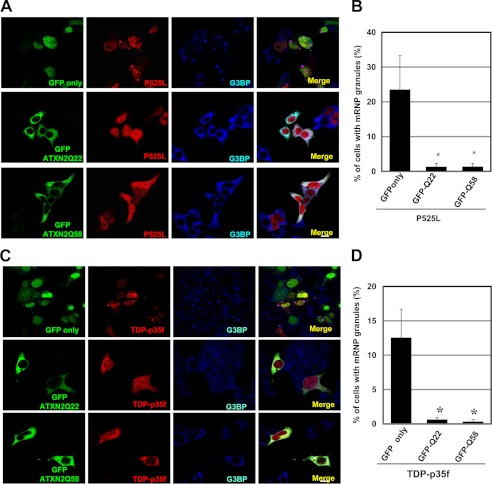
A recent study in both yeast and mammalian cells demonstrated that the altered expressions of ataxin-2 and a yeast ortholog of ataxin-2, Pab1-binding protein, affect the assembly of SGs and P-bodies (13). To address whether ataxin-2 may influence the mRNP granule formation of mutant FUS and TDP-p35f, we examined HeLa cells coexpressing ataxin-2 tagged with HA and mutant FUS or TDP-p35f tagged with V5. In cells expressing ataxin-2 with 22, 30, or 79 consecutive glutamines, the number of cells harboring mRNP granules of mutant FUS and TDP-p35f was decreased drastically (Fig. 4). Similar findings were observed in cells expressing GFP-ataxin-2 with 22 or 58 consecutive glutamines (Fig. 5).
FIGURE 4.
Ataxin-2 overexpression interferes with mRNP granules formed by mutant FUS and TDP-p35f. A and B, HeLa cells were transiently transfected with plasmid HA only (mock) or HA-ataxin-2 (Gln-22, Gln-30, or Gln-79) (red) and p525L FUS-V5 (A) or TDP-p35f-V5 (B) (green). To stain for exogenous ataxin-2 and mutant FUS and TDP-p35f, the cells were incubated with antibodies directed against HA (red) and V5-tag (green), followed by treatment with secondary antibodies. C and D, in ∼1000 transfected cells from five independent experiments, the percentage of cells harboring mRNP granules of mutant FUS (C) and TDP-p35f (D) were calculated. Note that the formation of mRNP granules was decreased in ataxin-2-expressing cells. Scale bar = 20 μm. Error bars show S.D. *, p < 0.0001, significant difference versus HA tag only.
FIGURE 5.
Overexpressed GFP-ataxin-2 interferes with the formation of mRNP granules containing mutant FUS and TDP-p35f. A, HeLa cells were transfected with plasmids for GFP only (pEGFP-N1) or GFP-ataxin-2 (Gln-22, Gln-58) and P525L FUS-V5 (A and B) or TDP-p35f-V5 (C and D). The cells were stained with anti-V5 (red) and the SG marker G3BP (blue) antibody. B and D, in ∼600 transfected cells from three independent experiments, the percentage of cells with mRNP granules of mutant FUS was calculated. Scale bar = 10 μm. Error bars show S.D. *, p < 0.003, significant difference versus GFP tag only.
We also examined whether the PAM2 motif and the Lsm domain of ataxin-2 are required for the inhibition of mRNP granule formation induced by mutant FUS. As shown in Fig. 6, ΔLsm+PAM2 completely abolished the effect of interference in mRNP granule formation. Therefore, these experiments indicated that an increased cellular ataxin-2 level potentially interferes with the formation of mRNP granules of the ALS/FTLD-U-linked molecules FUS and TDP-43, depending on the PAM2 motif and the Lsm domain.
