Background: Synapse formation and function is modulated by intrinsic and extrinsic non-autonomous factors.
Results: Astrocytes induce synapse formation through TGF-β1 pathway. TGF-β1 synaptogenic property is dependent on d-serine signaling.
Conclusion: TGF-β induces excitatory glutamatergic synapses in vertebrates.
Significance: This is a novel molecular mechanism that might impact synaptic function and shed light on new potential therapeutic targets for synaptic deficit diseases.
Keywords: Astrocytes, Glia, Neurotransmitters, Synapses, Transforming Growth Factor beta (TGFbeta)
Abstract
Assembly of synapses requires proper coordination between pre- and postsynaptic elements. Identification of cellular and molecular events in synapse formation and maintenance is a key step to understand human perception, learning, memory, and cognition. A key role for astrocytes in synapse formation and function has been proposed. Here, we show that transforming growth factor β (TGF-β) signaling is a novel synaptogenic pathway for cortical neurons induced by murine and human astrocytes. By combining gain and loss of function approaches, we show that TGF-β1 induces the formation of functional synapses in mice. Further, TGF-β1-induced synaptogenesis involves neuronal activity and secretion of the co-agonist of the NMDA receptor, d-serine. Manipulation of d-serine signaling, by either genetic or pharmacological inhibition, prevented the TGF-β1 synaptogenic effect. Our data show a novel molecular mechanism that might impact synaptic function and emphasize the evolutionary aspect of the synaptogenic property of astrocytes, thus shedding light on new potential therapeutic targets for synaptic deficit diseases.
Introduction
Human cognitive ability is associated with hugely complex synaptic connections (1). Conversely, synaptic dysfunctions may lead to several human neurodevelopmental disorders, including autism, schizophrenia, epilepsy, and others. Thus, understanding the mechanism by which synapses are formed, specified, and maintained is a key step, not only in understanding human cognitive advantages, but also in designing therapeutic approaches to repair the injured human nervous system. Despite compelling advances in the identification of cellular and molecular events in synapse formation, a fundamental question remains unresolved. Is the number of synapses an intrinsic property of neurons, or is it controlled by extrinsic signals or non-neuronal determinants?
The transforming growth factor β (TGF-β) superfamily is constituted by multifunctional polypeptide members, which perform critical functions in nervous system development and repair (2–9). Disturbances in TGF-β pathways have been associated with a broad spectrum of behavioral abnormalities, including cognitive impairment, affective disorders, and deficits in sensorimotor gating (10–13). Members of the TGF-β superfamily have been strongly implicated in synapse formation in invertebrates (14–18) and, more recently, in vertebrates (19–22).
Glial cells are key determinants in the formation, stabilization, and elimination of synapses, in either the peripheral or central nervous system (CNS) (23–32). It remains unclear whether glia-driven synaptogenesis is a general principle that applies to all neuronal cell types and brain regions as well as being a conserved feature for all mammals, including humans. The fact that TGF-β1 is secreted by central and peripheral glia (2, 3, 6) and is expressed in the embryonic and postnatal brain during the synaptic period (33) led us to investigate the hypothesis of TGF-β1 as a synaptogenic molecule for vertebrate neurons.
Here, we provide evidence that the TGF-β signaling pathway is involved in murine and human astrocytes synaptogenic property. Further, we unveil a mechanism underlying TGF-β1-induced synaptogenesis involving d-serine, a co-agonist of the N-methyl-d-aspartate (NMDA) subtype of glutamate receptor. Our results not only contribute to understanding the mechanism by which astrocytes and growth factors modulate synapse development but may potentially lead to the development of novel therapeutic approaches to neurological disorders, based on the pharmacological manipulation of the levels of excitatory amino acids.
EXPERIMENTAL PROCEDURES
Animals
For neuronal cultures, embryonic (embryonic day 14) mice were used and for astrocyte cultures, newborn (postnatal day 0) Swiss mice were used. All animal use protocols were approved by the Animal Use Ethics Committee of the Federal University of Rio de Janeiro.
Astrocyte Cultures
Primary astrocyte cultures were prepared from the cerebral cortex of newborn Swiss mice as described previously. Briefly, the mice were decapitated, the brain structures were removed, and the meninges were carefully stripped off. Tissues were washed in phosphate-buffered saline (PBS) with 0.6% glucose (Sigma) and dissociated into single cells in a medium consisting of Dulbecco's minimum essential medium (DMEM) and nutrient mixture F-12 (DMEM/F-12; Invitrogen), enriched with glucose (3.3 × 10−2 m), glutamine (2 × 10−3 m), and sodium bicarbonate (0.3 × 10−2 m). Dissociated cells were plated onto glass coverslips previously coated with poly-l-lysine (Sigma), on a 24-well plate (Corning Glass), in DMEM/F-12 medium supplemented with 10% fetal bovine serum (Invitrogen). The cultures were incubated at 37 °C in a humidified 5% CO2, 95% air chamber for 7–10 days until reaching confluence. After growing to confluence, cells were subjected to passages to generate pure astrocytic cultures. This protocol yields an astrocyte-enriched culture constituted by more than 95% glial fibrillary acidic protein (GFAP)3-positive cells.
Astrocyte Conditioned Medium
Astrocyte conditioned medium was prepared as described previously (34). After reaching confluence, secondary astrocyte cultures were thoroughly washed to eliminate residual serum, and the cultures were maintained without fetal bovine serum, for 1 day. The medium was then collected and centrifuged to remove cellular debris and used immediately. To analyze the effect of astrocyte conditioned medium in neuronal synapse formation, neuronal cultures were grown in Neurobasal medium for 12 days in vitro. After this period, the neuronal culture was preincubated with vehicle or the TGF-β pathway inhibitor, SB-431542, for 30 min. Then the neuronal cultures were maintained simultaneously in the presence of astrocyte conditioned medium and the inhibitor for additional 3 h, followed by fixation and immunostaining of synaptic proteins. SB-431542 (10 μm) (Sigma) was diluted in DMSO (Sigma). Cells were exposed to a maximum concentration of 0.4% DMSO. The addition of DMSO to astrocyte conditioned medium had no effect on synapse formation (data not shown).
Neuronal Cultures
Dissociated primary neuronal cultures were prepared from the cerebral cortex of embryonic day 14 Swiss mice and maintained in Neurobasal medium (Invitrogen) supplemented with B-27, penicillin, streptomycin, l-glutamine, fungizone, and cytosine arabinoside (0.65 μm; Sigma). Neurons were plated at a density of 50,000–150,000 neurons/well of 13-mm diameter and 800,000 cells/well of 35-mm diameter, previously coated with polylysine (10 μg/ml; Sigma). One-third of the medium was changed for fresh medium every 3 days. This protocol produces a neuron-enriched culture (98% of neurons and 2% of astrocytes).
Treatment of Neuronal Cultures
Neurons were treated with TGF-β1 (10 ng/ml; R&D Systems, Buckinghamshire, UK) or d-serine (0.4 mm; Sigma) for different times (0, 3, 6, and 9 days in vitro). For pharmacological inhibition assays, neuronal cultures were plated and incubated in the presence or absence of specific inhibitors for 3 days in vitro (10 μm 5,7-dichlorokynurenic acid; 10 μm MK-801 (Sigma). To analyze the effect of d-serine degradation on synapse formation, neuronal cultures were simultaneously treated with TGF-β1 and the enzyme d-amino acid oxidase (DAO) for 3 days in vitro (20 μg/ml; MP Biomedicals LLC, Illkirch, France). For assurance of DAO activity specificity, cultures were also treated with heat-inactivated DAO, boiled for 5 min. Removal of hydrogen peroxide derived from the degradation of d-serine was assayed by the addition of catalase (10 units/ml; Sigma).
Human Astrocyte Cultures
Adult primary human astrocytes were isolated from surgically resected anterior temporal lobe tissue, from patients selected for surgical treatment of temporal lobe epilepsy associated with hippocampus sclerosis. The selected patients were evaluated by video-electroencephalography monitoring with a 132-channel Nihon-Kohden® apparatus, and the ictal onset zone was concordant with neuroimaging and semiology data. The pathological tissue targeted in surgery for these cases is the gliotic hippocampus, and the anterior temporal lobe resection is used merely as a surgical pathway to the diseased area. As described previously (35), only healthy cortical tissue was used to produce astrocyte cultures. All patients gave written consent to the study, and the procedures were in agreement with the Brazilian Ministry of Health Ethics Committee (CONEP 2340). Experimental protocols were performed as described previously (36). Briefly, tissues were washed in DMEM medium, mechanically dissociated, chopped into small (<2-mm3) pieces with a sterile scalpel, and incubated in 10 ml of 0.25% trypsin solution at 37 °C for 10 min. After centrifugation for 10 min, the cell pellet was resuspended in DMEM/F-12 growth medium supplemented with 10% FCS and plated onto tissue culture plates in a humidified 5% CO2, 95% air atmosphere at 37 °C for 2 h in order to achieve adherence of microglial cells. The nonadherent astrocytes were transferred into other culture plates, previously coated with poly-l-lysine. Adherent astrocytes were allowed to grow by replacing the medium once a week. New passages of cells were generated by harvesting confluent astrocyte cultures using trypsin-EDTA solution (0.25% trypsin with EDTA; Invitrogen). Human astrocytes from up to the third passage were used in the study.
