Abstract
Gene duplication as a major source of novel genetic material plays an important role in evolution. In this study, we focus on duplicate genes in Aspergillus fumigatus, a ubiquitous filamentous fungus causing life-threatening human infections. We characterize the extent and evolutionary patterns of the duplicate genes in the genome of A. fumigatus. Our results show that A. fumigatus contains a large amount of duplicate genes with pronounced sequence divergence between two copies, and approximately 10% of them diverge asymmetrically, i.e. two copies of a duplicate gene pair diverge at significantly different rates. We use a Bayesian approach of the McDonald-Kreitman test to infer distributions of selective coefficients γ(=2Nes) and find that (1) the values of γ for two copies of duplicate genes co-vary positively and (2) the average γ for the two copies differs between genes from different gene families. This analysis highlights the usefulness of combining divergence and diversity data in studying the evolution of duplicate genes. Taken together, our results provide further support and refinement to the theories of gene duplication. Through characterizing the duplicate genes in the genome of A. fumigatus, we establish a computational framework, including parameter settings and methods, for comparative study of genetic redundancy and gene duplication between different fungal species.
Keywords: duplicate gene, Aspergillus fumigatus, positive selection, sequence diversity
Introduction
Eukaryotic genomes are characterized by the presence of numerous multigene families. In a typical eukaryotic species, more than a third of its proteins are encoded in genes that belong to multigene families formed by duplication of a single original gene.1–3 Gene duplication is believed to be a major evolutionary event that provides a source for genetic innovation.4 Duplicate genes may facilitate the development of phenotypic diversity in organismal evolution and are likely to play a prominent role in the adaptive evolution of eukaryotes.5,6 Computational analysis based on molecular evolution models may provide valuable information about gene evolution. By examining the extent of gene duplication, which is manifested by the frequency and magnitude of gene duplication events and the consequent evolutionary fates of gene pairs following the duplication events,7,8 we may gain novel insights into the evolution of organismal adaptation.5,9,10 One of the evolutionary patterns related to gene duplication is the difference in evolutionary rates between the two copies of duplicate genes, which has attracted great interest.9–16 Understanding the evolutionary forces underlying the different evolutionary rates of duplicated genes requires examination of this phenomenon in more organisms. With the plethora of genomic data, it is possible to study the features of gene duplication, including the asymmetric divergence of the duplicate genes, on a genome-wide scale. In the present study, we examined the extent and evolutionary patterns of duplicate genes in several fungal species.
Aspergillus fumigatus is a ubiquitous filamentous fungus and one of the most important opportunistic fungal pathogens. It plays an essential role in recycling environmental carbon and nitrogen by growing on organic debris in soil, its natural ecological niche.17 Due to the widespread distribution of small airborne conidia, A. fumigatus is inevitably inhaled into the airways and the lungs of human beings.18 These conidia are cleared by the innate immune system of healthy individuals but may cause invasive infection in immunosuppressed individuals. Aspergillosis represents a major cause of morbidity and mortality in patients receiving immunosuppressive therapy for autoimmune or neoplastic disease, in organ transplant recipients, and in AIDS patients.19,20 For many years, A. fumigatus was not thought to reproduce sexually, as neither mating nor meiosis had ever been observed.21 Recently, A. fumigatus was shown to possess a fully functional sexual reproductive cycle, and it was accordingly renamed Neosartorya fumigata.22 Considering that it has been known as an anamorph for the majority of its research history, we use A. fumigatus hereafter.
This study focused on examining the extent and evolutionary patterns of duplicate genes in A. fumigatus using evolutionary bioinformatics approaches. To gain a comprehensive and broad view across species, we compared the analytical results obtained in A. fumigatus with those obtained in four other fungal species, which were selected from a diverse phyletic background. The four species were Cryptococcus neoformans, Neurospora crassa,23Saccharomyces cerevisiae,24 and Schizosaccharomyces pombe25 (Table 1). These fungi have distinct life styles and diverse phenotypic characteristics. The brewer’s yeast S. cerevisiae and the fission yeast S. pombe have a life cycle characterized by a unicellular thallus that reproduces by budding and fission respectively. N. crassa is a filamentous ascomycetes growing hyphae apically and branching laterally. C. neoformans is a dimorphic fungus that is able to grow in either a yeast or hyphal mode in response to certain environmental conditions.26,27 We used one species within the Aspergillus genus, A. fischeri,28 as the closely related outgroup species for evolutionary analysis of A. fumigatus genes. Finally we used polymorphic data ascertained in 12 different strains of A. fumigatus to infer the population-scale selection coefficient for duplicate genes using a McDonald-Kreitman (MK) type analysis.29,30
Table 1.
Fungal species and sources of genomic and proteomic sequences.
| Species | Strain | Reference | Source* |
|---|---|---|---|
| A. fumigatus | Af293 | 66 | AspGD |
| C. neoformans var. grubii | H99 | C. neoformans sequencing project† | FGI |
| N. crassa | OR74A | 23 | FGI |
| S. cerevisiae | S288c | 24 | NCBI |
| S. pombe | 972h- | 25 | NCBI |
| A. fischeri | NRRL181 | 28 | NCBI |
Note:
Cryptococcus neoformans var. grubii H99 Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/).
AspGD, Aspergillus Genome Database, http://www.aspgd.org/; FGI, The Fungal Genome Initiative of Broad Institute, http://www.broadinstitute.org/scientific-community/science/projects/fungal-genome-initiative; NCBI, http://www.ncbi.nlm.nih.gov/genome.
Materials and Methods
Identification of duplicate genes
The computer program BLASTCLUST (BLAST score-based single-linkage clustering) was used to automatically and systematically cluster protein sequences. (For documentation on its use, see ftp://ftp.ncbi.nlm.nih.gov/blast/documents/blastclust.html.) Briefly, BLASTCLUST clustering is based on pairwise matches between protein sequences found using the BLAST algorithm. BLASTCLUST uses the default values for the BLAST parameters, including the matrix BLOSUM62, gap opening cost 11, gap extension cost 1, no low-complexity filtering, and e-value threshold 1e-6 for protein sequences. For each pair of sequences, the top-scoring alignment is evaluated according to the minimum length coverage (L = 0.0 to 1.0) and a similarity threshold (ie, the percent of identical residues, S = 3% to 100%) to determine whether the pair of sequences should be linked to each other, providing the base for clustering by the single-linkage method. Different combinations of L and S values influence the results of clustering for the same protein sequences. In order to obtain the best combination of L and S for most accurately clustering protein sequences in our study, we iterated a full range of possible values of the two parameters and ran BLASTCLUST to cluster two test sets of protein families (Supplementary Tables S1 and S2). The two test sets of protein families were constructed by randomly selecting proteins from A. fumigatus and C. neoformans proteomes. For each fungus, a total of 50 protein families were manually constructed using BLAST search against the fungus’ own proteome sequences and visual inspection. These protein families varied in size: 10 families contained 5 proteins or more, and 10 families each contained 4, 3, 2, and 1 protein(s). The two sets of protein families constructed for the two fungal species were used as two test sets to search for the optimized values for parameters L and S. During the search, L was set from 10% to 100% in intervals of 1% and S was set from 25% to 45% with a 1% interval. For each combination of parameters, the accuracy of the BLASTCLUST result was estimated using the percentage of protein families correctly clustered. For each family, correct clustering meant the complete match of gene members between BLASTCLUST clustering results and clusters in the original manually constructed training sets.
Estimation of divergence rates
Protein sequences were aligned using CLUSTALW (version 1.82) with the default parameters. To obtain the alignments of codons, the corresponding nucleotide-sequence alignments were derived by substituting the respective coding sequences from the protein sequences. The number of synonymous substitutions per synonymous site, dS, and the number of non-synonymous substitutions per nonsynonymous site, dN, were calculated using the maximum-likelihood method implemented in the codeml program of the PAML package.31 For each pair of genes, we repeated the computation of dS, dN and dN/dS 1000 times and took the median of the 1000 repeats as the final values. Pairs with dS ≥ 5.0 were eliminated because such high sequence divergence is often associated with problems such as difficulty in alignment, different codon usage biases, or nucleotide compositions in different sequences. The genetic distance between proteins dWAG based on the empirical WAG model32 were computed using MBEToolbox.33 Protein pairs with dWAG ≥ 2.0 were excluded from further analysis.
Test for asymmetric evolution
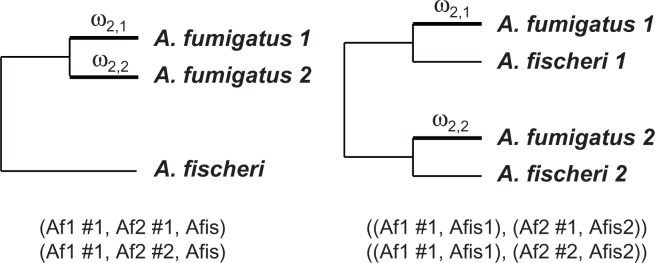
A total of 202 pairs of A. fumigatus duplicate genes with dS ≤ 5.0 between copies were used in this test. These genes’ orthologs were identified using reciprocal BLASTP search in A. fischeri. We found that 44 A. fumigatus duplicate gene pairs in which two copies of genes had the same orthologous gene in A. fischeri. In these cases, two copies of A. fumigatus duplicate genes and their A. fischeri orthologs formed sequence triplets (Fig. 1, left panel). There were 158 A. fumigatus gene pairs in which two copies of genes had two distinct orthologous genes in A. fischeri. In these cases, two copies of A. fumigatus duplicate genes and their corresponding A. fischeri orthologs formed sequence quadruplets (Fig. 1, right panel). The test for the asymmetric evolution was constituted as a relative rate test between a pair of A. fumigatus duplicate genes on an unrooted tree. A. fischeri orthologous sequence(s) were used as outgroup(s). The statistical tests were conducted with a codon-based branch-site model using Codeml program of the PAML package.31 We used the clade model C, which allows for two branch types (clades) and assumes three site classes: site class 0 of conserved sites with ω0 < 1, site class 1 of neutral sites with ω1 = 1, and site class 2 with different selective pressures (ω2,1 and ω2,2) in the two clades. We compared ω2,1 and ω2,2 between two copies of a pair of duplicate gene to detect the difference in the proportion of selected sites in the two clades.34 Likelihood ratio (LR) test was used to test for significance. To do this, two models were applied to the data: model 1 (ω2,1 = ω2,2) constrains the two ω2 values to be equal on the two sequences, and model 2 (ω2,1 ≠ ω2,2) estimates the two ω2 values as free parameters. Collected maximum likelihood values ML1 and ML2 from the two models were used to calculate the likelihood ratio, LR = 2(ln ML1 – lnML2). LR is then compared against the χ2 distribution with one degree of freedom.
Figure 1.
Outline of branch-site models used in the study.
Notes: The phylogenies illustrate the cases of two A. fumigatus duplicate genes with one A. fisheri ortholog (left) and two A. fumigatus duplicate genes with one A. fisheri orthologs (right). The two branches leading to the A. fumigatus duplicate genes are labeled with class 2 selective pressure measures ω2,1 and ω2,2, respectively. Phylogenies in Newick format are given under the trees. The labels #1 and #2 specify the two models in which ω2,1 = ω2,2 and ω2,1 ≠ ω2,2, respectively. Af A. fumigatus, Afis, A. fisheri.
Estimation of selection coefficients
We used the MKPRF test29 to estimate γ of duplicate genes in A. fumigatus. The default values of initial parameters as given in the web service of the program at http://cbsuapps.tc.cornell.edu/MKPRF.aspx were taken. Notably, we used the hierarchical model option FIXED_VARIANCE = 0 and the standard deviation (σ) of the Gaussian prior of γ at 8.0. Given that results of MKPRF may be sensitive to some initial values of parameters,35 we repeated the analysis using different values of σ at 1, 4, and 16. No qualitatively different results were produced in estimation of the means or 95% CIs of γ for duplicate genes in A. fumigatus. The coding SNPs in A. fumigatus genes were obtained from the A. fumigatus genome sequencing project at J. Craig Venter Institute (JCVI) in collaboration with the University of Manchester. These SNPs were ascertained from genome sequences of 12 strains Af293, A1163, AF10, AF210, AFB62, F11628, F11698, F12865, F14946, F15767, F15861, and F16867 using a SNP calling pipeline developed at JCVI. The released project data can be retrieved from the NCBI BioProject database (http://www.ncbi.nlm.nih.gov/bioproject) with IDs 14003, 18733, 46347, 52783, 9521, and 67101.