Overexpressed Ataxin-2 Increases the Cytoplasmic Distribution of Mutant FUS and TDP-p35f
Whether the formation of mRNP granules is pathogenic or protective in neurodegenerative diseases is controversial. Recent evidence has revealed that mRNP granules reduce intracellular levels of toxic proteins linked to neurodegeneration, suggesting that they may serve a neuroprotective function (19–21). To address this issue, we transfected HeLa cells with mutant FUS or TDP-p35f for 48 h, and the presence or absence of mRNP granules was determined using immunostaining. The levels of mutant FUS or TDP-p35f expression in randomly chosen cells were then determined according to the fluorescence intensities using confocal microscopy. No significant difference in the whole cellular levels of mutant FUS and TDP-p35f was seen between cells with and those without mRNP granules (overall cellular intensity: granules-/P525L, 2.16 ± 0.76 × 107 (n = 30) and granules+/P525L, 1.95 ± 0.74 × 107 (n = 30), p = 0.287; granules-/TDP-p35f, 0.78 ± 0.37 × 107 (n = 30) and granules+/p35f, 0.66 ± 0.31 × 107 (n = 30), p = 0.195). As shown in Fig. 7, the mean levels of diffuse cytoplasmic mutant FUS and p35f (with the exception of mRNP granules) in cells with mRNP granules were significantly low, suggesting that mRNP granule formation was linked to a decrease in diffuse cytoplasmic mutant FUS and TDP-p35f (mean cytosol/mean whole cellular intensity: granules-/P525L, 0.624 ± 0.25 and granules+/P525L, 0.47 ± 0.21, p = 0.012; granules-/TDP-p35f, 0.71 ± 0.27, granules+/TDP-p35f, 0.52 ± 0.19, p = 0.0021).
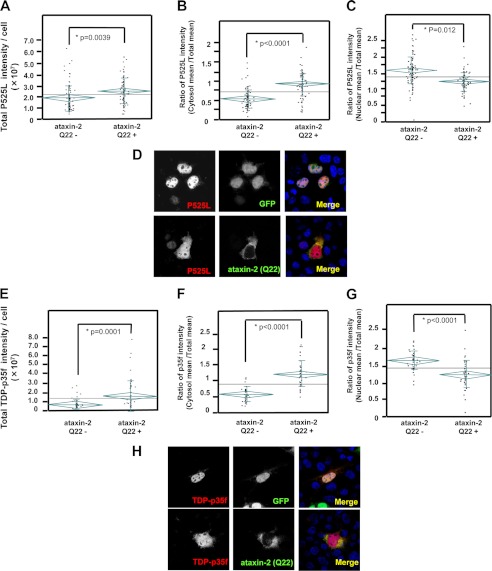
Next, we asked whether altered ataxin-2 levels affected the cytoplasmic levels of mutant FUS or TDP-p35f. Cells expressing mutant FUS or TDP-p35f with/without ataxin-2 (Gln-22) were used to assess the diffuse cytoplasmic levels of mutant FUS or TDP-p35f. The total cellular levels of TDP-p35f or mutant FUS in cells overexpressing ataxin-2 were significantly higher than those in cells that were not transfected with ataxin-2 (Fig. 8, A and E) (whole cellular intensity: empty vector/P525L, 1.99 ± 0.14 × 107 (n = 93) and ataxin-2 (Gln-22)/P525L, 2.62 ± 0.15 × 107 (n = 93), p = 0.0039; empty vector/TDP-p35f, 0.73 ± 0.56 × 107 (n = 40) and ataxin-2 (Gln-22)/TDP-p35f, 1.88 ± 1.69 × 107 (n = 40), p = 0.0001). This finding supports the results of a Western blot analysis, which showed increases in the TDP-p35f, and wild-type FUS and mutant FUS levels in the presence of ataxin-2 (Gln-22 and Gln-58) coexpression (Fig. 9A). A quantification analysis showed that ataxin-2 (Gln-58) increased the mutant FUS and TDP-p35f protein levels to a significantly greater degree than ataxin-2 (Gln-22), suggesting that the polyQ repeat of ataxin-2 might affect the levels of these proteins (Fig. 9, B and C). Interestingly, the expression level of full-length TDP-43 changed minimally compared with the changes in the expressions of the other molecules. A cellular compartment analysis (Fig. 8) revealed that the diffuse distribution of cytoplasmic TDP-p35f and mutant FUS clearly increased in the presence of ataxin-2 overexpression (mean cytosol/mean whole cellular intensity: empty vector/P525L, 0.61 ± 0.29 and ataxin-2 (Gln-22)/P525L, 1.05 ± 0.36, p < 0.0001; empty vector/TDP-p35f, 0.62 ± 0.25 and ataxin-2 (Gln-22)/TDP-p35f, 1.30 ± 0.43, p < 0.0001). Meanwhile, the nuclear distributions in cells with overexpressed ataxin-2 were significantly lower (mean nuclear/mean whole cellular intensity: empty vector/P525L, 1.50 ± 0.26 and ataxin-2 (Gln-22)/P525L, 1.13 ± 0.38, p = 0.012; empty vector/TDP-p35f, 1.56 ± 0.44 and ataxin-2 (Gln-22)/TDP-p35f, 1.23 ± 0.28, p < 0.0001]. Collectively, these findings suggest that ataxin-2 is a potent modifier of the cytoplasmic and nuclear distribution of TDP-p35f and mutant FUS.