Presynaptic Activity Analysis
Cells were washed with extracellular solution (150 mm NaCl, 4 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm glucose, 10 mm HEPES, pH 7.4). After that, the cells were incubated with a high potassium solution (97 mm NaCl, 57 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm glucose, 10 mm HEPES, pH 7.4) containing FM1-43 (5 μm; Molecular Probes, Paisley, UK) at room temperature for 5 min. Cells were washed twice with the extracellular solution and then fixed and observed by means of a TE2000 Nikon microscope.
d-Serine Level Analysis
The media of astrocyte or neuronal cultures were supplemented with 10 mm l-serine (Sigma) to maximize d-serine formation, in the presence or absence of 10 ng/ml TGF-β1 (R&D Systems). After 24 h, the conditioned medium was removed and treated with trichloroacetic acid (5% final concentration) to precipitate proteins and extract the free amino acids. Samples were centrifuged (20,000 × g for 5 min), the supernatants were extracted three times with water-saturated diethyl ether to remove trichloroacetic acid, and the amino acids were measured by HPLC, as described (37).
Transfection of Neuronal Cultures with shRNA
Sequences complementary to the rat homolog of the serine racemase gene were designed and cloned into the pENTR/U6 plasmid (Invitrogen). This vector induces the expression of short hairpin RNAs (shRNAs) in mammalian cells, under the control of the U6 promoter and RNA polymerase III. Two sequences targeting the serine racemase gene and one control sequence targeting the exogenous LacZ gene were chemically synthesized and cloned into the pENTR/U6 plasmid to generate the shRNA pENTR/U6 vectors: SR597-pENTR/U6 (top strand, CACCGCTCTCACCTATGCTGCTAAACGAATTTAGCAGCATAGGTGAGAGC), SR1063-pENTR/U6 (top strand, CACCGCTTAAATACCTGGCCTATTACGAATAATAGGCCAGGTATTTAAGC), and LacZ-pENTR/U6 (top strand, CACCGCTACACAAATCAGCGATTTCGAAAAATCGCTGATTTGTGTAG). For transfection, neuronal cultures were incubated for 4 h with 0.5 ml of Opti-MEM (Invitrogen) and Lipofectamine 2000 (Invitrogen) in the presence of 1 μg of SR597-pENTR/U6 plus 1 μg of SR1063-pENTR/U6 or in the presence of 1 μg of LacZ-pENTR/U6. After transfection, cells were maintained for an additional 48 h in the regular medium followed by treatment with 10 ng/ml TGF-β1 (R&D Systems) for 3 days and subsequent immunocytochemistry analysis.
ELISA of Neuronal and Astrocyte Conditioned Medium
Concentrations of murine TGF-β1 were measured in cell-free conditioned media derived from astrocyte and neuron cultures. A standardized sandwich ELISA protocol with matched antibody pairs from PeproTech and R&D Systems was carried out according to the manufacturers' instructions. Results are expressed as pg/ml. The experimental groups consisted of triplicate samples from three independent experiments.
Immunocytochemistry
After fixation with 4% paraformaldehyde for 15 min, the cultures were permeabilized with 0.2% Triton X-100 for 5 min at room temperature, and nonspecific sites were blocked with 10% bovine serum albumin (Sigma) for 1 h before immunoreactions with the following antibodies: rabbit anti-GFAP (1:500; DAKO Cytomation, Glostrup, Denmark); mouse anti-class III β-tubulin (1:1,000; Promega Corp., Madison, WI); mouse anti-α-synaptophysin (1:200; Chemicon International, Billerica, MA); rabbit anti-PSD-95 (1:100; Cell Signaling Technology, Beverly, MA); rabbit anti-serine racemase (1:500; Abcam, Inc., Cambridge, UK); rabbit anti-TGFβRII (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); guinea pig anti-glutamate-aspartate transporter (1:100; Chemicon International); mouse anti-human leukocyte antigen-ABC (α-HLA-ABC; 1:100; BD Biosciences). After primary antibody incubation, the cells were thoroughly washed with PBS and incubated with secondary antibodies for 2 h at room temperature. Secondary antibodies were Alexa Fluor 546 (goat anti-rabbit IgG, goat anti-mouse IgG; 1:1,000; Molecular Probes) or with Alexa Fluor 488 (goat anti-rabbit IgG, goat anti-guinea pig IgG, goat anti-mouse IgG; 1:300; Molecular Probes). Nuclei were counterstained with DAPI (Sigma). To analyze excitatory synapse formation, only DAPI-labeled neurons away from a distance equivalent of at least two diameters of the cell body were selected. After immunostaining, cells were observed with the aid of a TE2000 Nikon microscope and a Leica confocal microscope. Colocalization of synaptic proteins was analyzed as described under “Puncta Analysis.”
Puncta Analysis
Neurons at least two diameters away from the neighboring neuron were randomly selected and identified using the DAPI stain. After capturing 15–20 images/experimental condition, the green and red channels were aligned, and neurites of the same sizes (10 μm) were selected and quantified using the Puncta Analyzer plug-in in NIH ImageJ as described previously (24). The neurites were chosen randomly, following only one criterion; they had to be located at a distance of one cell body from the neuronal cell soma. In each image, 8–12 neurites were analyzed, totaling at least 1000 μm of neurite length analyzed per experimental condition. In each experiment, at least 30–40 neurons were analyzed per experimental condition. Experiments were done in duplicate, and each result represented the mean of at least three independent neuronal cultures.
Neuronal Morphometry and Quantitative Analysis
Cell density, neuron number, and neurite outgrowth were analyzed. Neuronal cultures were immunostained with the antibody against the neuronal marker β-tubulin III (1:1,000; Promega Corp.) and observed and counted using a TE2000 Nikon microscope and a Leica confocal microscope. Neurite length was measured as the summed distance from one cell body to the end of all of its neurites. At least 10 fields were analyzed per well. In all cases, at least 100 randomly chosen neurons were observed per well. The experiments were done in duplicate, and each result represents the mean of at least three independent experiments.
Statistical Analysis
GraphPad software, version 5.0 (GraphPad Software, La Jolla, CA), was used for statistical analysis of the quantitative data. Because all statistical tests involved multiple conditions, the analysis of variance test was applied in all comparisons, followed by a non-directional Tukey's post-test when statistical significance was achieved. A confidence interval of 95% was used, and a p value of <0.05 was considered statistically significant. Densitometry of blotted gels was performed using the program Un-Scan-It gel version 6.1 (Silk Scientific, Inc., Orem, UT). Data are reported as mean ± S.E., and error bars in the graphs represent S.E.
Electron Microscopy
Cortical neurons were fixed on coverslips for 4 h in 2.5% glutaraldehyde (Sigma) in 0.1 m cacodylate buffer (pH 7.3). After fixation, the cells were postfixed for 20 min in a solution containing 2% OsO4 and 0.8% potassium ferrocyanide in 0.3 m cacodylate buffer, washed in the same buffer, dehydrated, and flat-embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and observed in a Zeiss 900 electron microscope. Images were recorded in three independent experiments, and the number of synapses was counted by a investigator blinded to the origin of the images. The images were morphometrically analyzed using ImageJ software. Only excitatory synapses recognized by high postsynaptic density regions were evaluated.
Immunoblotting Assays
Protein concentration in cell extracts was measured by the BCATM protein assay kit (Cole-Parmer Canada Inc., Montreal, Canada). Forty micrograms of protein/lane was electrophoretically separated on a 12% SDS-polyacrylamide gel. After separation, the proteins were electrically transferred to a Hybond-P polyvinylidene difluoride transfer membrane (Amersham Biosciences) for 1.5 h. Nonspecific sites were blocked by membrane incubation in Tris-buffered saline-Tween 20 (TBS-T; Merck) containing 5% milk for 1 h. Primary antibodies were incubated in block solution overnight, followed by 2-h incubation with peroxidase-conjugated secondary antibodies. Proteins were observed using the enhancing chemiluminescence detection system (Super Signal West Pico Chemiluminescent Substrate, Pierce), and PVDF membranes were exposed to autoradiographic films. Primary antibodies were mouse anti-α-synaptophysin (1:500; Chemicon International), rabbit anti-PSD-95 (1:1,000; Cell Signaling Technology), and rabbit anti-serine racemase (1:1,000; Abcam, Inc.). Secondary peroxidase-conjugated antibodies were goat anti-rabbit IgG and goat anti-mouse IgG (1:3,000; Amersham Biosciences).
