Abstract
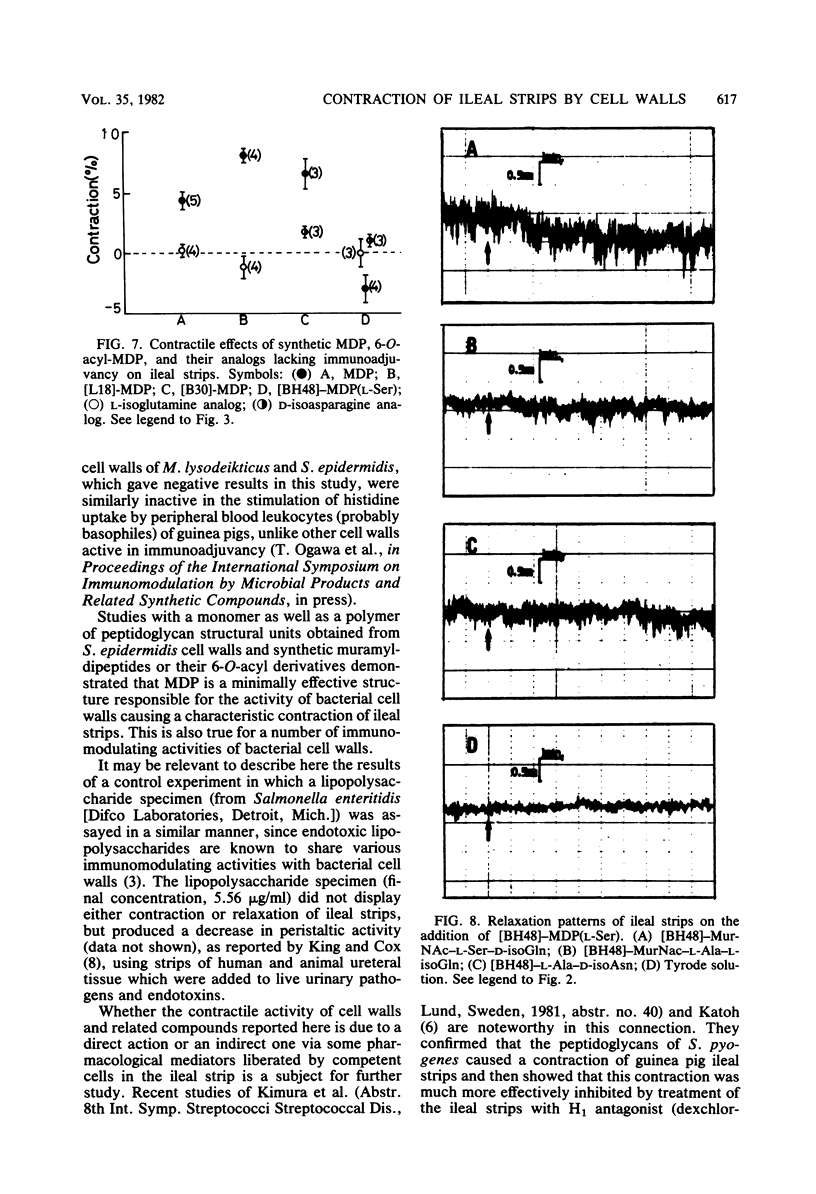
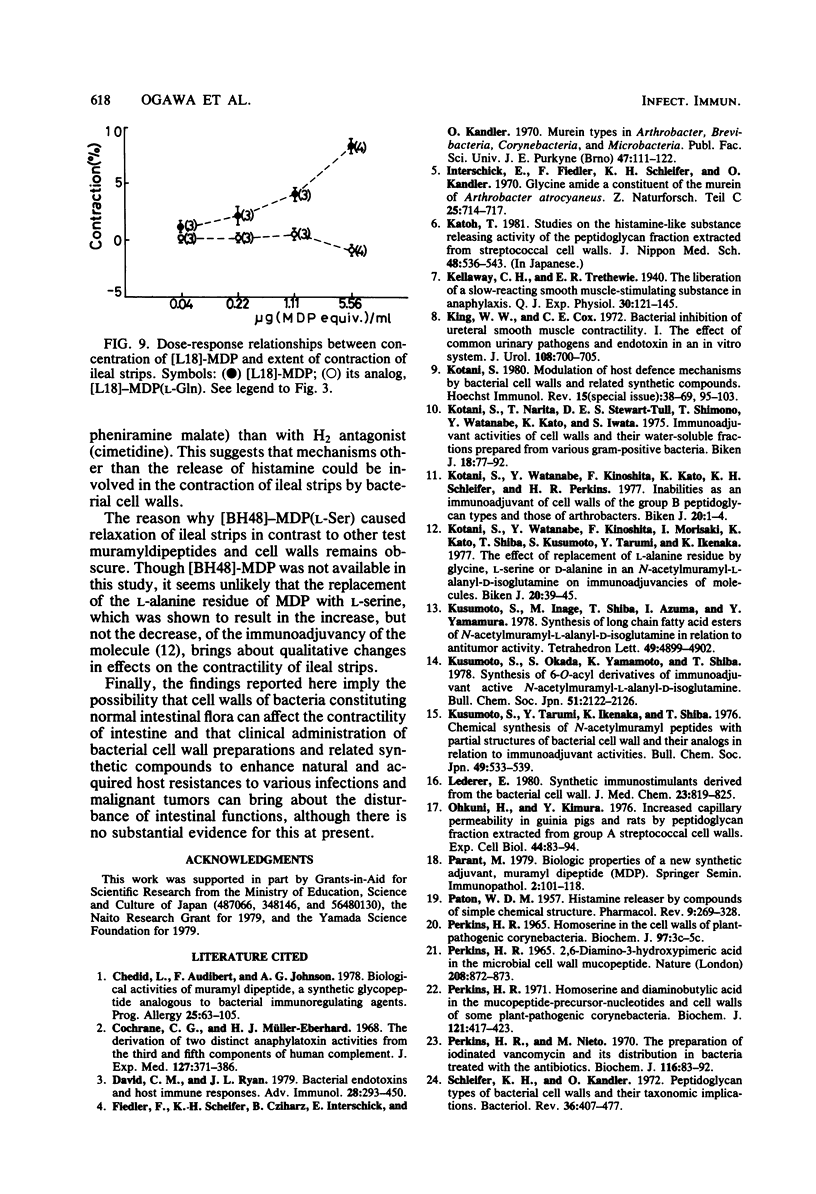
Cell walls isolated from four bacterial species (Streptococcus pyogenes, Lactobacillus plantarum, Streptomyces gardneri, and Nocardia corynebacteriodes), which exhibited the adjuvant effect of stimulating cellular and humoral immune responses against ovalbumin in guinea pigs, caused the slow-starting and long-lasting contraction of guinea pig ileal strips suspended in Tyrode solution. In contrast to these cell walls active in immunoadjuvancy, those isolated from five bacterial species (Micrococcus lysodeikticus, Staphylococcus epidermidis, Arthrobacter atrocyaneus, Corynebacterium insidiosum, and Ampullariella regularis), which lacked immunoadjuvancy at least in intact walls, caused no or very weak contraction of the ileal strips. Further study demonstrated that both a monomer and a polymer of disaccharide-stem peptides, which were obtained by enzymatic degradation of S. epidermis cell wall peptidoglycans, displayed similar contractile effects. It was finally revealed that guinea pig ileum strips showed a definite contractile response to N-acetylmuramyl-L-alanyl-D-isoglutamine (MDP) and 6-O-stearoyl- and 6-O-(2-tetradecylhexadecanoyl)-MDPs, but not to their analogs, whose C-terminal amino acid was L-isoglutamine or D-isoasparagine in place of D-isoglutamine and which lacked adjuvancy. 6-O-(3-Hydroxy-2-docosylhexacosanoyl)-MDP, on the other hand, caused a slow and lasting relaxation of the ileum strips, but its L-isoglutamine and D-isoasparagine analogs did not.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh T. [Studies on the histamine-like substance releasing activity of the peptidoglycan fraction extracted from streptococcal cell walls (author's transl)]. Nihon Ika Daigaku Zasshi. 1981 Aug;48(4):536–543. doi: 10.1272/jnms1923.48.536. [DOI] [PubMed] [Google Scholar]