FIGURE 8.
Overexpression of ataxin-2 increases cytoplasmic mutant FUS and TDP-p35f. HeLa cells transfected with GFP only or GFP-Ataxin-2 (Gln-22) (green) and p525L FUS-V5 (A–D) or TDP-p35f-V5 (E–H) were stained with anti-V5 antibody (red). The level of mutant FUS and TDP-p35f were quantified using confocal microscopy. The graphs indicate the intensity of all the cells (A and E), the ratio of the mean intensity of the cytosol/whole cell (B and F), or the mean intensity of the nucleus/whole cell (C and G). Note that the whole cell and cytosolic fluorescence intensities of mutant FUS (A and B) and TDP-p35f (E and F) in the cells that overexpressed ataxin-2 were significantly high, whereas the nuclear intensity (C and G) was low in the cells that overexpressed ataxin-2. Representative images are shown in D and H. The diamonds and vertical lines indicate the 95% prediction interval and S.D., respectively. The mean values among all the cells or the cells with/without ataxin-2 expression are shown using long horizontal lines on the graph and short horizontal lines in a diamond, respectively.
DISCUSSION
This study documents several lines of evidence regarding the molecular pathogenesis of the ALS-associated molecules TDP-p35f and mutant FUS and their modification by ataxin-2. First, ataxin-2 is assembled with SGs induced by arsenite in an Lsm domain and PAM2 motif-dependent manner (Fig. 3B). Second, the increased ataxin-2 levels interfered with the mRNP granule formation of TDP-p35f and mutant FUS depending on the Lsm domain and PAM2 motif (Figs. 4–6). Third, the cytoplasmic distributions of TDP-p35f and mutant FUS in cells with mRNP granules were significantly lower than those in cells without mRNP granules, suggesting that mRNP granule formation potentially decreases the diffuse cytoplasmic TDP-p35f and mutant FUS (Fig. 7). Fourth, the overexpression of ataxin-2 potentially increases the diffuse cytoplasmic distribution of TDP-p35f and mutant FUS, whereas it decreased the nuclear distributions of both proteins (Fig. 8), suggesting that an excessive ataxin-2 level enhances the disturbance of the RNA quality control system via an aberrant distribution of RNA binding proteins. These findings support a recent report that ataxin-2 enhances TDP-43 toxicity in animal, yeast, and fly models (11) and also explains the mechanism of ataxin-2 as a modulator of TDP-43 toxicity.
The proper control of RNA processing, degradation, and translation is a critical aspect of the regulation of gene expression and was shown recently to be an important part of the pathogenesis of neurodegeneration (22), including that which occurs in ALS (9, 10, 14, 23). Although there is no direct evidence showing a relation between mRNP granules, so-called SGs and P-bodies, and neurodegeneration, mRNP granules containing nontranslating mRNAs, translation regulators, and RNA degradation machinery, exhibit a dynamic cycle of distinct biochemical and compartmentalized mRNPs and mRNA in the cytosol with implications for the control of mRNA function (24). Although each granule has a distinct composition in the cytoplasm, they do not function and exist entirely separate from each other (25). The SGs and P-bodies, which are often juxtaposed with one another, share many mRNP components, such as Rck/p54 and eIF4E, suggesting a transition between these mRNP granules (26). An experiment in yeast indicated that SG assembly depends on P-body formation and suggested that SG is primarily formed from pre-existing P-bodies (27). Recent studies have shown that the concentration of the cytoplasmic RNA binding protein ataxin-2 is important for the assembly of mRNP granules (13). Nonhoff et al. (13) demonstrated that overexpressed ataxin-2 levels decreased with cellular P-body formation. Meanwhile, a reduction in ataxin-2 inhibited SG formation, suggesting that ataxin-2 was necessary for SG assembly. In contrast, this study showed that the overexpression of ataxin-2 drastically disrupted the mRNP granule formation of cytoplasmic TDP-p35f and mutant FUS, which were positive for SG markers as described in a previous paper (6, 7). The reason for this discrepancy remains unknown, but differences in the experimental conditions, cell lines, and expression levels are reasonable explanations. However, because SGs and P-bodies are not entirely separate from each other, as described above, one possibility is that mRNP granules of cytoplasmic TDP-p35f and mutant FUS also share at least some features of P-bodies with regard to the assembly regulated by ataxin-2. This hypothesis is supported by the data shown in Figs. 3 and 6, which show that the assembly machinery of ataxin-2 differs between SG-induced by arsenite and mRNP granules of cytoplasmic TDP-p35f and mutant FUS, indicating that TDP-p35f and mutant FUS mRNP granules have partially distinct features from well established arsenite-induced SG.