Electrophysiology
Whole-cell membrane currents were recorded at a membrane potential of −70 mV with an EPC-7 (List, Darmstadt, Germany) patch clamp system. Currents were low pass-filtered at 3 kHz and digitized with a LabMaster interface under the control of pClamp software (Axon Instruments, Foster City, CA). The standard extracellular solution was 165 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm d-glucose, 5 mm HEPES, and ∼2 mm NaOH, pH 7.3. This solution replaced the culture medium ∼20 min before the recordings and was continuously perfused at a rate of ∼1 ml/min throughout the experiments. Patch micropipettes were made from borosilicate glass capillaries (WPI, Sarasota, FL) in a P-97 horizontal puller (Sutter Instruments, Novato, CA). The intracellular solution was 80 mm CsCl, 80 mm CsF, 10 mm EGTA, 10 mm HEPES, and ∼26 mm CsOH, pH 7.3. Reported membrane potentials are corrected for a −7 mV liquid junction potential measured between solutions. The filled patch microelectrodes had resistances of 2–5 megaohms in the bath; the access resistance (28 ± 12 megaohms) was left uncompensated. Experiments were performed in 6–10 neurons from treated and untreated paired cultures, prepared from the same cell suspension, either at 3 or 12 days in vitro. Membrane capacitance was estimated from the analog compensation of the transient response to a hyperpolarizing test pulse. Approximately 2 min after achieving the whole-cell configuration, voltage-sensitive sodium currents were evoked by depolarizing square pulses to 0 mV (50 ms) from a holding potential of −70 mV. Leak currents were subtracted by a fractional (P/4) method. Immediately after this protocol, recordings of the spontaneous activity were made during 3 min for each neuron. Only cells that responded to the depolarizing pulse with a fast inactivating inward current were considered neurons and were used for the spontaneous activity experiments. Recordings were made at room temperature (∼23 °C). Current traces were analyzed with pClamp software. Synaptic currents larger than 4 times the baseline S.D. (noise) were detected by automatic template matching followed by manual removal of spurious events.
RESULTS
Astrocytes from Rodents and Humans Induce Synapse Formation between Cerebral Cortex Neurons through TGF-β Pathway
It remains unclear if the synaptogenic property of astrocytes also applies to humans. Here, we tested the involvement of TGF-β family members as synaptogenic factors derived from both human and murine astrocytes. Members of the TGF-β family elicit their bioactivity via a heteromeric receptor complex, consisting of TGF-β receptor (TGFβR) types I and II. TGFβRII is required for the transmembrane signaling of TGF-βs, whereas phosphorylation of TGFβRI by TGFβRII is required for activating Smad proteins and propagating the downstream cellular signal. To directly test the involvement of TGF-β family members as synaptogenic factors derived from astrocytes, we analyzed the effect of SB-431542, a TGF-β pathway inhibitor, in astrocyte-induced synaptogenesis. Neuronal cultures were grown in Neurobasal medium for 12 days in vitro; after this period, the neuronal culture was preincubated with vehicle or the TGF-β pathway inhibitor, SB-431542, for 30 min. Then the neuronal cultures were maintained simultaneously in the presence of astrocyte conditioned medium derived from either murine or human astrocytes and the inhibitor for an additional 3 h. Human astrocytes were obtained from normal subcortical white matter of the anterior left temporal lobe (Fig. 1A). Those cells highly express astrocyte markers, such as GFAP (Fig. 1B) and glutamate-aspartate transporter (GLAST) (Fig. 1C), and HLA (Fig. 1D), attesting to their human and astrocytic nature. The normal subcortical white matter was obtained from patients with drug-resistant temporal lobe epilepsy associated with hippocampal sclerosis who underwent surgery (Fig. 1A). As expected, murine conditioned medium increased the number of synapses, by 185%, as revealed by quantification of double-immunolabeled puncta for the pre- and postsynaptic proteins, synaptophysin and PSD-95, respectively (Fig. 2, A, B, and F). The addition of SB-431542 partially prevented this effect, showing that TGF-β signaling is involved in the synaptogenic effect of the murine astrocyte conditioned medium (Fig. 2, A–C and F). Interestingly, human conditioned medium increased by 260% the number of synapses of murine cerebral cortex neurons, whereas the addition of SB-431542 completely prevented this event, suggesting that the synaptogenic effect of human astrocyte conditioned medium also involves TGF-β signaling (Fig. 2, D–F). In order to measure synaptic functionality in the presence of human astrocyte conditioned medium, presynaptic activity was assayed by the fluorochrome FM1-43, which labels active presynaptic vesicles (Fig. 2, G–I). The addition of SB-431542 to non-conditioned medium had no effect on synapse number (data not shown).
FIGURE 1.

Human astrocyte cultures express typical markers. Adult primary astrocytes were isolated from human temporal lobe tissue derived from epileptic patients submitted to surgical procedures. A magnetic resonance image shows the brain region used for astrocyte isolation (A). The arrow represents the healthy subcortical area of the temporal lobe, from where tissue was obtained for astrocyte cultures. The arrowhead shows the atrophied hippocampal tissue affected by epileptic disorder. Human astrocytes were grown in DMEM/F-12 supplemented with 10% FCS, and the distribution of typical astrocytic proteins was analyzed by immunocytochemistry. Human astrocytes express the typical astrocyte marker GFAP (B), glutamate-aspartate transporter in a typical punctate distribution pattern in their membranes (C), and the HLA in a spread distribution pattern over the monolayer (D). Scale bars, 50 μm.
FIGURE 2.
Astrocyte-induced synapse formation is mediated by TGF-β pathway. Cerebral cortex neurons were maintained in the presence of DMEM/F-12 (Control; A and F) or murine (MACM) and human (HACM) astrocyte conditioned medium alone (B and D) or simultaneously with the TGF-β pathway inhibitor, SB-431542 (CM + SB; C and E). The number of synaptophysin/PSD-95 puncta was quantified after 12 days (F). Presynaptic activity for human astrocyte conditioned medium assays was evaluated by FM1-43 incubation followed by puncta quantification (G–I). Scale bars, 20 μm. *, p < 0.001; **, p < 0.0001. Error bars, S.E.
These data not only highlight the evolutionary aspect of the synaptogenic property of astrocytes but point to TGF-β signaling as a conserved synaptogenic glia-induced pathway present in invertebrates and vertebrates, including humans.
TGF-β1 Induces Synapse Formation between Cerebral Cortex Neurons
Among the TGF-β family, TGF-β1 has been strongly implicated in synapse formation in invertebrates (15) and in the vertebrate peripheral nervous system (51). We then investigated the possibility of TGF-β1 as a synaptogenic molecule for glutamatergic vertebrate synapses. The demonstration of high levels of TGF-β1 in astrocyte conditioned medium analyzed by ELISA (Fig. 3A) and the presence of TGFβRII protein in neuronal cultures support this hypothesis (Fig. 3, B–D).
FIGURE 3.

Levels of TGF-β1 secreted by astrocytes and TGFβRII distribution in cerebral cortex neurons. Levels of TGF-β1 present in neuronal (NCM) and astrocyte (ACM) conditioned media were measured by ELISAs (A). Astrocytes secrete higher amounts of TGF-β1 than neurons in vitro. β-Tubulin-III-positive neurons cultured for 12 days express TGFβRII in their membranes (B–D). Scale bar, 20 μm. **, p < 0.0001. Error bars, S.E.
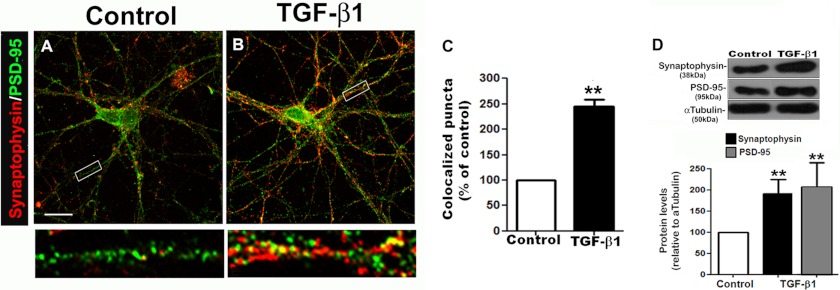
To test whether TGF-β1 alone could reproduce the effects of astrocyte conditioned medium, we cultured cerebral cortex neurons in the presence of TGF-β1 for 12 days and analyzed synapse formation by immunocytochemistry, Western blotting, electron microscopy, and electrophysiological assays. The presence of synapses in cerebral cortex cultures was first morphologically assessed by immunostaining for synaptophysin and PSD-95. After 3 days in culture (data not shown), the untreated neurons contained strikingly few sites of colocalized immunoreactivity, which progressively increased in number during 12 days in culture (Fig. 4, A–C). TGF-β1 enhanced the number of synaptic puncta after 3 (data not shown) and 12 days in culture (Figs. 4 (A–C) and 5 (A–E)). Levels of pre- and postsynaptic proteins were also increased by this treatment (Fig. 4D). Synapses induced by TGF-β1 were ultrastructurally identical to those from control cultures (Fig. 5, A–D and F). Pre- and postsynaptic specializations could be easily detected in both cultures. There was no difference in the average length of the postsynaptic densities (PSD), but their number increased by 150% (Fig. 5, E and F).