- King W. W., Cox C. E. Bacterial inhibition of ureteral smooth muscle contractility. I. The effect of common urinary pathogens and endotoxin in an in vitro system. J Urol. 1972 Nov;108(5):700–705. doi: 10.1016/s0022-5347(17)60844-9. [DOI] [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Kato K., Schleifer K. H. Inabilities as an immunoadjuvant of cell walls of the group B peptidoglycan types and those of arthrobacters. Biken J. 1977 Mar;20(1):1–4. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Morisaki I., Kato K. The effect of replacement of L-alanine residue by glycine, L-serine or D-alanine in an N-acetylmuramyl-L-alanyl-D-isoglutamine on immunoadjuvancies of molecules. Biken J. 1977 Jun;20(2):39–45. [PubMed] [Google Scholar]
- Lederer E. Synthetic immunostimulants derived from the bacterial cell wall. J Med Chem. 1980 Aug;23(8):819–825. doi: 10.1021/jm00182a001. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Ohkuni H., Kimura Y. Increased capillary permeability in guinea pigs and rats by peptidoglycan fraction extracted from Group A streptococcal cell walls. Exp Cell Biol. 1976;44(2):83–94. doi: 10.1159/000163102. [DOI] [PubMed] [Google Scholar]
- PATON W. D. Histamine release by compounds of simple chemical structure. Pharmacol Rev. 1957 Jun;9(2):269–328. [PubMed] [Google Scholar]
- Perkins H. R. 2,6-Diamino-3-hydroxypimelic acid in microbial cell wall mucopeptide. Nature. 1965 Nov 27;208(5013):872–873. doi: 10.1038/208872a0. [DOI] [PubMed] [Google Scholar]
- Perkins H. R. Homoserine and diaminobutyric acid in the mucopeptide-precursor-nucleotides and cell walls of some plant-pathogenic corynebacteria. Biochem J. 1971 Feb;121(3):417–423. doi: 10.1042/bj1210417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The preparation of iodinated vancomycin and its distribution in bacteria treated with the antibiotic. Biochem J. 1970 Jan;116(1):83–92. doi: 10.1042/bj1160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