Whether the aberrant accumulation of pathogenic proteins, so-called inclusion bodies, is pathogenic or protective in neurodegenerative diseases remains to be determined. Several lines of experimental evidence have indicated that inclusion bodies reduce intracellular levels of diffuse polyglutamine disease proteins, such as the overexpression of huntingtin or ataxin 1, suggesting that they may serve a neuroprotective function (19, 28). However, whether this is a common mechanism of protection in other neurodegenerative diseases remains unclear. Recent pathological findings have demonstrated that inclusion bodies of FTLD-U are histologically positive for mRNP granules markers, suggesting that mRNP granule biology plays an important role in inclusion body formation (29, 30). Interestingly, our present findings also revealed that the formation of mRNP granules, which interferes with increased ataxin-2 levels, substantially reduced the diffuse cytoplasmic distribution of overexpressed truncated TDP-43 and mutant FUS (Fig. 7), suggesting that the formation of mRNP granules may be neuroprotective via the attenuation of excessive pathogenic RNA binding protein.
Another important subject to be elucidated is the molecular mechanism responsible for the enhancement of the aberrant distribution of TDP-43 and FUS protein by the expression of ataxin-2, as shown in Fig. 8. We hypothesized several possible scenarios explaining the relation between the distribution of TDP-43, FUS proteins, and ataxin-2. First, the mRNP granules may potentially trap and incarcerate excessive diffuse cytoplasmic mRNA binding protein. Increased ataxin-2 expression inhibits the formation of mRNP granules (Figs. 4 and 5), thereby increasing the free levels of both mRNA binding proteins. This hypothesis is consistent with the findings in Fig. 7, which shows that the diffuse cytoplasmic TDP-p35f and mutant FUS levels in cells with mRNP granules were significantly lower than in those without mRNP granules. Second, the fact that the total cellular levels of cytoplasmic TDP-43 and FUS protein are also increased in the presence of overexpressed ataxin-2 (Figs. 8, A and E, and 9) suggests that ataxin-2 may modulate the stabilities of both proteins. This scenario is supported by a recent study showing that excess polyglutamine, such as huntingtin and SCA1, inhibits ubiquitin-proteasome system activity (31). Thus, increased levels of polyglutamine protein, such as ataxin-2, likely affect the degradation machinery of TDP-43 and FUS protein. Third, because the nuclear levels of both mRNA binding proteins in ataxin-2 overexpressed cells are clearly decreased (Fig. 8, C and G), ataxin-2 may affect the nuclear distribution of both proteins from the cytoplasm to the nucleus. A recent study has shown that the intranuclear transport mechanisms of TDP-43 and FUS mediate nuclear import receptors (importin and transportin, respectively) (32, 33). Thus, ataxin-2 may detain both proteins in cytoplasm via either a direct or an indirect interaction and consequently may lead to an insufficiency of nuclear TDP-43 and FUS. A recent paper reported the direct binding of TDP-43 and ATXN2 in a coimmunoprecipitation study (11), but the detailed interactions and modification of ataxin-2 on TDP-43 and FUS remain unknown. Although future studies are needed to clarify the molecular mechanisms of ataxin-2 on TDP-43 and FUS, we propose that the molecular basis for the enhancement of an aberrant distribution of TDP-43 and FUS mediated by ataxin-2 may be a key therapeutic target against ALS/FTLD-U.