FIGURE 4.
TGF-β1 induces synaptogenesis in cerebral cortex neuronal cultures. Cerebral cortex neurons were cultured for 12 days in the presence of Neurobasal medium alone or supplemented with 10 ng/ml of TGF-β1. The number of synaptophysin/PSD-95 puncta was quantified after 12 days (A–C). Levels of synaptic proteins were evaluated by Western blotting assays (D). Scale bar, 20 μm. **, p < 0.0001. Error bars, S.E.
FIGURE 5.
TGF-β1 induces ultrastructurally normal and functional synapses in vitro. Cerebral cortex neurons were cultured for 12 days in the presence of Neurobasal medium alone or supplemented with 10 ng/ml TGF-β1. Synapse formation was evaluated by electron microscopy quantification of the number of synapses (A, C, and E) and length of PSD density (B, D, and F), and electrophysiological assays (G–I). Arrowheads (A and C) and brackets (B and D) indicate postsynaptic density. In both cases, asymmetric synapses (presumably excitatory) structurally normal are observed. TGF-β1 increased the number of synapses, although their ultrastructure seemed to be indistinguishable from control synapses. Scale bars, 0.5 μm (A and C) and 0.2 μm (B and D). **, p < 0.0001. G–I, electrophysiological effects of TGF-β1 treatment. G, representative raw current traces illustrate differences in spontaneous activity recorded in the control (Ctrl) and TGF-β1-treated group. The distributions of the amplitude and interval between events of the two groups were significantly different (p < 0.001; Kolmogorov-Smirnov test; n = 5,177 and 8,580 events, from 19 and 18 neurons, respectively) (H). Averaged voltage-activated Na+ current traces obtained from five randomly chosen neurons in each condition illustrate the effect of TGF-β1 (p < 0.001 versus control) (I). Error bars, S.E.
To ascertain if the increase in colocalized puncta reflected the formation of functional synapses, cortical neurons with 3 and 12 days in culture were treated with TGF-β1 from day 1 and used in whole-cell patch clamp experiments. Spontaneous activity, Na+ currents, and membrane capacitance were recorded. Spontaneous postsynaptic currents in 12-day treated neurons (n = 19) showed significantly higher frequencies (2.5 ± 0.3 versus 1.6 ± 0.3 s−1 in control; p < 0.05, Student's t test) and amplitudes (130 ± 14 versus 75 ± 5 pA; p < 0.001, Student's t test) than control neurons (n = 18). The distributions of interevent intervals and amplitudes were also significantly different (Fig. 5, G and H). Treated and untreated cortical neurons cultured for 3 days showed either very few or (more often) no spontaneous currents. The mean voltage-activated Na+ currents in neurons treated with TGF-β1 (3,395 ± 233 pA, n = 7) were significantly greater than those of untreated neurons (1,374 ± 255 pA, n = 8; p < 0.001, two-way analysis of variance) in 12-day cultures (Fig. 5I) but not in 3-day cultures (data not shown). TGF-β1 also increased neuronal membrane capacitance, reflecting an increased membrane area. Measured capacitances were 15 ± 2.0 picofarads for untreated (n = 8) and 25 ± 2.7 picofarads for 12-day treated neurons (n = 7; p < 0.001, two-way analysis of variance). Again, no differences were observed in 3-day cultures. Together, these data demonstrate that cortical neurons are targets for TGF-β1, which induces formation of morphologically organized and functional synapses in vitro.
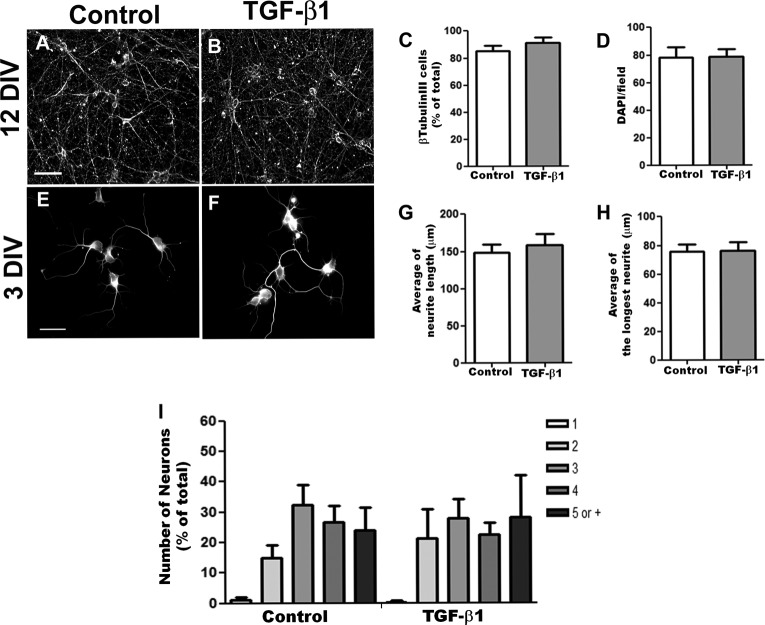
Several mechanisms are proposed to underlie the astrocyte effect on synapse number and efficacy, including increased dendritic maturation and neuronal arborization, enhanced neuronal survival, modulation of synapse elimination or stabilization, and secretion of gliotransmitters or neuromodulators (38–40). To determine whether the effect of TGF-β1 in synaptogenesis was due to an increase in dendritic arborization or neuronal density, the number of neurons and their arborization were evaluated. TGF-β1 did not induce significant changes in the number of total or β-tubulin-immunoreactive cells or in dendritic morphology, indicating that TGF-β1 increases synaptogenesis independently of dendrite outgrowth or neuronal survival (Fig. 6).
FIGURE 6.
TGF-β1 does not affect neuronal survival and neurite arborization. Cerebral cortex neurons were cultured for 12 and 3 days in control medium (Control; A and E) or in the presence of 10 ng/ml TGF-β1 (TGF-β1; B and F). Numbers of β-tubulin III cells (C), total cells (D), total neurite length (G and H) or neurites (I) were obtained using the ImageJ software. There was no statistical difference between the groups. DIV, days in vitro. Scale bars, 50 μm (A) and 30 μm (E). Error bars, S.E.
d-Serine Also Induces Synapse Formation between Cerebral Cortex Neurons
What mechanism underlies the synaptogenesis induced by TGF-β1? It is known that synaptic transmission mediated by the NMDA type of glutamate receptor participates in structural plasticity of synaptic networks (41, 42). To investigate if TGF-β1 synaptogenic property was dependent on NMDA receptor activity, we blocked the receptors with MK-801. Blockade of NMDA receptors completely abolished the effect of TGF-β1 on synaptogenesis (Fig. 7A).
FIGURE 7.
TGF-β1 synaptogenic property is dependent on NMDA receptor activity and involves an increase in the extracellular levels of the amino acid, d-serine. Cerebral cortex neurons were cultured in the presence of Neurobasal medium alone, with 10 ng/ml TGF-β1, and simultaneously with TGF-β1 and the NMDA receptor inhibitor MK-801 (TGF-β1 + MK). After 3 days, the number of synaptophysin/PSD-95 puncta was quantified (A). TGF-β1 increases the extracellular levels of d-serine in neuronal and astrocyte cultures. Extracellular levels of the neurotransmitter glutamate and the amino acid d-serine (B) were quantified by HPLC chromatography assays of neuronal and astrocyte conditioned medium. TGF-β1 raised the level of extracellular d-serine in either neuronal and astrocyte cultures. *, p < 0.001. Error bars, S.E.
d-Serine is the main endogenous ligand of the co-agonist site at the NMDA receptors and, together with glutamate, is required for NMDA receptor function in synaptic transmission and plasticity (43–46). We investigated whether d-serine mediates the effect of TGF-β1 on synaptogenesis.
TGF-β1 markedly increased the levels of extracellular d-serine from both neuronal and astrocyte cultures (Fig. 7B). Levels of the neurotransmitter glutamate were not altered by TGF-β1 in either cell type (Fig. 7B). d-Serine formation is mediated by the enzyme serine racemase, which converts l-serine to d-serine. TGF-β1 did not modulate serine racemase levels or distribution in either neurons or astrocytes (Fig. 8).
FIGURE 8.