Results
Extent of duplicate genes in fungal species
To compare the genome-wide extent of gene duplication across species, we obtained the protein sequences of complete proteomes of A. fumigatus, C. neoformans, N. crassa, S. cerevisiae, and S. pombe from various sources (Table 1). We computationally identified multigene families in each of those fungi by clustering proteins into family groups based on the sequence similarity between protein pairs. It is known that the clustering process is sensitive to the statistical criteria used in determining sequence homologs.36,37 For instance, when sequence homologs are determined by using the e-value of BLAST algorithm alone, without specifying the proportion of alignable regions, two non-homologous proteins are likely to be grouped into the same family as homologs due to domain sharing.38
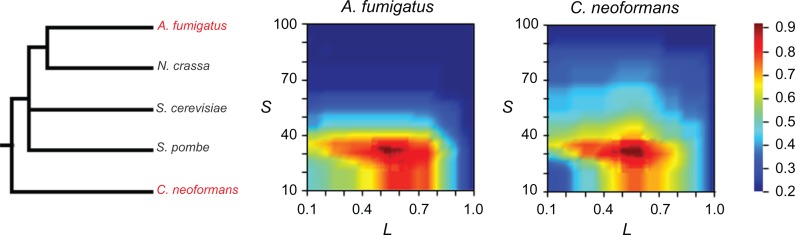
We adapted the program BLASTCLUST that takes two key parameters, sequence similarity (S) and the proportion of alignable regions (L), to ascertain the homologous relationship between a pair of protein sequences. To determine the optimized values of L and S, we manually created two sizable sets of gene families and used them as training data sets. The best combination of values of L and S were those values that produced the most accurate clustering results for the training data sets. That is to say, the results of automatic clustering by using BLASTCLUST with these L and S values were most similar to the results of manual clustering (Materials and Methods). The results of BLASTCLUST are given in Figure 2. It is noteworthy that although the test protein sets were constructed separately for A. fumigatus and C. neoformans, the two most diverged species in our analysis, highly similar values of optimized parameters were obtained: For A. fumigatus, the optimized alignable region between two proteins was between 52% and 55% of the longer protein and the optimized amino-acid similarity was between 31% and 32%, while for C. neoformans, the values were between 53% and 61% and between 30% and 32%, respectively (Fig. 2). Accordingly, we set our criteria of homologous sequences as the alignable region between two proteins to be at least 53% of the longer protein and the alignable region contain more than 31% amino-acid identities.
Figure 2.
Optimization of the values of L and S parameters for BLASTCLUST in A. fumigatus and C. neoformans.
Notes: Phylogenetic tree of the five fungal species included in this study, A. fumigatus, N. crassa, S. cerevisiae, S. pombe, and C. neoformans. The tree topology is taken from James et al.67 Heat maps show the percentage of accurately clustered gene families as a function of L and S values in the two tested species, A. fumigatus and C. neoformans.
The BLASTCLUST results showed that 25.9% of A. fumigatus proteins (2565 of 9887) belong to multigene families (including at least two genes). The percentages for C. neoformans, N. crassa, S. cerevisiae, and S. pombe are 18.1%, 15.4%, 29.5%, and 23.1%, respectively. S. cerevisiae showed a higher percentage of duplicate genes compared with A. fumigatus. Notably, S. cerevisiae showed a higher percentage of duple duplicate families than A. fumigatus and others, which is probably due to the whole genome duplication of S. cerevisiae.39 For triple duplicate families, A. fumigatus is higher than others. For tetra duplicate families, A. fumigatus is as large as S. pombe and higher than the others. For larger families, the percentage of A. fumigatus is also higher than that of other species. Taken together, A. fumigatus exhibits more proteins that belong to multigene families containing more than two genes than the other fungi in consideration (Table 2).
Table 2.
Distributions of protein-coding genes in singleton and multigene families.
| Species | A. fumigatus | C. neoformans | N. crassa | S. cerevisiae | S. pombe |
|---|---|---|---|---|---|
| Number of total genes | 9,887 | 6,968 | 9,733 | 5,863 | 5,010 |
| Number of total families | 8,077 | 6,106 | 8,742 | 4,733 | 4,256 |
| Number and % of multigene families | |||||
| n ≥ 5 | 100 (1.24%) | 46 (0.75%) | 54 (0.62%) | 47 (0.99%) | 33 (0.78%) |
| n = 4 | 59 (0.73%) | 28 (0.46%) | 25 (0.29%) | 27 (0.57%) | 31 (0.73%) |
| n = 3 | 145 (1.80%) | 65 (1.06%) | 84 (0.96%) | 70 (1.48%) | 53 (1.25%) |
| n = 2 | 451 (5.58%) | 261 (4.27%) | 341 (3.90%) | 455 (9.61%) | 285 (6.70%) |
| Number and % of singletons | |||||
| n = 1 | 7,322 (90.65%) | 5,706 (93.45%) | 8,238 (94.23%) | 4,134 (87.34%) | 3,854 (90.55%) |
Our clustering results for S. cerevisiae were comparable to those obtained in previous studies,40,41 but were higher than those obtained by Kondrashov et al,10 who used BLASTCLUST with alignments of at least 95% of their lengths and with a score density of 1.5 bits per position, which approximately corresponds to 75% identity. We found this setting is too strict to produce a sufficient number of multigene families for the non-S. cerevisiae fungal species we analyzed.
Age distribution of duplicate genes in fungal species
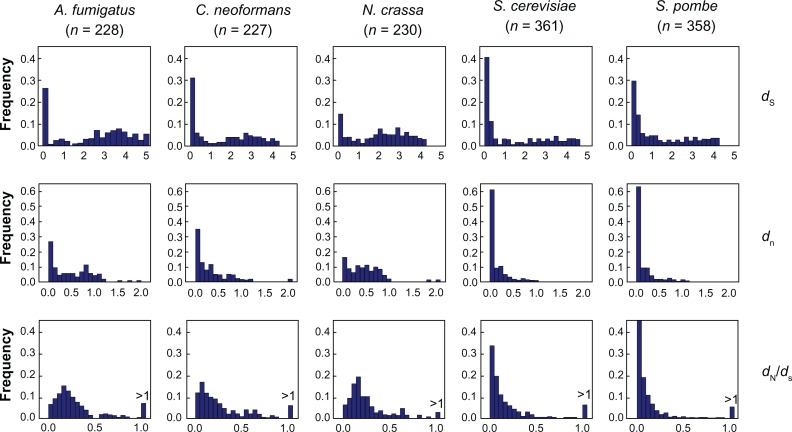
To obtain the age distribution of duplicate genes, we constructed the distribution of synonymous substitution rate (dS) for all fungal species, as dS increases approximately linearly with divergence time. We first computed dS between all pairs of duplicate genes in the same multigene family for all gene families. We then excluded gene pairs with dS ≥ 5.0 to avoid the problem of dS saturation. We followed the procedure described by Zhang et al8 to pick representative duplicate gene pair(s) from each gene family. The procedure guaranteed that the distribution and the mean of dS was not determined by those gene families with extremely large numbers of genes. From each family we picked the gene pair with smallest dS and excluded the pair from the gene family. We then repeated this process for the remaining genes within the same family until the last pair. A total of 228 pairs of duplicate genes in A. fumigatus were selected and analyzed. We plotted the relative frequency of gene pairs as a function of dS (Fig. 3).
Figure 3.
Distributions of synonymous substitution rate, dS, nonsynonymous substitution rate, dN, and the ratio between nonsynonymous and synonymous substitution rates, dN/dS, versus the frequency of total genes in A. fumigatus, C. neoformans, N. crassa, S. cerevisiae, and S. pombe.
Note: The numbers of gene pairs included in analysis are given in parentheses.
All fungal species except N. crassa displayed similar patterns in the distribution of dS between two copies of duplicate genes. The extremely young duplicate genes (with dS ranges between 0 and 0.25) were proportionally more abundant than duplicate genes of other ages. S. cerevisae showed the strongest degree of this enrichment: more than 40% of its duplicate genes belong to the extremely young duplicate genes, which may be attributed to the recent genome duplication of this fungus.39,42 The distributions of dS also showed that the frequency of slightly older duplicate genes (with dS > 0.25) dropped quickly with the increase of duplication age (ie, dS value). The similar shape of dS distributions in all non-N. crassa fungi suggested that the frequency of gene duplication, as a basic evolutionary parameter, may be constant in diverse fungal species. Unlike in other fungi, few duplicate genes in N. crassa had small dS, forming the distinct pattern shown in Fig. 3.
The abnormally low number of duplicate genes whose two copies are highly similar to each other in N. crassa may be attributed to the influence of the repeat-induced point mutation (RIP), which acts as a defense against selfish and mobile DNA by detecting and mutating both copies of the duplicated sequence.43,44 It has been proposed that RIP is distributed not only in Neurospora but also in other filamentous ascomycetes including A. fumigatus, but only one gene so far has been shown to be specifically essential for RIP in N. crassa.45
Selective pressure and sequence divergence of duplicate genes
To estimate the selective pressure acting on duplicate genes, we computed dN and dN/dS ratio between pairs of duplicate genes (Fig. 3). The ratio dN/dS measures the selection pressure to which a gene pair is subjected.46 Generally speaking, a dN/dS = 1 indicates that the duplicate genes are under few or no selective constraints. A dN/dS > 1 is strong evidence for positive selection (ie, replacement substitutions occur at a rate higher than expected by chance, so advantageous mutations have occurred during sequence divergence). In contrast, a dN/dS < 1 indicates purifying selection (ie, amino-acid replacement substitutions have been purged by natural selection because of their deleterious effects). As shown in Figure 3, all fungal species contained 2% to 7% duplicate genes with dN/dS > 1, suggesting that positive selection drives sequence divergence of duplicate genes to some extent. Now, considering only gene pairs with dN/dS < 1, the medians of dN/dS of two filamentous fungi, A. fumigatus and N. crassa, were significantly higher than those of duplicate genes in two yeasts, S. cerevisiae and S. pombe (P < 0.001 in all comparisons between filamentous fungi and yeasts, Kolmogorov-Smirnov [K–S] test). This indicates that purifying selection constraining the sequence divergence between two copies of duplicate genes is more relaxed in the two filamentous fungi than in the two yeasts. A more relaxed pattern of evolution in filamentous fungi, notably A. fumigatus, may be related to a recent reduction in the effective population size of the species possibly due to a lowered frequency (or, a loss) of sexual reproduction.47 A reduced population size would lead to a larger effect produced by genetic drift, which may have allowed additional duplicate copies to be maintained in A. fumigatus and to have evolved a subfunction or neofunction in opportunistic pathogenicity where gene copies would ultimately be maintained by positive selection. This pattern in A. fumigatus becomes more intriguing when considering that proportionally more duplicate genes in the two unicellular yeasts have extremely small dS, which is more likely to numerically inflate dN/dS values. The dimorphic fungus, C. neoformans, showed an intermediate dN/dS median. It is worth noting that although comparing the patterns of the distributions of evolutionary parameters gives an impression of the relationships between the fungal species under consideration, these relationships are not necessarily consistent with their evolutionary relationships (such as in Fig. 2) or morphological groups.
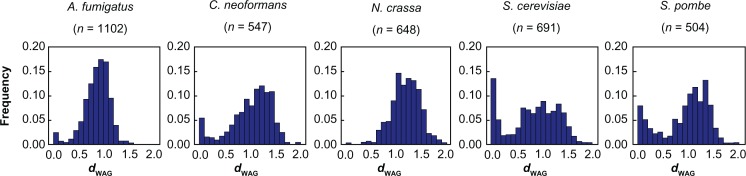
In the above analyses, we excluded gene pairs with highly diverged sequences. In order to get a full picture of genetic divergence between duplicate genes, we went back and included those diverged duplicate genes in the analysis. We computed the evolutionary distance (dWAG) between protein sequences of pairs of duplicate genes using the WAG model of protein divergence.32 We used the same procedure described above to pick pairs of duplicate genes with the smallest dWAG from each gene family. We included all sampled duplicate gene pairs with dWAG < 2 and plotted the frequency distribution of pairs of duplicate genes as a function of dWAG (Fig. 4). By using the protein distance, we included nearly twice as many gene pairs in S. cerevisiae and at least more than three times more gene pairs in other fungal species than in the previous analysis. In all fungal species, most gene pairs have a dWAG that ranges from 0.5 to 1.5. The distribution of dWAG in the two yeasts showed a bimodal pattern with a main peak at 0.5–1.5 and an extra peak at 0–0.2. The extra peaks of smaller dWAG in yeasts correspond to the same duplicate genes with small dN and dN/dS. The lack of the extra peaks in filamentous fungi suggests that large amounts of diverged duplicated genes are present in their genomes, which may be due to a high retention rate of duplicate genes and weak purifying selection. C. neoformans displayed a small dWAG peak, which is higher than those of filamentous fungi but lower than those of yeasts.