Growing evidence indicates that intermediate-length polyglutamine expansions (27–33 glutamine residues) in ataxin-2 are a genetic risk factor for ALS (11, 34–36). A recent study has also shown that intermediate-length ataxin-2 polyQ expansions activate stress-induced TDP-43 C-terminal cleavage and phosphorylation, linking the activation of caspase 3 (37). Elden et al. (11) demonstrated that polyQ repeat expansions increase ataxin-2 stability and enhance TDP-43 mislocalization, and we suggested that long-lifespan ataxin with expanded polyQ repeats may accumulate to a greater degree than ataxin-2 with a normal polyQ repeat, possibly enhancing toxicity. In this study, the longer polyQ length of ataxin-2 (Gln-58) significantly increased the levels of mutant FUS and TDP-p35f compared with ataxin-2 (Gln-22), indicating a polyQ repeat dependence (Fig. 9). However, Fig. 4 shows that a long polyQ repeat tends to enhance the inhibition of mRNP granule formation only slightly, and a significant repeat dependence was not observed. In this case, the potency of ataxin-2 may have been saturated in the overexpression studies, masking the polyQ repeat dependence. Future studies on the basis of native expression levels using knockin or patient-derived cells, such as induced pluripotent stem (iPS) or neuronal cells (iN), may be necessary to reveal the significance of polyQ repeat dependence in mRNP granule formation.
Although further investigations are required to elucidate the precise function of RNA granules in TDP-43 and FUS proteinopathies, our study contributes significantly toward a greater understanding of the molecular basis of interactions among the key proteins TDP-43, FUS, and ataxin-2 in ALS/FTLD-U. Further improvement of RNA quality control systems may serve as a novel therapeutic target for delaying or preventing neurodegeneration in ALS/FTLD-U.
Acknowledgments
We thank Dr. Manfred Schwab (German Cancer Research Center, Heidelberg, Germany) for providing the pcDNA-HA-Ataxin-2 Gln-22, Gln-33, and Gln-79; Dr. John Goodier (Department of Genetics, University of Pennsylvania School of Medicine) for providing RFP-TIA1; and Dr. Stefan M. Pulst (Department of Neurology, University of Utah, Salt Lake City, UT) for providing the pGFP-ataxin-2-(Q22) and -(Q58).
This work was supported by Eisai Co. Ltd, by Ministry of Education, Culture, Sports, Science and Technology of Japan Grant 23591254, by the ALS Foundation, and by the Japan ALS Association.

This article contains supplemental Figs. 1 and 2.
- FUS
- Fused in Sarcoma
- ALS
- amyotrophic lateral sclerosis
- FTLD-U
- frontotemporal lobar degeneration with ubiquitin-positive inclusion bodies
- mRNP
- messenger ribonucleoprotein
- SG
- stress granules
- Lsm
- Like Sm
- LsmAD
- Lsm-associated domain
- PABP
- poly(A)-binding protein.
REFERENCES
- 1. Kwiatkowski T. J., Jr., Bosco D. A., Leclerc A. L., Tamrazian E., Vanderburg C. R., Russ C., Davis A., Gilchrist J., Kasarskis E. J., Munsat T., Valdmanis P., Rouleau G. A., Hosler B. A., Cortelli P., de Jong P. J., Yoshinaga Y., Haines J. L., Pericak-Vance M. A., Yan J., Ticozzi N., Siddique T., McKenna-Yasek D., Sapp P. C., Horvitz H. R., Landers J. E., Brown R. H., Jr. (2009) Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 [DOI] [PubMed] [Google Scholar]
- 2. Neumann M., Sampathu D. M., Kwong L. K., Truax A. C., Micsenyi M. C., Chou T. T., Bruce J., Schuck T., Grossman M., Clark C. M., McCluskey L. F., Miller B. L., Masliah E., Mackenzie I. R., Feldman H., Feiden W., Kretzschmar H. A., Trojanowski J. Q., Lee V. M. (2006) Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 [DOI] [PubMed] [Google Scholar]
- 3. Sreedharan J., Blair I. P., Tripathi V. B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J. C., Williams K. L., Buratti E., Baralle F., de Belleroche J., Mitchell J. D., Leigh P. N., Al-Chalabi A., Miller C. C., Nicholson G., Shaw C. E. (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319, 1668–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vance C., Rogelj B., Hortobágyi T., De Vos K. J., Nishimura A. L., Sreedharan J., Hu X., Smith B., Ruddy D., Wright P., Ganesalingam J., Williams K. L., Tripathi V., Al-Saraj S., Al-Chalabi A., Leigh P. N., Blair I. P., Nicholson G., de Belleroche J., Gallo J. M., Miller C. C., Shaw C. E. (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., Oda T. (2006) TDP-43 is a component of ubiquitin-positive τ-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 [DOI] [PubMed] [Google Scholar]