TGF-β1 does not affect serine racemase distribution and levels. Cerebral cortex astrocytes and neurons were cultured in control medium (A–C and H–J) or in the presence of 10 ng/ml TGF-β1 (D–F and K–M). Serine racemase (Ser Rac) distribution and labeling intensity were evaluated by immunocytochemistry assays (B, E, I, and L), and protein levels were analyzed by Western blotting assays (G and N). There were no statistical differences in distribution, labeling intensity, and protein levels of serine racemase in astrocytes and neurons treated with TGF-β1 compared with control conditions. Scale bar, 30 μm. Error bars, S.E.
In order to address the effects of d-serine on synaptogenesis, cerebral cortex neurons were treated with d-serine, followed by synapse evaluation, as described previously. As shown for TGF-β1, d-serine increased the PSD/synaptophysin puncta by 218% (Fig. 9, A–C) and nearly doubled the levels of these pre- and postsynaptic proteins (Fig. 9D). d-Serine-induced synaptogenesis was mediated by the co-activation of NMDA receptors because the specific antagonist of the co-agonist site 5,7-dichlorokynurenic acid (DCK) and the NMDA receptor activity inhibitor, MK-801, completely blocked the effect (Fig. 9C). Cell viability assays revealed no drug toxicity at the concentrations used (data not shown). MK-801 and DCK alone had no effect on synapse formation (data not shown).
FIGURE 9.
d-Serine induces ultrastructurally organized and functional synapses. Cerebral cortex neurons were cultured in the presence of Neurobasal medium alone, with 0.4 mm d-serine alone, and simultaneously with d-serine and NMDA receptor inhibitors MK-801 and DCK (D-ser + MK and D-ser + DCK). After 12 days, the number of synaptophysin/PSD-95 puncta was quantified (A–C), and levels of synaptic proteins were evaluated by Western blotting assays (D). Synapse formation was also evaluated by quantitative electron microscopy (E–J) and electrophysiological assays (K–M). Number of synapses (I) and length of PSD density (J) were evaluated by electron microscopy. The arrowheads (E and G) and brackets (F and H) indicate postsynaptic density. In both cases, structurally normal synapses are observed. d-serine increased the number of synapses, although their ultrastructure seemed to be indistinguishable from control synapses. Scale bars, 20 μm (A and B), 0.5 μm (E and G), and 0.2 μm (F and H); *, p < 0.001; **, p < 0.0001. K–M, electrophysiological effects of d-serine treatment. Representative raw current traces illustrate differences in spontaneous activity recorded in the control (Ctrl) and d-serine-treated group (K). The distributions of the amplitude and interval between events of the two groups were significantly different (p < 0.001; Kolmogorov-Smirnov test; n = 5,644 and 6,127 events, from 25 and 21 neurons, respectively) (L). Averaged voltage-activated Na+ current traces obtained from five randomly chosen neurons in each condition illustrate the effect of TGF-β1 (p < 0.001 versus control) (M). Error bars, S.E.
Synapses induced by d-serine were ultrastructurally identical to those from the control- and TGF-β1-treated cultures (Fig. 9, E–H and J). There was no difference in PSD length, although the synapse number increased by 133% (Fig. 9, I and J). As shown for TGF-β1 and other synaptogenic molecules, such as thrombospondin (24), d-serine also did not induce significant changes in dendritic morphology or neuronal survival (Fig. 10). As seen with TGF-β1, treatment with d-serine for 12 days also significantly changed the distributions in both amplitude and interevent intervals of spontaneous synaptic currents (Fig. 9, K and L). However, this treatment did not significantly change voltage-activated currents or membrane capacitance (Fig. 9M).
FIGURE 10.
d-Serine does not affect neuronal survival and neurite arborization. Cerebral cortex neurons were cultured for 12 and 3 days in control medium (Control; A and E) or in the presence of 0.4 mm d-serine (D-ser; B and F). Numbers of β-tubulin III cells (C), total cells (D), total neurite length (G and H), or neurites (I) were obtained using the ImageJ software. There was no statistical difference between the groups. Scale bars, 50 μm (A) and 30 μm (E). DIV, days in vitro. Error bars, S.E.
TGF-β1-induced Cerebral Cortex Synapse Depends on d-Serine
To determine whether the increase in synaptogenesis induced by TGF-β1 resulted from increased d-serine release, the effects of TGF-β1 on synaptogenesis were evaluated after inducing degradation or impairing formation of d-serine. We reduced d-serine content by adding exogenous DAO, an enzyme that specifically degrades d-amino acids, and by down-expressing serine racemase by an shRNA approach. As shown previously, treatment of cortical neurons with TGF-β1 increased PSD/synaptophysin puncta by 90%, whereas pretreatment with DAO (20 μg/ml) completely prevented this effect (Fig. 11, A–F). Treatment with heat-inactivated DAO had no effect on the TGF-β synaptogenic property, attesting to the specificity of the enzyme (Fig. 11, D and F). Further, DAO or heat-inactivated DAO alone, in the absence of TGF-β, had no effect on synapse number (Fig. 11F).
FIGURE 11.
Synaptogenesis induced by TGF-β1 is mediated by d-serine. Cerebral cortex neurons were cultured for 3 days in the presence of control medium (Control; A and F), 10 ng/ml TGF-β1 (TGF-β1; B and F), or simultaneously with TGF-β1 and the d-serine degradation enzyme, DAO (TGF-β1 + DAO; C and F). To address DAO specificity, neurons were also simultaneously treated with TGF-β1 and heat-inactivated DAO (TGF-β1 + hiDAO; D and F). Neurons were also transfected with plasmidial vectors containing two different shRNA constructs against serine racemase enzyme mRNA (shSR1 and shSR2) or with control constructs (LacZ) (H). Rescue assays were addressed by adding 0.4 mm d-serine to either DAO- or shSR-treated TGF-β neuronal cultures (F and H). Neurons were also cultured in the presence of DAO, heat-inactivated DAO, and shSR1 and shSR2 alone (F and H). DAO synapses were evaluated by immunolabeling for synaptophysin and PSD-95 followed by quantification (A–F and H). Serine racemase knockdown levels were evaluated by Western blotting assays (G). Scale bar, 5 μm. *, p < 0.001; ***, p < 0.0001; ns, not significant. Error bars, S.E.
Loss of function with serine racemase shRNA completely abolished the TGF-β1 synaptogenic effect (Fig. 11H). Knockdown of serine racemase by shRNA decreased the levels of serine racemase by ∼50% (Fig. 11G). Measurement of d-serine levels by HPLC revealed that either DAO or shRNA treatment decreased the levels of extracellular d-serine (decrease of 18.8 and 41.4%, respectively). Further, the addition of d-serine completely rescued the DAO (Fig. 11, E and F) and serine racemase knockdown (Fig. 11H) effect in TGF-β synapse induction, thus supporting the hypothesis that this effect depends on d-serine synthesis. Interestingly, neurons transfected with serine racemase shRNA had a tendency to exhibit lower synaptogenic levels compared with LacZ control (although not statistically relevant), uncovering the basal role of d-serine in synapse formation (Fig. 11H). Together, these results demonstrate that the effect of TGF-β1 in synaptogenesis is dependent on d-serine levels.
DISCUSSION
Identification of signaling pathways involved in synapse formation and function provides basic knowledge that may help in manipulating neural circuitry during development and adulthood. In the present study, we described a novel non-cell-autonomous mechanism by which a growth factor controls synapse formation in vertebrates; by increasing the extracellular levels of the NMDA receptor co-agonist, d-serine, TGF-β1 raises the number of functional cerebral cortex neuronal synapses. Further, the data presented here address an attractive and unknown issue, the evolutionary conservation of astrocyte and TGF-β1 synaptogenic properties, thus providing new insights into the role of glial cells in neurodevelopmental disorders.
Members of the TGF-β superfamily have been implicated in synapse formation, especially in invertebrates (14–18). Recently, in vivo and in vitro assays using animals deficient for members of the TGF-β family revealed impaired synaptic development and cognition deficit (12, 19–22, 47, 48).
TGF-βs and their receptors are widely expressed in the embryonic and adult brain (33, 49). TGF-β1 expression was identified in proliferative zones and distributed over the entire rodent cortical plate from embryonic day 16 to postnatal day 30, including during the synaptogenic period. Both astrocytes and neurons are sources of TGF-β1 and express their receptors (2, 3, 50). This molecular arrangement supports the possibility that TGF-β1 is a local regulator of synapse formation in the cerebral cortex. Our data corroborate the recent identification of TGF-β1 as a Schwann cell-derived synaptogenic factor in the Xenopus and rat peripheral nervous systems (51) and suggest that TGF-β members might also represent glia-derived synaptogenic factors for the vertebrate CNS in addition to others already described (24, 52–55).
Here we found that synapses induced by TGF-β1 are both pre- and postsynaptically active, as revealed by the increased incidence and mean amplitude of spontaneous synaptic currents. This is a significant observation because several astrocyte-derived synaptogenic molecules identified so far induce presynaptic differentiation but postsynaptically inactive synapse (24, 52).