Figure 4.
Distributions of evolutionary distance, dWAG, versus frequency of total genes in A. fumigatus, C. neoformans, N. crassa, S. cerevisiae, and S. pombe.
Note: The numbers of gene pairs included in analysis are given in parentheses.
Asymmetric evolution of duplicate genes in A. fumigatus
To assess the asymmetric evolution between two copies of duplicate genes in A. fumigatus, we adapted a codon-based test (Materials and Methods). Codon-based tests take into account the ratio between the rate of nonsynonymous and synonymous substitution and give a more direct measure of the strength of selection and functional constraint in the genes. It is believed that codon-based tests are more sensitive than nucleotide- and amino acid-based tests.48,49 Among the studied duplicate gene pairs in A. fumigatus, 202 pairs had unambiguous orthlogous gene(s) identified in A. fischeri, a close relative of A. fumigatus.28
We used the 202 gene pairs in the test for asymmetric evolution and used the clade model C implemented in Codeml to compute the values of selective pressure ω for both branches of two copies of duplicate genes in A. fumigatus.34 In this branch-site model, the parameter of selective pressure ω is classified into three categories: ω0, ω1, and ω2. Among them, ω2 is allowed to vary in value during model optimization. Codeml estimates the value for ω2 and simultaneously assigns the portions of sites that belong to each of the ω classes. We used a likelihood ratio (LR) test to determine the asymmetric evolution between two copies of duplicate genes; we then examined the values of ω2 at two branches for the two copies of duplicate genes on gene trees (Fig. 1).
The LR test suggested that 24 out of 202 (11.8%) duplicate gene pairs in A. fumigatus have a significant P value < 0.05 (χ2 test), indicating that they evolved at significantly different rates, that is, under asymmetric evolution (Table 3). Among them, 18 gene pairs had at least one copy with ω2 > 1, and the remaining 6 gene pairs had no copy with ω2 > 1. These results indicated that a small portion of duplicate genes are under asymmetric evolution and most asymmetrically evolved genes (18/24 = 75%) are driven by positive selection acting on one copy of the duplicate genes. The portion of ω2 sites ranged widely from 4% to 78.5% among gene pairs (Table 3). Among the 24 gene pairs, 17 pairs remained significant after applying the Bonferroni correction at the 5% level, which suggests that 8.4% gene pairs (17 out of 202) represents a lower boundary for the fraction of duplicate genes evolving by an asymmetric means. Again, most of these highly significant gene pairs (15 out of 17) contain at least one copy of genes with ω2 > 1.
Table 3.
Asymmetrically evolved duplicate gene pairs in A. fumigatus. ω2,1 and ω2,2 are variables of the selective pressure, class 2 of dN/dS, for two copies of duplicate genes in the branch-site model.
| Gene pair | ω2,1 | Systematic name | Functional description | ω2,2 | Systematic name | Functional description | Portion of ω2 sites | P-value‡ |
|---|---|---|---|---|---|---|---|---|
| 1 | >10 | Afu1g10880 | P-type calcium ATPase | >10 | Afu3g10690 | Calcium-translocating P-type ATPase | 65.6% | <0.0001 |
| 2 | >10 | Afu1g00650† | Extracellular alpha-1,3-glucanase/mutanase | 0.0263 | Afu7g08510* | Extracellular alpha-1,3-glucanase/mutanase | 61.8% | <0.0001 |
| 3 | >10 | Afu4g02720* | GPI anchored glycosyl hydrolase | 0.00001 | Afu3g00340 | Glycosyl hydrolase | 5.4% | <0.0001 |
| 4 | >10 | Afu8g04160 | Folylpolyglutamate synthetase | 0.00001 | Afu6g09440 | Tetrahydrofolylpolyglutamate synthase | 4.0% | <0.0001 |
| 5 | >10 | Afu4g00860 | Cell surface protein | 0.00001 | Afu6g12180 | Conserved hypothetical protein | 5.4% | <0.0001 |
| 6 | >10 | Afu1g00810* | Allergen Asp F7-like | 0.00001 | Afu4g06670 | Allergen Asp f 7 Precursor | 28.2% | <0.0001 |
| 7 | >10 | Afu1g05720 | C-14 sterol reductase | 0.2819 | Afu1g03150 | C-14 sterol reductase | 4.0% | <0.0001 |
| 8 | >10 | Afu6g02240 | ORF, Uncharacterized | 0.1749 | Afu3g03740* | Protein kinase | 9.8% | <0.0001 |
| 9 | 5.7 | Afu7g05110 | Hypothetical protein | 0.00001 | Afu6g09410* | Conserved hypothetical protein | 15.1% | <0.0001 |
| 10 | 5.1 | Afu3g02420 | ThiJ/PfpI family transcriptional regulator | 0.00001 | Afu4g01400 | ThiJ/PfpI family protein | 10.3% | <0.0001 |
| 11 | 3.4 | Afu2g10840† | RTA1 domain protein | 0.0183 | Afu6g11810† | RTA1 domain protein | 31.6% | <0.0001 |
| 12 | 4.1 | Afu5g14920* | Hypothetical protein | 0.0001 | Afu5g07980 | Hypothetical protein | 19.8% | <0.0001 |
| 13 | 2.0 | Afu7g08250* | C6 finger domain protein | 0.00001 | Afu2g08040 | C6 finger domain protein | 30.3% | <0.0001 |
| 14 | 1.2 | Afu6g09300 | C2H2 finger domain protein | 0.0001 | Afu4g14400† | C2H2 finger domain protein | 18.5% | <0.0001 |
| 15 | 1.1 | Afu1g01610 | Conserved hypothetical protein | 0.00001 | Afu4g02880 | Conserved hypothetical protein | 34.5% | <0.001 |
| 16 | 0.0295 | Afu5g11240 | Oxidoreductase, short chain dehydrogenase/reductase family | 0.0001 | Afu6g09140 | Oxidoreductase, short-chain dehydrogenase/reductase family | 15.1% | <0.0001 |
| 17 | 0.0001 | Afu3g15280 | Methyltransferase | 0.0000 | Afu2g04380 | LaeA-like methyltransferase | 78.5% | <0.0001 |
| 18 | 0.0001 | Afu5g14930* | Conserved hypothetical protein | 0.0000 | Afu1g16030* | Conserved hypothetical protein | 8.5% | 0.002 |
| 19 | 0.0001 | Afu5g02410 | ATP-dependent RNA helicase fal1 | 0.0000 | Afu3g07200 | DEAD/DEAH box RNA helicase | 69.2% | 0.004 |
| 20 | 0.0001 | Afu4g10240 | Small nuclear ribonucleoprotein SmD3 | 0.0000 | Afu2g12020* | U6 snRNA-associated Sm-like protein LSM4 | 53.7% | 0.007 |
| 21 | 1.8 | Afu8g07150 | Arsenic resistance protein ArsH | 0.3139 | Afu1g16120† | Arsenic resistance protein ArsH, flavoprotein | 21.9% | 0.009 |
| 22 | 0.2531 | Afu6g07320 | MFS multidrug transporter | 0.1189 | Afu6g09110 | MFS multidrug transporter | 31.1% | 0.027 |
| 23 | 2.2 | Afu5g14230* | C6 transcription factor | 0.00001 | Afu1g11290 | Transcription regulator | 6.2% | 0.028 |
| 24 | >10 | Afu5g03930 | Alcohol dehydrogenase | 0.0001 | Afu1g11020† | L-arabinitol 4-dehydrogenase | 32.8% | 0.047 |
Notes: The symbol
indicates negatively selected genes identified by using MKPRF; the symbol
indicates positively selected genes identified by using MKPRF. P values were obtained by likelihood ratio test between model ω2,1 = ω2,2 and ω2,1 ≠ ω2,2 (Materials and Methods).
Duplicate genes with P < 0.0001 remain significant after Bonferroni correction.
We hypothesized that asymmetric evolution of duplicate genes is likely to be associated with the functional divergence between two copies of the genes. If true, the difference in gene function-related measures (such as the level of gene expression) between two copies of asymmetrically divergent duplicate genes should be greater than that in non-asymmetrically divergent duplicate genes. To test this, we obtained the gene expression data of A. fumigatus in shake cultures from the RNA sequencing (RNA-seq) study by Gibbons et al.50 We log-transformed the RNA-seq RPKM values and quantile-normalized them across samples, then we computed the absolute value of the difference between two copies of each pair of duplicate genes, |Δe|. We found that the values of |Δe| for the 24 asymmetrically diverged duplicate genes are higher than those for the other 178 pairs of duplicate genes (P = 0.03, K-S test). This result suggests that the asymmetric sequence divergence of A. fumigatus duplicate genes may be associated with the expression divergence. In most asymmetrically diverged duplicate genes (17 out of 24), the fast evolving copy has a higher expression level compared with the slowly evolving copy. Although this portion is not significant statistically (P = 0.053, Fisher’s exact one-sided test), the correlated divergence of sequence and expression seems consistent with the theoretical expectation.
Distribution of selection coefficients of duplicate genes in A. fumigatus
To estimate the distribution of selection intensities among duplicate genes in A. fumigatus, we used the MKPRF test29,30 to compare the number of synonymous and nonsynonymous polymorphisms within 12 A. fumigatus strains and the number of synonymous and nonsynonymous fixed differences between A. fumigatus and A. fischeri. The MKPRF program uses a Markov chain Monte Carlo algorithm to sample for the posterior distribution of parameters in the models based on Poisson random field (PRF) theory.29,51,52 For each gene, we used the program to estimate the value of population-effective selection coefficient γ (=2Nes, where Ne is the effective population size and s is the selection coefficient in a Wright-Fisher genic selection model) and the 95% confidence interval (CI) of γ for each gene. If a gene has its 95% CI of γ completely above 0, the gene appears to have been evolving under positive selection. On the other hand, if the 95% CIs of γ are completely below 0, the gene appears to be under negative selection.
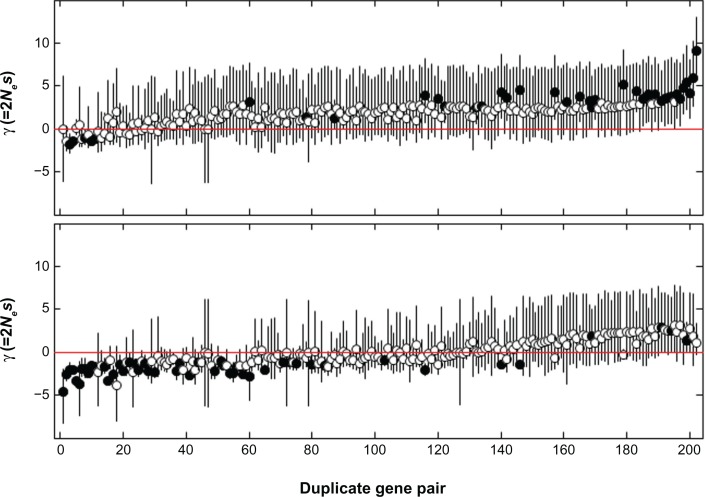
The means of γ varied among genes in different families, as well as between two copies of duplicate genes in the same family (Fig. 5). Among A. fumigatus’ 202 duplicate gene pairs, 38 (18.8%) contained a gene with CI of γ > 0 indicating positive selection, and 49 (24.2%) contained a gene with CI of γ < 0 indicating negative selection (supplementary Table S3). For comparison, in the study of human protein-coding genes, MKPRF analysis discovered 304 (9.0%) out of 3377 tested loci showing evidence of positive selection and 813 (13.5%) out of 6033 loci showing evidence of negative selection.30 Similarly, most of the genes, in either A. fumigatus or human, showed no evidence of selection according to MKPRF with a 5% cutoff, indicating weak negative selection and/or balancing selection operating on mutations at these genes. Four pairs contained one gene under negative selection and the other gene under positive selection. Despite the existence of these pairs with gene(s) under selection, the overall correlation between γ values of two copies of duplicate genes was strong and significant (Spearman’s ρ = 0.405, P = 3.1 × 10−9). This indicates that two copies of duplicate genes had significantly more similar selection intensities than randomly selected pairs of genes overall.