- 6. Ito D., Seki M., Tsunoda Y., Uchiyama H., Suzuki N. (2011) Nuclear transport impairment of ALS-linked mutations in FUS/TLS. Ann. Neurol. 69, 152–162 [DOI] [PubMed] [Google Scholar]
- 7. Nishimoto Y., Ito D., Yagi T., Nihei Y., Tsunoda Y., Suzuki N. (2010) Characterization of alternative isoforms and inclusion body of the TAR DNA-binding protein-43. J. Biol. Chem. 285, 608–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito D., Suzuki N. (2011) Conjoint pathological cascades mediated by the ALS/FTLD-U linked RNA-binding proteins. Neurology 77, 1636–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polymenidou M., Lagier-Tourenne C., Hutt K. R., Huelga S. C., Moran J., Liang T. Y., Ling S. C., Sun E., Wancewicz E., Mazur C., Kordasiewicz H., Sedaghat Y., Donohue J. P., Shiue L., Bennett C. F., Yeo G. W., Cleveland D. W. (2011) Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat. Neurosci. 14, 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tollervey J. R., Curk T., Rogelj B., Briese M., Cereda M., Kayikci M., König J., Hortobágyi T., Nishimura A. L., Zupunski V., Patani R., Chandran S., Rot G., Zupan B., Shaw C. E., Ule J. (2011) Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci. 14, 452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elden A. C., Kim H. J., Hart M. P., Chen-Plotkin A. S., Johnson B. S., Fang X., Armakola M., Geser F., Greene R., Lu M. M., Padmanabhan A., Clay-Falcone D., McCluskey L., Elman L., Juhr D., Gruber P. J., Rüb U., Auburger G., Trojanowski J. Q., Lee V. M., Van Deerlin V. M., Bonini N. M., Gitler A. D. (2010) Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albrecht M., Golatta M., Wüllner U., Lengauer T. (2004) Structural and functional analysis of ataxin-2 and ataxin-3. Eur. J. Biochem. 271, 3155–3170 [DOI] [PubMed] [Google Scholar]
- 13. Nonhoff U., Ralser M., Welzel F., Piccini I., Balzereit D., Yaspo M. L., Lehrach H., Krobitsch S. (2007) Ataxin-2 interacts with the DEAD/H-box RNA helicase DDX6 and interferes with P-bodies and stress granules. Mol. Biol. Cell 18, 1385–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Igaz L. M., Kwong L. K., Lee E. B., Chen-Plotkin A., Swanson E., Unger T., Malunda J., Xu Y., Winton M. J., Trojanowski J. Q., Lee V. M. (2011) Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J. Clin. Invest. 121, 726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ito D., Suzuki N. (2007) Molecular pathogenesis of seipin/BSCL2-related motor neuron diseases. Ann. Neurol. 61, 237–250 [DOI] [PubMed] [Google Scholar]
- 16. Ito D., Walker J. R., Thompson C. S., Moroz I., Lin W., Veselits M. L., Hakim A. M., Fienberg A. A., Thinakaran G. (2004) Characterization of stanniocalcin 2, a novel target of the mammalian unfolded protein response with cytoprotective properties. Mol. Cell Biol. 24, 9456–9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kedersha N., Anderson P. (2007) Mammalian stress granules and processing bodies. Methods Enzymol. 431, 61–81 [DOI] [PubMed] [Google Scholar]
- 18. Satterfield T. F., Pallanck L. J. (2006) Ataxin-2 and its Drosophila homolog, ATX2, physically assemble with polyribosomes. Hum. Mol. Genet. 15, 2523–2532 [DOI] [PubMed] [Google Scholar]
- 19. Arrasate M., Mitra S., Schweitzer E. S., Segal M. R., Finkbeiner S. (2004) Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810 [DOI] [PubMed] [Google Scholar]
- 20. Orr H. T. (2004) Neurodegenerative disease. Neuron protection agency. Nature 431, 747–748 [DOI] [PubMed] [Google Scholar]
- 21. Taylor J. P., Tanaka F., Robitschek J., Sandoval C. M., Taye A., Markovic-Plese S., Fischbeck K. H. (2003) Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum. Mol. Genet. 12, 749–757 [DOI] [PubMed] [Google Scholar]