We found that TGF-β1 increased voltage-activated sodium currents, which may reflect a general effect of TGF-β1 on neuronal maturation, akin to the transient up-regulation of potassium channels in cerebellar granule neurons (56). These data, together with the fact that TGF-β1 is not synaptogenic for retinal ganglion cells (24) despite being synaptogenic for neuromuscular junction (51) and cerebral cortical neurons (shown here), support the concept that the TGF-β1 effect is cell-specific.
Interestingly, inhibition of TGF-β1 receptor activity only partly impaired the synaptogenic effect of astrocyte conditioned medium on cortical neurons. The remaining activity is likely to be due to other astrocyte-derived factors already described, including thrombospondin, cholesterol, hervin, and d-serine, the latter described here (24, 53–55, 57–60). However, like d-serine and unlike most of these molecules, TGF-β1 induces ultrastructurally normal and post-synaptically active synapses. The present study may suggest that TGF-β1 itself is an instructive molecule for excitatory synapse formation although not the only molecule secreted by astrocytes.
Our data are in apparent contradiction with those generated from forebrain-specific Smad4 knock-out mice, which show an enhancement of paired pulse facilitation in excitatory synaptic transmission and stronger paired pulse depression of GABAA currents in the hippocampus (12). It is also possible that TGF-β1-triggered Smad4 signaling promotes synaptogenesis in developing neurons but later down-modulates the short term plasticity of mature synapses, which we did not examine. Furthermore, astrocytes have been reported to enhance both excitatory and inhibitory synapses, suggesting that through activation of different signaling pathways, astrocytes balance the number of excitatory and inhibitory inputs (54, 61). In fact, we cannot completely rule out an effect of TGF-β1 on inhibitory synapses between the cortical neurons in our study.
Several mechanisms are proposed to mediate synapse formation, including enhancement in dendritic arborization, neuronal survival, modulation of stabilization, and elimination of dendritic spines (38–40). In contrast to our observations on cortical neurons, TGF-β1 has been shown to induce neurite outgrowth and dendritic spine formation in hippocampal neurons in vitro (62). However, as we observed, thrombospondin also does not affect glutamatergic axon length or branching, either in hippocampal neurons or in retinal ganglion cells (24, 58, 63). In contrast, glia-derived cholesterol induces synapse from retinal ganglion cells through increasing neurite outgrowth and dendritic differentiation (30). Additionally, astrocytes have been shown to increase inhibitory synapses by enhancing GABAergic axon length and branching (58), raising the possibility that astrocytes might promote inhibitory and excitatory synaptogenesis through different pathways and molecules.
Our results suggest that the TGF-β1-increased number of synapses is not attributable to an increase in the number or density of neurons or the result of nonspecific trophic effects on cell death, although TGF-β members are potent neuronal survival factors. A possibility is that trophic stimulation in our culture system might have elicited the maximum effect for neuronal survival and neurite outgrowth. A similar explanation has been proposed for the TGF-β1 effect at the neuron-muscular junction (51).
Although our data have shown that most TGF-β1 activity in excitatory synapse formation is mediated by d-serine, we cannot rule out the possibility that TGF-β1 might itself have a residual synaptogenic activity. TGF-β1 binds to several extracellular matrix proteins and their receptors, leading to the possibility that binding to adhesion molecules facilitates the recruitment of pre- and postsynaptic molecules at new contact sites. In fact, additional pathways have been proposed to explain TGF-β1 modulation of synapse efficacy, such as induction of synapsin phosphorylation and redistribution in invertebrates (15) or binding to the nerve-derived heparin sulfate proteoglycan, agrin (51). Further, TGF-β1 has been reported to increase the levels of thrombospondin in astrocytes (64). The fact that d-serine increased synaptic currents similarly to TGF-β1 but failed to increase inward voltage-gated currents also suggests that not all effects of TGF-β1 in the cortical neurons were mediated by d-serine. Although this might occur, the loss of function and pharmacological inhibition assays for serine racemase and d-serine, respectively, suggest that most TGF-β1 synaptogenic activity depends on d-serine. Increased d-serine levels induced by TGF-β1 were not explained by changes in total serine racemase protein levels in both neurons and astrocytes. On the other hand, several regulatory mechanisms and protein interactions have been proposed to play a role in regulating serine racemase activity and d-serine availability. For example, the scaffolding molecule PICK1 (protein interacting with protein kinase C1) has been shown to bind to serine racemase (65), leading to an increase in d-serine formation (66) Interactions between PICK1 and PKC have been shown to reduce serine racemase activity by phosphorylation of serine amino acid residues (67). Recent findings revealed PICK1 as an important regulator of TGF-β signaling (68). It remains to be investigated whether the increase in d-serine levels yielded by the TGF-β1 pathway is related to modulation of serine racemase regulator function.
d-Serine is the dominant endogenous co-agonist of NMDA receptors in several brain regions, and its production and secretion are involved in synaptic plasticity in the hippocampus, prefrontal cortex, and hypothalamus (69). Here we identified a previously unknown activity of d-serine as a synaptogenic molecule. Inhibition of the NMDA receptor activity by the uncompetitive antagonist MK-801 suggests that the synaptogenic effect of d-serine relies on its co-activation of postsynaptic currents. Interestingly, d-serine levels in the rodent brain peak around postnatal day 7, a period of intense synaptogenesis. Consistent with a role of d-serine in synapse formation, recent evidence demonstrated that serine racemase knock-out mice show memory deficit and impairment in cortical dendrite morphology (70).
Indirect evidence is provided by the observation that TGF-β1 knock-out mice with the NIH genetic background (which rescues TGF-β1 mice from embryonic lethality) show reduced synaptic density, although this phenomenon might be attributed to the extensive neuronal death found in these animals (7). Further in vivo support for a correlation between TGF-β1 and glutamatergic responses is provided by the recent demonstration of increased levels of NR1 and NR2A/B receptor subunits in the hippocampus of TGF-β1-overexpressing mice (62) and association between deficits in TGF-β pathways and neuropsychiatric disorders (10, 12, 13).
It remains unclear whether the glia-synaptogenic property, initially described for rodents, is a general principle that applies to other mammals, including primates. Previously, murine astrocytes have been shown to induce neuronal maturation and synapse formation from human embryonic stem cells (71). It is not clear, however, if this is an autonomous characteristic of human neurons because synaptogenic properties of human astrocytes have not been shown so far. This hypothesis was recently strengthened by the observation that astrocytes derived from Down syndrome fetuses are directly involved in the development of spine malformations and reduced synaptic density (72). However, this is indirect evidence, and direct proof of the involvement of healthy human astrocytes in synapse development is still lacking. We showed here that human astrocytes increase the number of functional synapses between murine cerebral cortex neurons revealed by puncta analysis and FM1-43-labeled vesicular secretion.
It is of note that the pharmacological inhibitor of TGFβR partially impaired the synaptogenic activity of murine astrocytes but fully prevented that of human astrocytes. This suggests that the TGF-β pathway might represent a more important role for human astrocyte-induced synapses than for mice, thus implying that the synaptogenic property of glial cells may be a conserved aspect of synaptic biology.
Our data suggest that the synaptogenic effect of TGF-β1 involves the activation of a cascade, which includes an increase in the release of d-serine and the activation of the co-agonist site of NMDA-glutamate receptors. Exogenous administration of d-serine enhances mechanisms related to synaptic plasticity, both in vitro and in vivo (37, 67, 73). Based on our present findings, we speculate that increased synaptogenesis can participate in the cognitive improvement induced by d-serine administration. Our work raises the question of whether manipulation of TGF pathways and/or d-serine levels might represent a potential tool to restore synapses in the normal brain or enhance the regeneration of new synapses in aged, degenerating, and acutely injured CNS.
Acknowledgments
We thank Marcelo Meloni and Adiel Batista do Nascimento for technical assistance and Drs. Wanderley de Sousa and Márcia Attias for electronic microscopy facilities. We also thank Dr. Janet W. Reid (JWR Associates, Biological Consulting and Editing Services) for revising the English text.
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Institute of Glia (iGLIA/CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
- GFAP
- glial fibrillary acidic protein
- DCK
- 5,7- dichlorokynurenic acid
- DAO
- d-amino acid oxidase enzyme
- TGFβRI
- TGFβ1 receptor type I
- TGFβRII
- TGFβ1 receptor type II
- PSD
- postsynaptic densities.