Figure 5.
Means of the posterior distributions of the selection coefficient γ (dots) and the 95% CIs (vertical lines) for duplicate genes in A. fumigatus.
Notes: For each pair of duplicate genes, the copy with larger γ is plotted in the upper panel and the other copy with smaller γ is plotted in the lower panel. Genes that are under positive (95% CIs of γ > 0) or negative selection (95% CIs of γ < 0) are indicated with filled circles. Duplicate gene pairs (x-axis) are ranked by the average values of γ for the two copies of duplicate genes.
The percentage of genes under positive selection identified using MKPRF was slightly higher than that identified using the codon-based LR test (18.8% versus 11.8%). Interestingly we found inconsistency between the MKPRF estimation of γ and the codon-based estimation of ω2 among genes. In Table 3, we marked those positively and negatively selected genes ascertained by using MKPRF. Among those asymmetrically evolved gene pairs identified using the LR test, all copies with a smaller ω2 than the other copies were under negative selection according to the LR test (as indicated by ω2 < 1). However, there were several cases, in which these negatively selected genes, such as Afu1g11020 and Afu6g11810, were found to be under positive selection using MKPRF. On the other hand, several genes, such as Afu4g02720 and Afu1g00810, with a greater ω2 than their duplicated copies due to positive selection as indicated by ω2 < 1 were reported to be under negative selection by MKPRF (Table 3).
Discussion
The completion of genome sequencing and the discovery of the sexual cycle in A. fumigatus have placed the foundations for the fungus as an emerging model organism for studying the biology, ecology, and pathogenicity of filamentous fungi. Despite these advents, we still know very little about the function of most of A. fumigatus genes. Given the slow pace associated with the experimental determination of gene functions, computer-based analysis serves as an initial screening in the characterization of roles of genes. In this study, we systematically assessed the extent of duplicate genes in the genomes of A. fumigatus and four other fungal species. We also systematically studied the molecular evolutionary forces associated with the divergence of duplicate genes in A. fumigatus. We focused on the role of natural selection on newly created and long-established gene pairs, as well as the role of ongoing selection in shaping nucleotide diversity of duplicate genes in population-genetic samples, which provides insights into the microevolutionary dynamics of these loci.
In examining the extent of gene duplication, we searched for the optimal parameter values for the clustering algorithm we employed. The optimization was performed against sizable sets of gene families that were manually constructed. The optimized values of two key parameters, L and S, for A. fumigatus and C. neoformans, were found to be nearly identical. It was, therefore, justified to apply the same criteria determined by the two optimized parameters to all fungal species considered in our study. This guaranteed the clustering of individual proteins into multi-gene families by a consistent and objective means, making it feasible to compare the size of multigene families across species. Our results revealed complex patterns in differences in the family size distributions among different fungal species. More than a quarter of A. fumigatus genes were found to be members of multigene families compared with nearly 30% for baker’s yeast S. cerevisiae and about 15% for another filamentous fungus N. crassa. The other two fungal species, C. neoformans and S. pombe, showed intermediate ratios. There is no apparent link between the extent of gene duplication in the genomes of species and the features in life style and morphology (eg, unicellar yeasts versus multicellar hypha) of these fungal species.
The extremely high ratio for S. cerevisiae may be attributed to the whole genome duplication ca. 10 million years ago.39,42 The previous comparative genomic analysis on several other yeast species, inducing Candida glabrata, Kluyveromyces lactis, Debaryomyces hansenii, and Yarrowia lipolytica, also revealed the influence of other evolutionary mechanisms, tandem gene repeat formation, segmental duplication, and extensive gene loss on the formation of gene duplication patterns.42 On the other hand, the extremely low ratio for N. crassa may be attributed to the strong influence of RIP mutation.43,44 As mentioned, RIP may be widely present in many other fungal species.45 However, the impact of RIP on duplicate genes seems more pronounced in Neurospora than other fungi in question. Examining the age distribution of duplicate genes gives a sense of the average rate of duplication and the scale of duplication events.53,54 Our results showed that the genomes of yeasts have been shaped by genome duplication or large-scale gene duplications in the recent evolution, whereas the genome of A. fumigatus contains more functionally divergent genes that may be resulted from ancient gene duplications.
Increasing evidence indicates that two copies of duplicate genes can assume unequal roles in divergence.11 Although the functional significance of asymmetric divergence is still unclear, it has been argued that some form of evolutionary asymmetry is required for functional diversification of duplicate genes.55 The study of asymmetric evolution of duplicate genes is important for determining the evolutionary processes that have occurred in the genome in order to obtain clues as to the development of the unequal roles of two copies of the same genes. Previous studies on asymmetric evolution between two copies of duplicate genes led to inconsistent conclusions (compare the studies of Kondrashov et al,10 Hughes and Hughes,12 and Van de Peer13 with the study by Conant and Wagner11). For example, Kondrashov et al10 found that no duplicate genes (n = 15) in S. cerevisiae showed signs of asymmetric evolution, or differential evolutionary rates between duplicate genes, and concluded that both copies of duplicate genes typically evolved at the same rates. Conant and Wagner11 found 21% (n = 14) showed significant signatures of asymmetric evolution in the same species, and suggested asymmetric divergence between two copies of duplicate genes is not uncommon. The discrepancy may be due to the small sample size and the sensitivity of methods used in different studies (eg, Kondrashov et al10 used a distance-based method, while Conant and Wagner11 used a codon-based model approach), as well as the way in which outgroup sequences were selected.
We conducted our test in A. fumigatus genes using the codon-based LR test with a branch-site model. We used orthologous sequences from the closely related species A. fischeri as outgroups. Our LR test identified asymmetric evolution in 12% of duplicate genes in A. fumigatus. This result is consistent with that of Conant and Wagner.11 Our result supports the general conclusion of several previous theoretical and empirical studies,56–58 indicating that positive selection plays a role in the evolutionary histories of duplicate genes. The fate of duplicate gene pairs may be determined in the initial phases of duplicate gene evolution during the period of reinforced asymmetric evolution. If true, young duplicate genes should be more likely to be driven by positive selection to fixation. In other words, positively selected duplicate genes should be younger. However, our result did not provide evidence supporting this aspect of the theory. Positively selected duplicate genes were not younger than other duplicate genes in A. fumigatus, as shown in that the distribution of dS between two copies of asymmetrically evolved duplicate genes did not differ from that between two copies of randomly selected duplicate genes (P > 0.05, K-S test). Our results could suggest that positive selection can occur both in the short period of asymmetric evolution directly following duplication but can also occur after the period of asymmetric evolution, which correlates with the models advocating neofunctionalization, in which one copy develops a novel function, as well as subfunctionalization, in which the copies share the function of the original unduplicated copy.59
A close examination of functions of those duplicate genes suggested to be under asymmetric evolution cover a broad range of functionalities, including dehydrogenases, ATPases, glucanases, mutanases, glycosyl hydrolases, as well as genes that encode cell surfaces proteins, arsenic resistance proteins, and transcriptional factors. Recent studies have shown that many of these protein classes are important to the evolved opportunistic pathogenic nature of A. fumigatus and are important virulence factors. Kumar et al60 examined the secretory proteins of A. fumigatus showing that glucanases, mutanases, and hydrolases are associated with virulence. Hydrolases have also been shown to be involved in ergot alkaloid synthesis, a complex family of mycotoxins with a variety of pathogenicity functions.61 However, functions of most of these genes are putatively assigned and need further experimental validation; also many genes encode hypothetical gene products or proteins with unknown function. These again underscore the need of functional analysis of duplicate genes in A. fumigatus.
Taking advantage of the availability of sequence polymorphisms ascertained among multiple A. fumigatus strains, we applied the MKPRF method, an extension of the MK test, to infer the selection coefficients for individual genes. The MK framework allows examining the levels and patterns of nucleotide polymorphisms and increases the sensitivity of detecting natural selection in protein-coding genes compared with methods using the dN/dS ratio alone.62 It is noteworthy that MKPRF estimates γ also using the divergence between genes and their orthologs but not using the divergence between two copies of duplicate genes. We obtained the selection coefficients γ for individual genes in duplicate gene pairs and found that two copies of duplicate genes have highly correlated γ. This result suggests that two copies of duplicate genes are under selection of largely equal strength. The strength of selection is characteristic of individual gene families, determined by the functions of the families. This is consistent with the conclusion of a previous study that gene duplication (and loss) is highly constrained by the functional properties of genes.63 MKPRF test also showed that 18.8% of duplicate genes are under positive selection, and 24.2% are under negative selection. These figures are supported by multiple independent repetition of MKPRF with different initial parameters. Strikingly, there are marked discrepancies between the results of MKPRF and LR tests. Many genes that were detected to be under positive selection in one test were not detected or were detected to be under negative selection in the other. We believe that these discrepancies are most likely rooted from the methodological difference between the MKPRF and the LR test.35 If there is a biological reason behind the discrepancies, it would suggest a high turnover rate among positive, negative, and neutral selections that act on duplicate genes during different periods of evolution. The two tests happen to be more sensitive to different spectrums of the selective signal generated during the complex evolutionary process. Nevertheless, caution should be taken when interpreting the MKPRF results. MKPRF requires several assumptions in order to apply PRF theory. Some of these assumptions might not hold with our polymorphism data. For example, the sample size (n = 12) of A. fumigatus strains from which SNPs were ascertained is not large enough to allow low-frequency SNPs to be discovered. Also the influence of gene conversion between two copies of duplicate genes on the results is unclear. In addition, these strains are clinical isolates that may have experienced severe bottlenecks of population size during transmission and strain establishment. Bottleneck-induced drift may result in an elevated rate of fixation of slightly deleterious mutations, which in turn can lead to the biased results of MK test.64,65 In the future, repeating this analysis using SNPs discovered in more environmental stains of A. fumigatus is desired.
In summary, we conducted a systematic examination on the extent of duplicate genes in A. fumigatus and showed the difference in the size of multiple gene families between A. fumigatus and other fungal species. The established bioinformatics procedure and optimized parameters for the clustering program are ready to be adapted for other studies. We used A. fumigatus genes as examples to refine the theories of gene duplication and showed that negative selection contributes to the fixation and persistence of the duplicate genes, while positive selection may also play a role in sequence and functional divergence of duplicate genes.
Acknowledgments
The authors thank Natalie Fedorova, William Nierman, and Paul Bowyer for providing the polymorphism data of A. fumigatus, and Suman Pakala for technical assistance. The authors acknowledge the Texas A&M Supercomputing Facility (http://sc.tamu.edu/) for providing computing resources useful in conducting the research reported in this paper.
Footnotes
Author Contributions
Conceived and designed the experiments: JC and EY. Analyzed the data: EY and JC. Wrote the first draft of the manuscript: EY and JC. Contributed to the writing of the manuscript: AH. Agree with manuscript results and conclusions: EY, AH, and JC. Jointly developed the structure and arguments for the paper: AH and JC. Made critical revisions and approved final version: JC, AH, and EY. All authors reviewed and approved of the final manuscript.
Funding
EY is partially supported by CVM Postdoctoral Trainee Research Grant (02-144002-03504) at Texas A&M University.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Supplementary Data
Table S1.