- 22. Todd P. K., Paulson H. L. (2010) RNA-mediated neurodegeneration in repeat expansion disorders. Ann. Neurol. 67, 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lagier-Tourenne C., Polymenidou M., Cleveland D. W. (2010) TDP-43 and FUS/TLS. Emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 19, R46–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buchan J. R., Parker R. (2009) Eukaryotic stress granules. The ins and outs of translation. Mol. Cell 36, 932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kedersha N., Stoecklin G., Ayodele M., Yacono P., Lykke-Andersen J., Fritzler M. J., Scheuner D., Kaufman R. J., Golan D. E., Anderson P. (2005) Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 169, 871–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swisher K. D., Parker R. (2010) Localization to, and effects of Pbp1, Pbp4, Lsm12, Dhh1, and Pab1 on stress granules in Saccharomyces cerevisiae. PLoS ONE 5, e10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buchan J. R., Muhlrad D., Parker R. (2008) P bodies promote stress granule assembly in Saccharomyces cerevisiae. J. Cell Biol. 183, 441–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klement I. A., Skinner P. J., Kaytor M. D., Yi H., Hersch S. M., Clark H. B., Zoghbi H. Y., Orr H. T. (1998) Ataxin-1 nuclear localization and aggregation. Role in polyglutamine-induced disease in SCA1 transgenic mice. Cell 95, 41–53 [DOI] [PubMed] [Google Scholar]
- 29. Fujita K., Ito H., Nakano S., Kinoshita Y., Wate R., Kusaka H. (2008) Immunohistochemical identification of messenger RNA-related proteins in basophilic inclusions of adult-onset atypical motor neuron disease. Acta Neuropathol. 116, 439–445 [DOI] [PubMed] [Google Scholar]
- 30. Liu-Yesucevitz L., Bilgutay A., Zhang Y. J., Vanderweyde T., Vanderwyde T., Citro A., Mehta T., Zaarur N., McKee A., Bowser R., Sherman M., Petrucelli L., Wolozin B. (2010) Tar DNA binding protein-43 (TDP-43) associates with stress granules. Analysis of cultured cells and pathological brain tissue. PLoS ONE 5, e13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bennett E. J., Bence N. F., Jayakumar R., Kopito R. R. (2005) Global impairment of the ubiquitin-proteasome system by nuclear or cytoplasmic protein aggregates precedes inclusion body formation. Mol. Cell 17, 351–365 [DOI] [PubMed] [Google Scholar]
- 32. Dormann D., Rodde R., Edbauer D., Bentmann E., Fischer I., Hruscha A., Than M. E., Mackenzie I. R., Capell A., Schmid B., Neumann M., Haass C. (2010) ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 29, 2841–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishimura A. L., Zupunski V., Troakes C., Kathe C., Fratta P., Howell M., Gallo J. M., Hortobágyi T., Shaw C. E., Rogelj B. (2010) Nuclear import impairment causes cytoplasmic trans-activation response DNA-binding protein accumulation and is associated with frontotemporal lobar degeneration. Brain 133, 1763–1771 [DOI] [PubMed] [Google Scholar]
- 34. Van Damme P., Veldink J. H., van Blitterswijk M., Corveleyn A., van Vught P. W., Thijs V., Dubois B., Matthijs G., van den Berg L. H., Robberecht W. (2011) Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2. Neurology 76, 2066–2072 [DOI] [PubMed] [Google Scholar]
- 35. Corrado L., Mazzini L., Oggioni G. D., Luciano B., Godi M., Brusco A., D'Alfonso S. (2011) ATXN-2 CAG repeat expansions are interrupted in ALS patients. Hum. Genet. 130, 575–580 [DOI] [PubMed] [Google Scholar]
- 36. Lee T., Li Y. R., Chesi A., Hart M. P., Ramos D., Jethava N., Hosangadi D., Epstein J., Hodges B., Bonini N. M., Gitler A. D. (2011) Evaluating the prevalence of polyglutamine repeat expansions in amyotrophic lateral sclerosis. Neurology 76, 2062–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hart M. P., Gitler A. D. (2012) ALS-associated ataxin 2 polyq expansions enhance stress-induced caspase 3 activation and increase TDP-43 pathological modifications. J. Neurosci. 32, 9133–9142 [DOI] [PMC free article] [PubMed] [Google Scholar]