REFERENCES
- 1. McAllister A. K. (2007) Dynamic aspects of CNS synapse formation. Annu. Rev. Neurosci. 30, 425–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Sampaio e Spohr T. C., Martinez R., da Silva E. F., Neto V. M., Gomes F. C. (2002) Neuro-glia interaction effects on GFAP gene. A novel role for transforming growth factor-β1. Eur. J. Neurosci. 16, 2059–2069 [DOI] [PubMed] [Google Scholar]
- 3. Sousa Vde O., Romão L., Neto V. M., Gomes F. C. (2004) Glial fibrillary acidic protein gene promoter is differently modulated by transforming growth factor-β1 in astrocytes from distinct brain regions. Eur. J. Neurosci. 19, 1721–1730 [DOI] [PubMed] [Google Scholar]
- 4. Gomes F. C., Sousa Vde O., Romão L. (2005) Emerging roles for TGF-beta1 in nervous system development. Int. J. Dev. Neurosci. 23, 413–424 [DOI] [PubMed] [Google Scholar]
- 5. Stipursky J., Gomes F. C. (2007) TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro. Implications for radial glia development. Glia 55, 1023–1033 [DOI] [PubMed] [Google Scholar]
- 6. Romão L. F., Sousa Vde O., Neto V. M., Gomes F. C. (2008) Glutamate activates GFAP gene promoter from cultured astrocytes through TGF-β1 pathways. J. Neurochem. 106, 746–756 [DOI] [PubMed] [Google Scholar]
- 7. Brionne T. C., Tesseur I., Masliah E., Wyss-Coray T. (2003) Loss of TGF-β1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40, 1133–1145 [DOI] [PubMed] [Google Scholar]
- 8. Shi Y., Massagué J. (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113, 685–700 [DOI] [PubMed] [Google Scholar]
- 9. Garcia C. M., Darland D. C., Massingham L. J., D'Amore P. A. (2004) Endothelial cell-astrocyte interactions and TGF β are required for induction of blood-neural barrier properties. Brain Res. Dev. Brain Res. 152, 25–38 [DOI] [PubMed] [Google Scholar]
- 10. Ashwood P., Enstrom A., Krakowiak P., Hertz-Picciotto I., Hansen R. L., Croen L. A., Ozonoff S., Pessah I. N., Van de Water J. (2008) Decreased transforming growth factor beta1 in autism. A potential link between immune dysregulation and impairment in clinical behavioral outcomes. J. Neuroimmunol. 204, 149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graciarena M., Depino A. M., Pitossi F. J. (2010) Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFβ1 down-regulation. Brain. Behav. Immun. 24, 1301–1309 [DOI] [PubMed] [Google Scholar]
- 12. Sun M., Gewirtz J. C., Bofenkamp L., Wickham R. J., Ge H., O'Connor M. B. (2010) Canonical TGF-β signaling is required for the balance of excitatory/inhibitory transmission within the hippocampus and prepulse inhibition of acoustic startle. J. Neurosci. 30, 6025–6035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krieglstein K., Zheng F., Unsicker K., Alzheimer C. (2011) More than being protective. Functional roles for TGF-β/activin signaling pathways at central synapses. Trends Neurosci. 34, 421–429 [DOI] [PubMed] [Google Scholar]
- 14. Aberle H., Haghighi A. P., Fetter R. D., McCabe B. D., Magalhães T. R., Goodman C. S. (2002) Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33, 545–558 [DOI] [PubMed] [Google Scholar]
- 15. Chin J., Angers A., Cleary L. J., Eskin A., Byrne J. H. (2002) Transforming growth factor β1 alters synapsin distribution and modulates synaptic depression in Aplysia. J. Neurosci. 22, RC220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Packard M., Mathew D., Budnik V. (2003) Wnts and TGF β in synaptogenesis. Old friends signalling at new places. Nat. Rev. Neurosci. 4, 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCabe B. D., Hom S., Aberle H., Fetter R. D., Marques G., Haerry T. E., Wan H., O'Connor M. B., Goodman C. S., Haghighi A. P. (2004) Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron 41, 891–905 [DOI] [PubMed] [Google Scholar]
- 18. Sanyal S., Kim S. M., Ramaswami M. (2004) Retrograde regulation in the CNS. Neuron-specific interpretations of TGF-β signaling. Neuron 41, 845–848 [DOI] [PubMed] [Google Scholar]
- 19. Fukushima T., Liu R. Y., Byrne J. H. (2007) Transforming growth factor-β2 modulates synaptic efficacy and plasticity and induces phosphorylation of CREB in hippocampal neurons. Hippocampus 17, 5–9 [DOI] [PubMed] [Google Scholar]
- 20. Sun M., Thomas M. J., Herder R., Bofenkamp M. L., Selleck S. B., O'Connor M. B. (2007) Presynaptic contributions of chordin to hippocampal plasticity and spatial learning. J. Neurosci. 27, 7740–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heupel K., Sargsyan V., Plomp J. J., Rickmann M., Varoqueaux F., Zhang W., Krieglstein K. (2008) Loss of transforming growth factor-β2 leads to impairment of central synapse function. Neural Dev. 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fong S. W., McLennan I. S., McIntyre A., Reid J., Shennan K. I., Bewick G. S. (2010) TGF-β2 alters the characteristics of the neuromuscular junction by regulating presynaptic quantal size. Proc. Natl. Acad. Sci. U.S.A. 107, 13515–13519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfrieger F. W., Barres B. A. (1996) New views on synapse-glia interactions. Curr. Opin. Neurobiol. 6, 615–621 [DOI] [PubMed] [Google Scholar]
- 24. Christopherson K. S., Ullian E. M., Stokes C. C., Mullowney C. E., Hell J. W., Agah A., Lawler J., Mosher D. F., Bornstein P., Barres B. A. (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 [DOI] [PubMed] [Google Scholar]
- 25. Eroglu C., Barres B. A. (2010) Regulation of synaptic connectivity by glia. Nature 468, 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Araque A., Parpura V., Sanzgiri R. P., Haydon P. G. (1999) Tripartite synapses. Glia, the unacknowledged partner. Trends Neurosci. 22, 208–215 [DOI] [PubMed] [Google Scholar]
- 27. Stipursky J., Romão L., Tortelli V., Neto V. M., Gomes F. C. (2011) Neuron-glia signaling. Implications for astrocyte differentiation and synapse formation. Life Sci. 89, 524–531 [DOI] [PubMed] [Google Scholar]
- 28. Pyka M., Wetzel C., Aguado A., Geissler M., Hatt H., Faissner A. (2011) Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur. J. Neurosci. 33, 2187–2202 [DOI] [PubMed] [Google Scholar]
- 29. Halassa M. M., Fellin T., Takano H., Dong J. H., Haydon P. G. (2007) Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 27, 6473–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goritz C., Mauch D. H., Pfrieger F. W. (2005) Multiple mechanisms mediate cholesterol-induced synaptogenesis in a CNS neuron. Mol. Cell Neurosci. 29, 190–201 [DOI] [PubMed] [Google Scholar]
- 31. Chung W. S., Barres B. A. (2011) The role of glial cells in synapse elimination. Curr. Opin. Neurobiol. 3, 438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santello M., Bezzi P., Volterra A. (2011) TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69, 988–1001 [DOI] [PubMed] [Google Scholar]
- 33. Mecha M., Rabadán M. A., Peña-Melián A., Valencia M., Mondéjar T., Blanco M. J. (2008) Expression of TGF-βs in the embryonic nervous system. analysis of interbalance between isoforms. Dev. Dyn. 237, 1709–1717 [DOI] [PubMed] [Google Scholar]
- 34. Gomes F. C., Garcia-Abreu J., Galou M., Paulin D., Moura Neto V. (1999) Neurons induce GFAP gene promoter of cultured astrocytes from transgenic mice. Glia 26, 97–108 [DOI] [PubMed] [Google Scholar]
- 35. Sebollela A., Freitas-Correa L., Oliveira F. F., Paula-Lima A. C., Saraiva L. M., Martins S. M., Mota L. D., Torres C., Alves-Leon S., de Souza J. M., Carraro D. M., Brentani H., De Felice F. G., Ferreira S. T. (2012) Amyloid-β oligomers induce differential gene expression in adult human brain slices. J. Biol. Chem. 287, 7436–7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. De Groot C. J., Langeveld C. H., Jongenelen C. A., Montagne L., Van Der Valk P., Dijkstra C. D. (1997) Establishment of human adult astrocyte cultures derived from postmortem multiple sclerosis and control brain and spinal cord regions. Immunophenotypical and functional characterization. J. Neurosci. Res. 49, 342–354 [DOI] [PubMed] [Google Scholar]
- 37. Panizzutti R., De Miranda J., Ribeiro C. S., Engelender S., Wolosker H. (2001) A new strategy to decrease N-methyl-d-aspartate (NMDA) receptor coactivation. Inhibition of d-serine synthesis by converting serine racemase into an eliminase. Proc. Natl. Acad. Sci. U.S.A. 98, 5294–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fletcher T. L., De Camilli P., Banker G. (1994) Synaptogenesis in hippocampal cultures. Evidence indicating that axons and dendrites become competent to form synapses at different stages of neuronal development. J. Neurosci. 14, 6695–6706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pfrieger F. W. (2010) Role of glial cells in the formation and maintenance of synapses. Brain Res. Rev. 63, 39–46 [DOI] [PubMed] [Google Scholar]