Lists of gene families in A. fumigatus used for parameter optimization of BLASTCLUST.
| Family | No of Genes | Gene ID |
|---|---|---|
| 1 | 21 | Afu8g06290, Afu2g18080, Afu8g01040, Afu8g00310, Afu6g00780, Afu3g09430, Afu4g02640, Afu4g14870, Afu6g00790, Afu6g09470, Afu6g14660, Afu7g08200, Afu4g00820, Afu5g00270, Afu6g09480, Afu6g14650, Afu3g09440, Afu4g14880, Afu3g15360, Afu4g14370, Afu7g06935 |
| 2 | 10 | Afu6g09480, Afu6g14650, Afu2g18080, Afu8g01040, Afu8g00310, Afu8g06290, Afu3g09440, Afu4g14880, Afu3g15360, Afu5g00270 |
| 3 | 9 | Afu6g03030, Afu2g01580, Afu6g03490, Afu2g15520, Afu6g11480, Afu1g13660, Afu2g00140, Afu3g02620, Afu3g03280 |
| 4 | 7 | Afu7g04180, Afu3g00680, Afu1g13440, Afu3g14590, Afu5g07360, Afu5g01470, Afu7g08470 |
| 5 | 6 | Afu7g04080, Afu4g10950, Afu1g12650, Afu2g11350, Afu6g14200, Afu8g04000 |
| 6 | 5 | Afu2g00530, Afu3g07550, Afu5g13060, Afu8g01120, Afu3g00670 |
| 7 | 5 | Afu2g11610, Afu2g11620, Afu7g06380, Afu8g07070, Afu3g07380 |
| 8 | 5 | Afu2g00710, Afu4g10130, Afu2g03230, Afu3g00900, Afu2g13460 |
| 9 | 5 | Afu8g07260, Afu2g17320, Afu3g07220, Afu3g00170, Afu6g00520 |
| 10 | 5 | Afu1g10910, Afu7g00250, Afu1g02550, Afu2g14990, Afu1g13390 |
| 11 | 4 | Afu1g11350, Afu5g09940, Afu3g03500, Afu4g01360 |
| 12 | 4 | Afu6g11890, Afu4g00540, Afu3g13580, Afu7g08580 |
| 13 | 4 | Afu1g14170, Afu3g00380, Afu1g16700, Afu6g06660 |
| 14 | 4 | Afu3g01440, Afu4g03340, Afu5g07620, Afu3g13940 |
| 15 | 4 | Afu1g10790, Afu7g04720, Afu6g13760, Afu5g10520 |
| 16 | 4 | Afu6g03890, Afu3g02270, Afu2g18030, Afu2g00200 |
| 17 | 4 | Afu5g00410, Afu3g00240, Afu2g15490, Afu5g00380 |
| 18 | 4 | Afu6g12950, Afu2g04010, Afu4g03190, Afu5g14300 |
| 19 | 4 | Afu1g12400, Afu5g03110, Afu1g12190, Afu5g02850 |
| 20 | 4 | Afu4g00730, Afu8g01530, Afu2g14320, Afu3g13130 |
| 21 | 3 | Afu2g11270, Afu3g00910, Afu1g15440 |
| 22 | 3 | Afu3g10910, Afu3g07030, Afu6g09910 |
| 23 | 3 | Afu1g17570, Afu1g04870, Afu6g03200 |
| 24 | 3 | Afu2g05880, Afu1g10930, Afu5g11020 |
| 25 | 3 | Afu8g00770, Afu7g05550, Afu2g09450 |
| 26 | 3 | Afu2g09520, Afu6g11600, Afu6g07480 |
| 27 | 3 | Afu3g01170, Afu7g01590, Afu5g13810 |
| 28 | 3 | Afu7g00420, Afu8g06100, Afu8g06590 |
| 29 | 3 | Afu2g00120, Afu2g03820, Afu2g00540 |
| 30 | 3 | Afu6g00500, Afu8g00930, Afu4g01290 |
| 31 | 2 | Afu3g07860, Afu4g14070 |
| 32 | 2 | Afu1g04780, Afu1g12910 |
| 33 | 2 | Afu2g07940, Afu6g10990 |
| 34 | 2 | Afu6g07640, Afu1g04460 |
| 35 | 2 | Afu1g11290, Afu5g14230 |
| 36 | 2 | Afu7g04800, Afu2g12640 |
| 37 | 2 | Afu8g06080, Afu4g03410 |
| 38 | 2 | Afu2g00920, Afu2g12770 |
| 39 | 2 | Afu7g05740, Afu6g05210 |
| 40 | 2 | Afu1g12050, Afu4g09540 |
| 41 | 1 | Afu1g01350 |
| 42 | 1 | Afu4g01470 |
| 43 | 1 | Afu5g04090 |
| 44 | 1 | Afu2g04580 |
| 45 | 1 | Afu1g06000 |
| 46 | 1 | Afu5g06690 |
| 47 | 1 | Afu5g08030 |
| 48 | 1 | Afu2g11880 |
| 49 | 1 | Afu3g12050 |
| 50 | 1 | Afu1g14980 |
Table S2.
Lists of gene families in C. neoformans used for parameter optimization of BLASTCLUST.
| Family | No of Genes | Gene ID |
|---|---|---|
| 1 | 9 | CNAG_00792T0, CNAG_00823T0, CNAG_01575T0, CNAG_02262T0, CNAG_02430T0, CNAG_02977T0, CNAG_03450T0, CNAG_03503T0, CNAG_07781T0 |
| 2 | 7 | CNAG_02883T0, CNAG_03130T0, CNAG_03315T0, CNAG_05348T0, CNAG_05968T0, CNAG_05998T0, CNAG_06606T0 |
| 3 | 6 | CNAG_00099T0, CNAG_03341T0, CNAG_03962T0, CNAG_04052T0, CNAG_05825T0, CNAG_06182T0 |
| 4 | 6 | CNAG_00550T0, CNAG_00770T0, CNAG_01642T0, CNAG_01916T0, CNAG_02682T0, CNAG_05201T0 |
| 5 | 6 | CNAG_00859T0, CNAG_02018T0, CNAG_06944T0, CNAG_07002T0, CNAG_07753T0, CNAG_07893T0 |
| 6 | 6 | CNAG_01495T0, CNAG_05320T0, CNAG_05321T0, CNAG_05329T0, CNAG_06530T0, CNAG_07626T0 |
| 7 | 5 | CNAG_00308T0, CNAG_03420T0, CNAG_03960T0, CNAG_04141T0, CNAG_04988T0 |
| 8 | 5 | CNAG_01500T0, CNAG_01714T0, CNAG_03389T0, CNAG_06249T0, CNAG_06876T0 |
| 9 | 5 | CNAG_04474T0, CNAG_06536T0, CNAG_06537T0, CNAG_06538T0, CNAG_06539T0 |
| 10 | 5 | CNAG_05369T0, CNAG_06931T0, CNAG_06936T0, CNAG_06985T0, CNAG_07707T0 |
| 11 | 4 | CNAG_00122T0, CNAG_01940T0, CNAG_02189T0, CNAG_05264T0 |
| 12 | 4 | CNAG_00575T0, CNAG_04981T0, CNAG_05015T0, CNAG_05256T0 |
| 13 | 4 | CNAG_00789T0, CNAG_00980T0, CNAG_03477T0, CNAG_05911T0 |
| 14 | 4 | CNAG_00862T0, CNAG_05319T0, CNAG_05376T0, CNAG_05383T0 |
| 15 | 4 | CNAG_00863T0, CNAG_02475T0, CNAG_04561T0, CNAG_07389T0 |
| 16 | 4 | CNAG_01373T0, CNAG_01740T0, CNAG_02196T0, CNAG_05925T0 |
| 17 | 4 | CNAG_01968T0, CNAG_03797T0, CNAG_07447T0, CNAG_07613T0 |
| 18 | 4 | CNAG_01840T0, CNAG_03787T0, CNAG_04948T0, CNAG_06914T0 |
| 19 | 4 | CNAG_02217T0, CNAG_03326T0, CNAG_06487T0, CNAG_07499T0 |
| 20 | 4 | CNAG_05276T0, CNAG_01699T0, CNAG_05690T0, CNAG_05563T0 |
| 21 | 3 | CNAG_00063T0, CNAG_04828T0, CNAG_06745T0 |
| 22 | 3 | CNAG_00919T0, CNAG_01040T0, CNAG_02966T0 |
| 23 | 3 | CNAG_01635T0, CNAG_02016T0, CNAG_04225T0 |
| 24 | 3 | CNAG_01681T0, CNAG_04276T0, CNAG_04982T0 |
| 25 | 3 | CNAG_02552T0, CNAG_06172T0, CNAG_07445T0 |
| 26 | 3 | CNAG_02958T0, CNAG_06241T0, CNAG_07865T0 |
| 27 | 3 | CNAG_03277T0, CNAG_05316T0, CNAG_06623T0 |
| 28 | 3 | CNAG_04326T0, CNAG_06374T0, CNAG_06638T0 |
| 29 | 3 | CNAG_05937T0, CNAG_05941T0, CNAG_07703T0 |
| 30 | 3 | CNAG_06297T0, CNAG_06298T0, CNAG_06388T0 |
| 31 | 2 | CNAG_01487T0, CNAG_01511T0 |
| 32 | 2 | CNAG_00005T0, CNAG_02012T0 |
| 33 | 2 | CNAG_02086T0, CNAG_02087T0 |
| 34 | 2 | CNAG_02241T0, CNAG_05453T0 |
| 35 | 2 | CNAG_02899T0, CNAG_03007T0 |
| 36 | 2 | CNAG_02896T0, CNAG_03311T0 |
| 37 | 2 | CNAG_03355T0, CNAG_05590T0 |
| 38 | 2 | CNAG_05450T0, CNAG_05454T0 |
| 39 | 2 | CNAG_06010T0, CNAG_06018T0 |
| 40 | 2 | CNAG_06524T0, CNAG_06821T0 |
| 41 | 1 | CNAG_01563T0 |
| 42 | 1 | CNAG_03763T0 |
| 43 | 1 | CNAG_04049T0 |
| 44 | 1 | CNAG_04610T0 |
| 45 | 1 | CNAG_05721T0 |
| 46 | 1 | CNAG_06603T0 |
| 47 | 1 | CNAG_06620T0 |
| 48 | 1 | CNAG_06828T0 |
| 49 | 1 | CNAG_07687T0 |
| 50 | 1 | CNAG_07766T0 |
Table S3.
Gamma and 59% CIs of MKPRF results for 202 duplicate gene pairs in A. fumigatus.