- 40. Barker A. J., Ullian E. M. (2010) Astrocytes and synaptic plasticity. Neuroscientist 16, 40–50 [DOI] [PubMed] [Google Scholar]
- 41. Rajan I., Witte S., Cline H. T. (1999) NMDA receptor activity stabilizes presynaptic retinotectal axons and postsynaptic optic tectal cell dendrites in vivo. J. Neurobiol. 38, 357–368 [DOI] [PubMed] [Google Scholar]
- 42. Sin W. C., Haas K., Ruthazer E. S., Cline H. T. (2002) Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419, 475–480 [DOI] [PubMed] [Google Scholar]
- 43. Henneberger C., Papouin T., Oliet S. H., Rusakov D. A. (2010) Long-term potentiation depends on release of d-serine from astrocytes. Nature 463, 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Panatier A., Theodosis D. T., Mothet J. P., Touquet B., Pollegioni L., Poulain D. A., Oliet S. H. (2006) Glia-derived d-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784 [DOI] [PubMed] [Google Scholar]
- 45. Yang Y., Ge W., Chen Y., Zhang Z., Shen W., Wu C., Poo M., Duan S. (2003) Contribution of astrocytes to hippocampal long-term potentiation through release of d-serine. Proc. Natl. Acad. Sci. U.S.A. 100, 15194–15199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fossat P., Turpin F. R., Sacchi S., Dulong J., Shi T., Rivet J. M., Sweedler J. V., Pollegioni L., Millan M. J., Oliet S. H., Mothet J. P. (2012) Glial d-serine gates NMDA receptors at excitatory synapses in prefrontal cortex. Cereb. Cortex 22, 595–606 [DOI] [PubMed] [Google Scholar]
- 47. Lacmann A., Hess D., Gohla G., Roussa E., Krieglstein K. (2007) Activity-dependent release of transforming growth factor-β in a neuronal network in vitro. Neuroscience 150, 647–657 [DOI] [PubMed] [Google Scholar]
- 48. Vashlishan A. B., Madison J. M., Dybbs M., Bai J., Sieburth D., Ch'ng Q., Tavazoie M., Kaplan J. M. (2008) An RNAi screen identifies genes that regulate GABA synapses. Neuron 58, 346–361 [DOI] [PubMed] [Google Scholar]
- 49. Vincze C., Pál G., Wappler E. A., Szabó E. R., Nagy Z. G., Lovas G., Dobolyi A. (2010) Distribution of mRNAs encoding transforming growth factors-β1, -2, and -3 in the intact rat brain and after experimentally induced focal ischemia. J. Comp. Neurol. 518, 3752–3770 [DOI] [PubMed] [Google Scholar]
- 50. Tomoda T., Shirasawa T., Yahagi Y. I., Ishii K., Takagi H., Furiya Y., Arai K. I., Mori H., Muramatsu M. A. (1996) Transforming growth factor-β is a survival factor for neonate cortical neurons. Coincident expression of type I receptors in developing cerebral cortices. Dev. Biol. 179, 79–90 [DOI] [PubMed] [Google Scholar]
- 51. Feng Z., Ko C. P. (2008) Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-β1. J. Neurosci. 28, 9599–9609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kucukdereli H., Allen N. J., Lee A. T., Feng A., Ozlu M. I., Conatser L. M., Chakraborty C., Workman G., Weaver M., Sage E. H., Barres B. A., Eroglu C. (2011) Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. U.S.A. 108, E440–E449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beattie E. C., Stellwagen D., Morishita W., Bresnahan J. C., Ha B. K., Von Zastrow M., Beattie M. S., Malenka R. C. (2002) Control of synaptic strength by glial TNFα. Science 295, 2282–2285 [DOI] [PubMed] [Google Scholar]
- 54. Garrett A. M., Weiner J. A. (2009) Control of CNS synapse development by γ-protocadherin-mediated astrocyte-neuron contact. J. Neurosci. 29, 11723–11731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mauch D. H., Nägler K., Schumacher S., Göritz C., Müller E. C., Otto A., Pfrieger F. W. (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science 294, 1354–1357 [DOI] [PubMed] [Google Scholar]
- 56. Zhuang P., Li Y., Hallett M. (2004) Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin. Neurophysiol. 115, 2542–2557 [DOI] [PubMed] [Google Scholar]
- 57. Fester L., Zhou L., Bütow A., Huber C., von Lossow R., Prange-Kiel J., Jarry H., Rune G. M. (2009) Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus 19, 692–705 [DOI] [PubMed] [Google Scholar]
- 58. Hughes E. G., Elmariah S. B., Balice-Gordon R. J. (2010) Astrocyte secreted proteins selectively increase hippocampal GABAergic axon length, branching, and synaptogenesis. Mol. Cell Neurosci. 43, 136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hama H., Hara C., Yamaguchi K., Miyawaki A. (2004) PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron 41, 405–415 [DOI] [PubMed] [Google Scholar]
- 60. Eroglu C., Allen N. J., Susman M. W., O'Rourke N. A., Park C. Y., Ozkan E., Chakraborty C., Mulinyawe S. B., Annis D. S., Huberman A. D., Green E. M., Lawler J., Dolmetsch R., Garcia K. C., Smith S. J., Luo Z. D., Rosenthal A., Mosher D. F., Barres B. A. (2009) Gabapentin receptor α2δ-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Elmariah S. B., Oh E. J., Hughes E. G., Balice-Gordon R. J. (2005) Astrocytes regulate inhibitory synapse formation via Trk-mediated modulation of postsynaptic GABAA receptors. J. Neurosci. 25, 3638–3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bae J. J., Xiang Y. Y., Martinez-Canabal A., Frankland P. W., Yang B. B., Lu W. Y. (2011) Increased transforming growth factor-beta1 modulates glutamate receptor expression in the hippocampus. Int. J. Physiol. Pathophysiol. Pharmacol. 3, 9–20 [PMC free article] [PubMed] [Google Scholar]
- 63. Ullian E. M., Sapperstein S. K., Christopherson K. S., Barres B. A. (2001) Control of synapse number by glia. Science 291, 657–661 [DOI] [PubMed] [Google Scholar]
- 64. Ikeda H., Miyatake M., Koshikawa N., Ochiai K., Yamada K., Kiss A., Donlin M. J., Panneton W. M., Churchill J. D., Green M., Siddiqui A. M., Leinweber A. L., Crews N. R., Ezerskiy L. A., Rendell V. R., Belcheva M. M., Coscia C. J. (2010) Morphine modulation of thrombospondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J. Biol. Chem. 285, 38415–38427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fujii K., Maeda K., Hikida T., Mustafa A. K., Balkissoon R., Xia J., Yamada T., Ozeki Y., Kawahara R., Okawa M., Huganir R. L., Ujike H., Snyder S. H., Sawa A. (2006) Serine racemase binds to PICK1: potential relevance to schizophrenia. Mol. Psychiatry 11, 150–157 [DOI] [PubMed] [Google Scholar]
- 66. Zhuang Z., Yang B., Theus M. H., Sick J. T., Bethea J. R., Sick T. J., Liebl D. J. (2010) EphrinBs regulate d-serine synthesis and release in astrocytes. J. Neurosci. 30, 16015–16024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vargas-Lopes C., Madeira C., Kahn S. A., Albino do Couto I., Bado P., Houzel J. C., De Miranda J., de Freitas M. S., Ferreira S. T., Panizzutti R. (2011) Protein kinase C activity regulates d-serine availability in the brain. J. Neurochem. 116, 281–290 [DOI] [PubMed] [Google Scholar]
- 68. Zhao B., Wang Q., Du J., Luo S., Xia J., Chen Y. G. (2012) PICK1 promotes caveolin-dependent degradation of TGF-β type I receptor. Cell Res. 22, 1467–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schell M. J., Brady R. O., Jr., Molliver M. E., Snyder S. H. (1997) d-Serine as a neuromodulator. Regional and developmental localizations in rat brain glia resemble NMDA receptors. J. Neurosci. 17, 1604–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. DeVito L. M., Balu D. T., Kanter B. R., Lykken C., Basu A. C., Coyle J. T., Eichenbaum H. (2011) Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav. 10, 210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Johnson M. A., Weick J. P., Pearce R. A., Zhang S. C. (2007) Functional neural development from human embryonic stem cells. Accelerated synaptic activity via astrocyte coculture. J. Neurosci. 27, 3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Garcia O., Torres M., Helguera P., Coskun P., Busciglio J. (2010) A role for thrombospondin-1 deficits in astrocyte-mediated spine and synaptic pathology in Down's syndrome. PLoS One 5, e14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bado P., Madeira C., Vargas-Lopes C., Moulin T. C., Wasilewska-Sampaio A. P., Maretti L., de Oliveira R. V., Amaral O. B., Panizzutti R. (2011) Effects of low-dose d-serine on recognition and working memory in mice. Psychopharmacology 218, 461–470 [DOI] [PubMed] [Google Scholar]