|
Gene 1
|
Gene 2
|
||||
|---|---|---|---|---|---|
| Gene ID | gamma | 95% CI | Gene ID | gamma | 95% CI |
| Afu2g12020 | −4.66 | [1.64, −8.35] | Afu4g10240 | −0.09 | [3.15, −6.15] |
| Afu5g04060 | −3.80 | [2.14, −8.08] | Afu3g12850 | 1.95 | [2.25, −1.52] |
| Afu5g02180 | −3.66 | [1.54, −7.36] | Afu2g07620 | 0.41 | [1.80, −2.04] |
| Afu5g14920 | −3.39 | [0.15, −3.69] | Afu5g07980 | 1.23 | [1.84, −1.33] |
| Afu1g00350 | −3.38 | [0.25, −3.90] | Afu8g06210 | 0.05 | [0.45, −0.67] |
| Afu5g05510 | −2.88 | [1.26, −5.66] | Afu7g03750 | 3.19 | [2.02, 0.03] |
| Afu6g11710 | −2.65 | [0.28, −3.19] | Afu7g08440 | 2.37 | [2.11, −0.90] |
| Afu3g02800 | −2.55 | [0.46, −3.42] | Afu6g02480 | 0.67 | [1.82, −1.82] |
| Afu2g17900 | −2.53 | [0.41, −3.30] | Afu2g00160 | −1.43 | [0.77, −2.58] |
| Afu1g16080 | −2.53 | [0.27, −3.04] | Afu5g14990 | 2.78 | [2.06, −0.45] |
| Afu5g00280 | −2.43 | [0.27, −2.94] | Afu2g05190 | −0.61 | [1.22, −2.18] |
| Afu7g01690 | −2.41 | [0.35, −3.07] | Afu8g05220 | 2.61 | [2.09, −0.65] |
| Afu3g00670 | −2.39 | [0.59, −3.44] | Afu5g13060 | 2.60 | [2.05, −0.60] |
| Afu6g12820 | −2.37 | [2.06, −6.42] | Afu2g12200 | 0.65 | [1.79, −1.77] |
| Afu6g02420 | −2.35 | [2.02, −6.26] | Afu6g13170 | −0.25 | [1.80, −2.65] |
| Afu2g17430 | −2.33 | [0.37, −3.01] | Afu5g09600 | 1.36 | [1.89, −1.24] |
| Afu6g09410 | −2.17 | [0.16, −2.47] | Afu7g05110 | 0.38 | [0.89, −0.87] |
| Afu4g08240 | −2.17 | [0.58, −3.18] | Afu2g13270 | 1.75 | [2.26, −1.84] |
| Afu2g00920 | −2.16 | [0.49, −3.04] | Afu2g12770 | 2.10 | [2.14, −1.22] |
| Afu1g17580 | −2.15 | [0.48, −3.02] | Afu3g15040 | 0.98 | [1.85, −1.53] |
| Afu2g08910 | −2.10 | [0.44, −2.88] | Afu1g02350 | −1.82 | [0.59, −2.78] |
| Afu8g06580 | −2.10 | [0.37, −2.77] | Afu8g04910 | 0.66 | [1.80, −1.76] |
| Afu1g16030 | −2.09 | [0.14, −2.35] | Afu5g14930 | −1.39 | [0.20, −1.76] |
| Afu8g06630 | −2.07 | [0.34, −2.68] | Afu1g16115 | 3.90 | [1.96, 0.73] |
| Afu6g14200 | −2.06 | [1.04, −3.71] | Afu1g12650 | 1.87 | [2.25, −1.64] |
| Afu8g01400 | −2.05 | [0.31, −2.61] | Afu1g09980 | 2.48 | [2.12, −0.80] |
| Afu5g09580 | −2.04 | [1.04, −3.71] | Afu1g17250 | 1.71 | [2.24, −1.77] |
| Afu4g00570 | −1.99 | [0.57, −2.96] | Afu3g02060 | −1.09 | [0.46, −1.83] |
| Afu1g03280 | −1.91 | [0.58, −2.88] | Afu6g11920 | −1.18 | [0.56, −2.04] |
| Afu2g17360 | −1.70 | [0.59, −2.63] | Afu4g02750 | 1.93 | [2.18, −1.48] |
| Afu5g11080 | −1.67 | [0.45, −2.42] | Afu2g15140 | −0.57 | [0.97, −1.86] |
| Afu5g00370 | −1.66 | [0.17, −1.97] | Afu5g00930 | −1.14 | [0.42, −1.83] |
| Afu8g04110 | −1.63 | [0.75, −2.76] | Afu1g00440 | 2.58 | [2.10, −0.70] |
| Afu5g02870 | −1.60 | [1.06, −3.13] | Afu5g09290 | 1.47 | [1.83, −1.11] |
| Afu5g14230 | −1.59 | [0.30, −2.13] | Afu1g11290 | 1.77 | [1.83, −0.88] |
| Afu8g01560 | −1.55 | [0.74, −2.68] | Afu2g11250 | −1.03 | [1.17, −2.58] |
| Afu2g13020 | −1.53 | [0.78, −2.68] | Afu6g07710 | 1.77 | [2.25, −1.78] |
| Afu6g09370 | −1.52 | [0.14, −1.78] | Afu1g00150 | −1.41 | [0.13, −1.66] |
| Afu4g02720 | −1.52 | [0.45, −2.27] | Afu3g00340 | 2.45 | [2.13, −0.91] |
| Afu7g06620 | −1.50 | [0.77, −2.64] | Afu4g00600 | 1.70 | [1.86, −0.89] |
| Afu3g12770 | −1.50 | [0.98, −2.89] | Afu1g14210 | 1.78 | [2.25, −1.78] |
| Afu1g00950 | −1.49 | [0.19, −1.84] | Afu7g07090 | 4.31 | [1.93, 1.24] |
| Afu4g10000 | −1.48 | [0.52, −2.33] | Afu2g03090 | −0.37 | [1.29, −2.02] |
| Afu5g03930 | −1.46 | [0.76, −2.61] | Afu1g11020 | 3.52 | [1.66, 0.93] |
| Afu5g01230 | −1.45 | [0.62, −2.40] | Afu3g01030 | 2.26 | [2.16, −1.11] |
| Afu7g08510 | −1.42 | [0.17, −1.73] | Afu1g00650 | 4.47 | [1.85, 1.48] |
| Afu4g14510 | −1.37 | [0.63, −2.37] | Afu2g11120 | 0.81 | [1.51, −1.17] |
| Afu3g10960 | −1.37 | [0.76, −2.49] | Afu2g03670 | 2.21 | [1.83, −0.47] |
| Afu1g16040 | −1.36 | [0.15, −1.63] | Afu5g14940 | −0.33 | [0.30, −0.84] |
| Afu8g06640 | −1.34 | [0.47, −2.11] | Afu3g02400 | 1.99 | [2.23, −1.50] |
| Afu6g10820 | −1.34 | [0.81, −2.48] | Afu2g17560 | 2.58 | [2.09, −0.67] |
| Afu8g02500 | −1.33 | [0.64, −2.31] | Afu1g17010 | 1.91 | [2.22, −1.58] |
| Afu1g01670 | −1.32 | [0.47, −2.08] | Afu7g01980 | 0.11 | [1.84, −2.33] |
| Afu3g11480 | −1.31 | [1.12, −2.81] | Afu3g07560 | 2.32 | [2.15, −1.03] |
| Afu8g06930 | −1.27 | [0.26, −1.73] | Afu6g12160 | 0.88 | [1.45, −1.09] |
| Afu1g01260 | −1.24 | [0.55, −2.09] | Afu5g09470 | 1.07 | [1.82, −1.45] |
| Afu6g02790 | −1.24 | [0.66, −2.23] | Afu3g13680 | 1.55 | [1.59, −0.62] |
| Afu2g00880 | −1.24 | [0.44, −1.95] | Afu1g12450 | 1.87 | [1.84, −0.72] |
| Afu7g08250 | −1.19 | [0.21, −1.58] | Afu2g08040 | 1.79 | [1.81, −0.81] |
| Afu5g01040 | −1.18 | [1.12, −2.70] | Afu6g09970 | −0.61 | [1.25, −2.20] |
| Afu5g01160 | −1.17 | [0.65, −2.17] | Afu8g01180 | 0.57 | [1.44, −1.32] |
| Afu8g04470 | −1.16 | [0.66, −2.13] | Afu6g11320 | 1.79 | [2.31, −1.80] |
| Afu7g00390 | −1.13 | [0.49, −1.91] | Afu3g01120 | −0.66 | [0.98, −1.94] |
| Afu5g00980 | −1.13 | [0.82, −2.31] | Afu1g10370 | 0.97 | [1.82, −1.53] |
| Afu7g06660 | −1.11 | [0.56, −1.99] | Afu6g13800 | −0.20 | [0.76, −1.27] |
| Afu7g06750 | −1.10 | [1.13, −2.61] | Afu1g06590 | 0.83 | [1.79, −1.63] |
| Afu6g03980 | −1.06 | [0.67, −2.05] | Afu2g16330 | 0.35 | [1.37, −1.47] |
| Afu3g03740 | −1.05 | [0.30, −1.57] | Afu6g02240 | 1.17 | [1.35, −0.63] |
| Afu5g01440 | −1.01 | [1.81, −3.52] | Afu6g12500 | 1.94 | [2.22, −1.50] |
| Afu6g09140 | −1.01 | [1.82, −3.52] | Afu5g11240 | 2.80 | [2.07,−0.45] |
| Afu6g04790 | −1.00 | [1.85, −3.53] | Afu5g02150 | −0.07 | [3.18, −6.33] |
| Afu3g13650 | −0.98 | [0.69, −2.00] | Afu8g05805 | −0.94 | [0.59, −1.85] |
| Afu4g02700 | −0.97 | [0.69, −2.00] | Afu4g13750 | 1.05 | [1.50, −0.99] |
| Afu1g00810 | −0.97 | [0.31, −1.50] | Afu4g06670 | 2.54 | [2.07, −0.68] |
| Afu6g00410 | −0.94 | [0.53, −1.76] | Afu7g00440 | 0.44 | [1.20, −1.12] |
| Afu7g01920 | −0.94 | [0.71, −1.97] | Afu1g02460 | 2.15 | [2.19, −1.21] |
| Afu7g07000 | −0.90 | [0.52, −1.73] | Afu3g08140 | 2.73 | [2.05, −0.47] |
| Afu8g01190 | −0.89 | [0.48, −1.66] | Afu1g17630 | 2.05 | [1.80, −0.57] |
| Afu5g09100 | −0.88 | [0.90, −2.10] | Afu1g12940 | 1.24 | [2.43, −2.75] |
| Afu6g09850 | −0.87 | [0.87, −2.09] | Afu6g10310 | 2.37 | [2.12, −0.96] |
| Afu8g04080 | −0.87 | [1.18, −2.43] | Afu1g00470 | 2.49 | [2.10, −0.79] |
| Afu4g08410 | −0.84 | [1.22, −2.41] | Afu1g13280 | 1.86 | [2.26, −1.68] |
| Afu7g00300 | −0.83 | [0.38, −1.48] | Afu7g00260 | 0.34 | [1.14, −1.19] |
| Afu4g14830 | −0.81 | [0.90, −2.04] | Afu4g14810 | 2.53 | [2.12, −0.76] |
| Afu8g06040 | −0.81 | [0.91, −2.05] | Afu2g02390 | 2.60 | [2.01, −0.54] |
| Afu3g12790 | −0.80 | [1.21, −2.35] | Afu4g11390 | 1.75 | [2.26, −1.85] |
| Afu2g07710 | −0.80 | [1.21, −2.39] | Afu1g09240 | 2.76 | [2.08, −0.45] |
| Afu4g01400 | −0.78 | [1.20, −2.34] | Afu3g02420 | 2.03 | [1.84, −0.63] |
| Afu2g12790 | −0.75 | [1.20, −2.34] | Afu6g09880 | 3.65 | [1.70, 1.01] |
| Afu1g09930 | −0.71 | [1.79, −3.13] | Afu6g10260 | 1.96 | [2.20, −1.41] |
| Afu5g01550 | −0.69 | [0.56, −1.54] | Afu4g14720 | 0.38 | [0.80, −0.74] |
| Afu7g01470 | −0.69 | [1.81, −3.08] | Afu1g10340 | 1.25 | [2.46, −2.73] |
| Afu6g10480 | −0.63 | [1.77, −3.02] | Afu8g03890 | 1.20 | [2.43, −2.79] |
| Afu5g01210 | −0.62 | [0.75, −1.70] | Afu3g02600 | 1.39 | [0.93, 0.03] |
| Afu3g03310 | −0.62 | [0.97, −1.91] | Afu5g05640 | 4.22 | [1.95, 1.07] |
| Afu1g03150 | −0.56 | [0.96, −1.83] | Afu1g05720 | 1.93 | [1.78, −0.69] |
| Afu8g02310 | −0.54 | [0.96, −1.85] | Afu4g01550 | 2.63 | [1.22, 0.78] |
| Afu3g01610 | −0.52 | [1.29, −2.15] | Afu4g09300 | 1.02 | [1.83, −1.46] |
| Afu1g15120 | −0.50 | [0.67, −1.48] | Afu6g12910 | 1.98 | [1.81, −0.62] |
| Afu3g00450 | −0.49 | [0.80, −1.61] | Afu1g12460 | 2.33 | [2.14, −1.01] |
| Afu2g07550 | −0.47 | [1.79, −2.85] | Afu1g13600 | −0.27 | [1.31, −1.95] |
| Afu6g09440 | −0.44 | [1.29, −2.06] | Afu8g04160 | 1.79 | [1.84, −0.82] |
| Afu5g02410 | −0.36 | [1.28, −2.02] | Afu3g07200 | 1.96 | [1.83, −0.67] |
| Afu2g16320 | −0.36 | [0.82, −1.5] | Afu4g06050 | 2.59 | [2.09, −0.65] |
| Afu7g08290 | −0.31 | [0.35, −0.89] | Afu7g07030 | 1.28 | [0.87, 0.02] |
| Afu5g09950 | −0.30 | [1.01, −1.66] | Afu1g10900 | 0.87 | [1.83, −1.59] |
| Afu7g05650 | −0.30 | [1.32, −1.99] | Afu7g04870 | 1.06 | [1.85, −1.46] |
| Afu8g00680 | −0.26 | [1.02, −1.64] | Afu3g03620 | 2.51 | [2.16, −0.81] |
| Afu6g09300 | −0.24 | [0.26, −0.69] | Afu4g14400 | 5.15 | [1.75, 2.31] |
| Afu6g00270 | −0.22 | [1.33, −1.91] | Afu1g11260 | 2.14 | [2.18, −1.23] |
| Afu5g01340 | −0.21 | [1.02, −1.57] | Afu4g08720 | 1.35 | [1.83, −1.23] |
| Afu5g00460 | −0.19 | [0.41, −0.86] | Afu6g13390 | 0.96 | [1.50, −1.06] |
| Afu4g03550 | −0.19 | [1.04, −1.58] | Afu6g04370 | 2.28 | [2.20, −1.14] |
| Afu8g02530 | −0.17 | [1.30, −1.86] | Afu4g04010 | 2.68 | [2.09, −0.58] |
| Afu4g04810 | −0.13 | [3.17, −6.33] | Afu2g15570 | −0.02 | [3.17, −6.24] |
| Afu1g13780 | −0.09 | [3.16, −6.21] | Afu2g13860 | −0.07 | [3.16, −6.21] |
| Afu1g04950 | −0.08 | [3.15, −6.19] | Afu5g06700 | 2.59 | [2.12, −0.68] |
| Afu5g06580 | −0.07 | [1.32, −1.79] | Afu4g11780 | 2.39 | [2.11, −0.89] |
| Afu7g05950 | −0.06 | [3.17, −6.21] | Afu6g14240 | 0.85 | [2.64, −3.79] |
| Afu6g03520 | −0.05 | [3.19, −6.20] | Afu2g01400 | 0.67 | [1.84, −1.79] |
| Afu5g09210 | −0.04 | [1.82, −2.44] | Afu7g04930 | 2.66 | [2.14, −0.66] |
| Afu5g11430 | 0.02 | [1.81, −2.36] | Afu1g02090 | 1.71 | [2.32, −1.93] |
| Afu3g08960 | 0.07 | [1.31, −1.68] | Afu4g03260 | 2.60 | [1.61, 0.19] |
| Afu4g01040 | 0.09 | [1.41, −1.70] | Afu6g02990 | 0.22 | [1.82, −2.22] |
| Afu3g11280 | 0.10 | [1.83, −2.33] | Afu8g01410 | 0.73 | [1.84, −1.77] |
| Afu3g02780 | 0.12 | [1.36, −1.66] | Afu5g00420 | 0.95 | [0.70, −0.10] |
| Afu2g11420 | 0.12 | [1.34, −1.64] | Afu2g12500 | 1.25 | [1.83, −1.29] |
| Afu4g08230 | 0.14 | [0.57, −0.74] | Afu2g02950 | 2.37 | [1.81, −0.32] |
| Afu4g11060 | 0.15 | [1.37, −1.61] | Afu3g15380 | 2.44 | [1.24, 0.62] |
| Afu5g00760 | 0.16 | [1.37, −1.59] | Afu4g04180 | 2.67 | [2.07, −0.60] |
| Afu8g00410 | 0.17 | [1.43, −1.66] | Afu2g01750 | 0.26 | [1.81, −2.17] |
| Afu2g01170 | 0.23 | [1.80, −2.19] | Afu2g05340 | 1.41 | [1.80, −1.14] |
| Afu3g10690 | 0.25 | [1.14, −1.26] | Afu1g10880 | 1.74 | [1.83, −0.88] |
| Afu8g05750 | 0.29 | [1.00, −1.05] | Afu3g01040 | 2.51 | [2.14, −0.84] |
| Afu4g03460 | 0.31 | [1.81, −2.11] | Afu1g17060 | 0.92 | [1.56, −1.11] |
| Afu6g03320 | 0.33 | [1.42, −1.52] | Afu1g12620 | 2.60 | [2.07, −0.61] |
| Afu6g11560 | 0.44 | [1.82, −2.01] | Afu2g15440 | 2.24 | [2.18, −1.18] |
| Afu2g04070 | 0.49 | [1.02, −0.91] | Afu6g03720 | 3.75 | [1.81, 0.91] |
| Afu4g00860 | 0.50 | [1.45, −1.40] | Afu6g12180 | 1.26 | [1.78, −1.29] |
| Afu2g04380 | 0.51 | [1.81, −1.90] | Afu3g15280 | 1.08 | [1.81, −1.43] |
| Afu3g00920 | 0.55 | [1.44, −1.33] | Afu6g14140 | 2.13 | [2.20, −1.28] |
| Afu4g03321 | 0.66 | [1.82, −1.82] | Afu7g06080 | 2.10 | [2.18, −1.29] |
| Afu2g10140 | 0.72 | [1.46, −1.23] | Afu3g14010 | 2.18 | [2.16, −1.22] |
| Afu4g00800 | 0.74 | [1.80, −1.71] | Afu4g00990 | 2.37 | [2.12, −0.95] |
| Afu5g06230 | 0.76 | [1.82, −1.74] | Afu4g03370 | 1.32 | [1.81, −1.21] |
| Afu2g11600 | 0.82 | [1.82, −1.65] | Afu1g13320 | 0.99 | [1.83, −1.53] |
| Afu5g13290 | 0.82 | [1.82, −1.68] | Afu2g15730 | 2.52 | [1.86, −0.18] |
| Afu3g11560 | 0.88 | [1.79, −1.58] | Afu2g13050 | 2.12 | [2.21, −1.32] |
| Afu6g02630 | 0.89 | [2.64, −3.68] | Afu1g15620 | 3.14 | [2.00, 0.06] |
| Afu6g07320 | 0.95 | [1.47, −1.04] | Afu6g09110 | 2.39 | [2.13, −0.91] |
| Afu6g08550 | 0.95 | [1.49, −1.05] | Afu6g00120 | 4.37 | [1.50, 1.96] |
| Afu4g11890 | 0.99 | [1.82, −1.47] | Afu6g02840 | 2.62 | [2.11, −0.68] |
| Afu8g07150 | 0.99 | [1.84, −1.52] | Afu1g16120 | 3.28 | [2.01, 0.14] |
| Afu8g05740 | 1.04 | [1.01, −0.39] | Afu2g00420 | 1.48 | [1.84, −1.07] |
| Afu5g10180 | 1.05 | [1.79, −1.46] | Afu4g01530 | 3.34 | [1.52, 1.07] |
| Afu4g01340 | 1.06 | [1.81, −1.38] | Afu1g11830 | 2.33 | [2.15, −1.05] |
| Afu8g02350 | 1.07 | [0.95, −0.29] | Afu3g02570 | 9.08 | [1.86, 5.79] |
| Afu5g05480 | 1.20 | [2.41, −2.77] | Afu3g10740 | 1.99 | [2.22, −1.47] |
| Afu2g10100 | 1.21 | [2.46, −2.83] | Afu1g06830 | 1.36 | [2.36, −2.45] |
| Afu3g01340 | 1.24 | [2.45, −2.71] | Afu2g15290 | 1.44 | [2.37, −2.37] |
| Afu5g11230 | 1.25 | [2.46, −2.74] | Afu5g12130 | 1.83 | [2.25, −1.71] |
| Afu8g04650 | 1.27 | [2.43, −2.75] | Afu2g17450 | 2.14 | [2.17, −1.22] |
| Afu8g06160 | 1.35 | [0.89, 0.06] | Afu1g01050 | 5.42 | [1.96, 2.18] |
| Afu6g05040 | 1.41 | [2.34, −2.37] | Afu5g01870 | 2.05 | [2.19, −1.37] |
| Afu1g14380 | 1.45 | [2.37, −2.34] | Afu7g00840 | 2.58 | [2.11, −0.66] |
| Afu4g12010 | 1.46 | [1.53, −0.66] | Afu1g00490 | 4.07 | [1.97, 0.91] |
| Afu4g13310 | 1.49 | [1.57, −0.69] | Afu5g12770 | 2.20 | [2.17, −1.20] |
| Afu1g00540 | 1.54 | [1.78, −1.00] | Afu8g04060 | 1.74 | [1.85, −0.86] |
| Afu2g10920 | 1.57 | [2.32, −2.09] | Afu3g03410 | 1.95 | [2.24, −1.52] |
| Afu8g04070 | 1.66 | [1.85, −0.92] | Afu1g00480 | 2.98 | [2.01, −0.19] |
| Afu3g07830 | 1.67 | [2.27, −1.88] | Afu1g06710 | 2.55 | [2.08, −0.69] |
| Afu2g10840 | 1.81 | [1.29, 0.01] | Afu6g11810 | 2.47 | [1.61, 0.10] |
| Afu4g12050 | 1.84 | [2.22, −1.62] | Afu1g00530 | 3.55 | [1.99, 0.39] |
| Afu4g09700 | 1.89 | [2.21, −1.61] | Afu6g07000 | 3.57 | [1.84, 0.71] |
| Afu8g04090 | 1.91 | [2.22, −1.58] | Afu1g00460 | 2.36 | [2.16, −0.99] |
| Afu8g04020 | 1.93 | [1.85, −0.71] | Afu1g00610 | 3.99 | [1.95, 0.88] |
| Afu3g01560 | 1.93 | [2.22, −1.55] | Afu8g02200 | 5.98 | [1.94, 2.69] |
| Afu2g01230 | 1.97 | [2.22, −1.46] | Afu8g02270 | 4.73 | [1.84, 1.78] |
| Afu3g13130 | 2.00 | [2.19, −1.45] | Afu8g01530 | 2.15 | [2.21, −1.29] |
| Afu8g04100 | 2.00 | [2.19, −1.45] | Afu1g00450 | 2.27 | [2.12, −1.05] |
| Afu4g07330 | 2.01 | [2.19, −1.46] | Afu2g11750 | 2.43 | [2.14, −0.87] |
| Afu1g05270 | 2.02 | [2.21, −1.44] | Afu4g09890 | 2.72 | [1.87, −0.07] |
| Afu1g01990 | 2.10 | [2.19, −1.37] | Afu6g12970 | 2.11 | [2.16, −1.27] |
| Afu1g01610 | 2.22 | [1.83, −0.41] | Afu4g02880 | 2.80 | [2.04, −0.42] |
| Afu6g07070 | 2.24 | [2.13, −1.11] | Afu6g11610 | 2.61 | [2.07, −0.63] |
| Afu1g00310 | 2.26 | [1.61, −0.07] | Afu3g07160 | 2.51 | [2.18, −0.85] |
| Afu2g14590 | 2.26 | [2.17, −1.13] | Afu2g02110 | 2.53 | [1.87, −0.19] |
| Afu7g06400 | 2.29 | [2.16, −1.08] | Afu8g05910 | 2.38 | [2.08, −0.87] |
| Afu3g03080 | 2.29 | [2.12, −1.05] | Afu6g14540 | 2.57 | [2.12, −0.76] |
| Afu1g11560 | 2.34 | [2.15, −1.04] | Afu3g14820 | 4.04 | [1.80, 1.19] |
| Afu8g04130 | 2.39 | [2.12, −0.88] | Afu1g00410 | 2.92 | [2.04, −0.26] |
| Afu1g16090 | 2.41 | [2.08, −0.83] | Afu5g15000 | 2.58 | [2.06, −0.63] |
| Afu8g04120 | 2.43 | [2.13, −0.86] | Afu1g00420 | 2.94 | [2.03, −0.26] |
| Afu8g04040 | 2.46 | [1.81, −0.25] | Afu1g00590 | 3.69 | [1.95, 0.59] |
| Afu7g00280 | 2.48 | [1.66, 0.04] | Afu8g05090 | 3.76 | [1.99, 0.62] |
| Afu5g07560 | 2.68 | [1.84, −0.06] | Afu4g14360 | 3.45 | [1.99, 0.31] |
| Afu2g17160 | 2.73 | [1.81, −0.03] | Afu3g13630 | 4.11 | [1.80, 1.25] |
| Afu5g15010 | 2.78 | [2.04, −0.39] | Afu1g16100 | 2.88 | [2.02, −0.28] |
| Afu6g14450 | 2.84 | [1.78, 0.11] | Afu3g01970 | 3.24 | [1.54, 0.89] |
| Afu5 g15020 | 2.86 | [2.09, −0.37] | Afu1g16110 | 3.05 | [2.03, −0.14] |
| Afu8 g06380 | 3.10 | [2.01, −0.04] | Afu7g08610 | 3.50 | [1.92, 0.39] |
| Afu5 g15040 | 3.14 | [2.06, −0.04] | Afu1g16170 | 3.16 | [2.04, −0.03] |
References
- 1.Grant D, Cregan P, Shoemaker RC. Genome organization in dicots: Genome duplication in Arabidopsis and synteny between soybean and Arabidopsis. P Natl Acad Sci U S A. 2000;97:4168–73. doi: 10.1073/pnas.070430597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidow A. Gen(om)e duplications in the evolution of early vertebrates. Curr Opin Genet Dev. 1996;6:715–22. doi: 10.1016/s0959-437x(96)80026-8. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe KH, Shields DC. Molecular evidence for an ancient duplication of the entire yeast genome. Nature. 1997;387:708–13. [Google Scholar]
- 4.Ohno S. Evolution by Gene Duplication. New York, NY: Springer-Verlag; 1970. [Google Scholar]
- 5.Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–5. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- 6.Han MV, Demuth JP, McGrath CL, Casola C, Hahn MW. Adaptive evolution of young gene duplicates in mammals. Genome Res. 2009;19:859–67. doi: 10.1101/gr.085951.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer A, Schartl M. Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol. 1999;11:699–704. doi: 10.1016/s0955-0674(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Gu Z, Li WH. Different evolutionary patterns between young duplicate genes in the human genome. Genome Biol. 2003;4:R56. doi: 10.1186/gb-2003-4-9-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dermitzakis ET, Clark AG. Differential selection after duplication in mammalian developmental genes. Mol Biol Evol. 2001;18:557–62. doi: 10.1093/oxfordjournals.molbev.a003835. [DOI] [PubMed] [Google Scholar]
- 10.Kondrashov FA, Rogozin IB, Wolf YI, Koonin EV. Selection in the evolution of gene duplications. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-2-research0008. RESEARCH0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conant GC, Wagner A. Asymmetric sequence divergence of duplicate genes. Genome Res. 2003;13:2052–8. doi: 10.1101/gr.1252603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes MK, Hughes AL. Evolution of Duplicate Genes in a Tetraploid Animal. Xenopus laevis Mol Biol Evol. 1993;10:1360–9. doi: 10.1093/oxfordjournals.molbev.a040080. [DOI] [PubMed] [Google Scholar]
- 13.Robinson-Rechavi M, Laudet V. Evolutionary rates of duplicate genes in fish and mammals. Mol Biol Evol. 2001;18:681–3. doi: 10.1093/oxfordjournals.molbev.a003849. [DOI] [PubMed] [Google Scholar]
- 14.Van de Peer Y, Taylor JS, Braasch I, Meyer A. The ghost of selection past: Rates of evolution and functional divergence of anciently duplicated genes. J Mol Evol. 2001;53:436–46. doi: 10.1007/s002390010233. [DOI] [PubMed] [Google Scholar]
- 15.Gaut BS, Zhang LQ, Vision TJ. Patterns of nucleotide substitution among simultaneously duplicated gene pairs in Arabidopsis thaliana. Mol Biol Evol. 2002;19:1464–73. doi: 10.1093/oxfordjournals.molbev.a004209. [DOI] [PubMed] [Google Scholar]
- 16.Li WH, Zhang P, Gu ZL. Different evolutionary patterns between young duplicate genes in the human genome. Genome Biol. 2003;4(9):R56. doi: 10.1186/gb-2003-4-9-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latge JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–50. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segal BH. Medical Progress Aspergillosis. New Engl J Med. 2009;360:1870–84. doi: 10.1056/NEJMra0808853. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ami R, Lewis RE, Kontoyiannis DP. Enemy of the (immunosuppressed) state: an update on the pathogenesis of Aspergillus fumigatus infection. British Journal of Haematology. 2010;150:406–17. doi: 10.1111/j.1365-2141.2010.08283.x. [DOI] [PubMed] [Google Scholar]
- 20.Hohl TM, Feldmesser M. Aspergillus fumigatus: Principles of pathogenesis and host defense. Eukaryot Cell. 2007;6:1953–63. doi: 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyer PS, Paoletti M. Reproduction in Aspergillus fumigatus: sexuality in a supposedly asexual species? Med Mycol. 2005;43:S7–14. doi: 10.1080/13693780400029015. [DOI] [PubMed] [Google Scholar]
- 22.O’Gorman CM, Fuller HT, Dyer PS. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature. 2009;457:471–4. doi: 10.1038/nature07528. [DOI] [PubMed] [Google Scholar]
- 23.Galagan JE, Calvo SE, Borkovich KA, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–68. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- 24.Goffeau A, Barrell BG, Bussey H, et al. Life with 6000 genes. Science. 1996;274:546, 563–47. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 25.Wood V, Gwilliam R, Rajandream MA, et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–80. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- 26.Wickes BL, Mayorga ME, Edman U, Edman JC. Dimorphism and haploid fruiting in Cryptococcus neoformans: Association with the alpha-mating type. P Natl Acad Sci U S A. 1996;93:7327–31. doi: 10.1073/pnas.93.14.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen K, Cox GM, Wang P, Toffaletti DL, Heitman J. Sexual cycle of Cryptococcus neoformans var. grubii and virulence of congenic a and alpha isolates. Infect Immun. 2003;71:4831–41. doi: 10.1128/IAI.71.9.4831-4841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedorova ND, Khaldi N, Joardar VS, et al. Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet. 2008;4:e1000046. doi: 10.1371/journal.pgen.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bustamante CD, Nielsen R, Sawyer SA, Olsen KM, Purugganan MD, Hartl DL. The cost of inbreeding in Arabidopsis. Nature. 2002;416:531–4. doi: 10.1038/416531a. [DOI] [PubMed] [Google Scholar]
- 30.Bustamante CD, Fiedel-Alon A, Williamson S, et al. Natural selection on protein-coding genes in the human genome. Nature. 2005;437:1153–7. doi: 10.1038/nature04240. [DOI] [PubMed] [Google Scholar]
- 31.Yang ZH. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–6. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 32.Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol. 2001;18:691–9. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- 33.Cai JJ, Smith DK, Xia X, Yuen KY. MBEToolbox 2.0: An enhanced version of a MATLAB toolbox for Molecular Biology and Evolution. Evol Bioinform. 2006;2:179–82. [PMC free article] [PubMed] [Google Scholar]
- 34.Bielawski JP, Yang Z. A maximum likelihood method for detecting functional divergence at individual codon sites, with application to gene family evolution. J Mol Evol. 2004;59:121–32. doi: 10.1007/s00239-004-2597-8. [DOI] [PubMed] [Google Scholar]
- 35.Li YF, Costello JC, Holloway AK, Hahn MW. “Reverse ecology” and the power of population genomics. Evolution. 2008;62:2984–94. doi: 10.1111/j.1558-5646.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubin GM, Yandell MD, Wortman JR, et al. Comparative genomics of the eukaryotes. Science. 2000;287:2204–15. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hughes AL, Friedman R. Gene duplication and the structure of eukaryotic genomes. Genome Res. 2001;11:373–81. doi: 10.1101/gr.155801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li WH, Gu ZL, Cavalcanti A, Chen FC, Bouman P. Extent of gene duplication in the genomes of Drosophila, nematode, and yeast. Mol Biol Evol. 2002;19:256–62. doi: 10.1093/oxfordjournals.molbev.a004079. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe KH, Seoighe C. Extent of genomic rearrangement after genome duplication in yeast. P Natl Acad Sci U S A. 1998;95:4447–52. doi: 10.1073/pnas.95.8.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu Z, Cavalcanti A, Chen FC, Bouman P, Li WH. Extent of gene duplication in the genomes of Drosophila, nematode, and yeast. Mol Biol Evol. 2002;19:256–62. doi: 10.1093/oxfordjournals.molbev.a004079. [DOI] [PubMed] [Google Scholar]
- 41.Friedman R, Hughes AL. Gene duplication and the structure of eukaryotic genomes. Genome Res. 2001;11:373–81. doi: 10.1101/gr.155801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dujon B, Sherman D, Fischer G, et al. Genome evolution in yeasts. Nature. 2004;430:35–44. doi: 10.1038/nature02579. [DOI] [PubMed] [Google Scholar]
- 43.Nelson MA, Kang S, Braun EL, et al. Expressed sequences from conidial, mycelial, and sexual stages of Neurospora crassa. Fungal Genet Biol. 1997;21:348–63. doi: 10.1006/fgbi.1997.0986. [DOI] [PubMed] [Google Scholar]
- 44.Galagan JE, Calvo SE, Borkovich KA, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003;422:859–68. doi: 10.1038/nature01554. [DOI] [PubMed] [Google Scholar]
- 45.Clutterbuck AJ. Genomic evidence of repeat-induced point mutation (RIP) in filamentous ascomycetes. Fungal Genet Biol. 2011;48:306–26. doi: 10.1016/j.fgb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Yang Z, Bielawski JP. Statistical methods for detecting molecular adaptation. Trends Ecol Evol. 2000;15:496–503. doi: 10.1016/S0169-5347(00)01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paoletti M, Rydholm C, Schwier EU, et al. Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Curr Biol. 2005;15:1242–8. doi: 10.1016/j.cub.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 48.Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol. 1994;11:725–36. doi: 10.1093/oxfordjournals.molbev.a040153. [DOI] [PubMed] [Google Scholar]
- 49.Muse SV, Gaut BS. A likelihood approach for comparing synonymous and nonsynonymous nucleotide substitution rates, with application to the chloroplast genome. Mol Biol Evol. 1994;11:715–24. doi: 10.1093/oxfordjournals.molbev.a040152. [DOI] [PubMed] [Google Scholar]
- 50.Gibbons JG, Beauvais A, Beau R, McGary KL, Latgé JP, Rokas A. Global transcriptome changes underlying colony growth in the opportunistic human pathogen Aspergillus fumigatus. Eukaryot Cell. 2012;11:68–78. doi: 10.1128/EC.05102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrier M, Bustamante CD, Yu JY, Purugganan MD. Selection on rapidly evolving proteins in the Arabidopsis genome. Genetics. 2003;163:723–33. doi: 10.1093/genetics/163.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawyer SA, Hartl DL. Population genetics of polymorphism and divergence. Genetics. 1992;132:1161–76. doi: 10.1093/genetics/132.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lynch M, Conery JS. The evolutionary demography of duplicate genes. Journal of Structural and Functional Genomics. 2003;3:35–44. [PubMed] [Google Scholar]
- 54.Blanc G, Wolfe KH. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. The Plant Cell. 2004;16:1667–78. doi: 10.1105/tpc.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krakaue DC, Nowak MA. Evolutionary preservation of redundant duplicated genes. Seminars in Cell and Developmental Biology. 1999;10:555–9. doi: 10.1006/scdb.1999.0337. [DOI] [PubMed] [Google Scholar]
- 56.Han MV, Demuth JP, McGrath CL, Casola C, Hahn MW. Adaptive evolution of young gene duplicates in mammals. Genome Res. 2009;19:859–67. doi: 10.1101/gr.085951.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hahn MW. Distinguishing among evolutionary models for the maintenance of gene duplicates. J Hered. 2009;100:605–17. doi: 10.1093/jhered/esp047. [DOI] [PubMed] [Google Scholar]
- 58.Kondrashov FA. Gene duplication as a mechanism of genomic adaptation to a changing environment. Proc R Soc B. 2012 Sep; doi: 10.1098/rspb.2012.1108. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–45. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar A, Ahmed R, Singh PK, Shukla PK. Identification of virulence factors and diagnostic markers using immunosecretome of Aspergillus fumigatus. J Proteomics. 2011;74:1104–12. doi: 10.1016/j.jprot.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Coyle CM, Panaccione DG. An ergot alkaloid biosynthesis gene and clustered hypothetical genes from Aspergillus fumigatus. Appl Environ Microb. 2005;71:3112–8. doi: 10.1128/AEM.71.6.3112-3118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kryazhimskiy S, Plotkin JB. The population genetics of dN/dS. PLoS Genet. 2008;4:e1000304. doi: 10.1371/journal.pgen.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wapinski I, Pfeffer A, Friedman N, Regev A. Natural history and evolutionary principles of gene duplication in fungi. Nature. 2007;449:54–61. doi: 10.1038/nature06107. [DOI] [PubMed] [Google Scholar]
- 64.Eyre-Walker A, Keightley PD. Estimating the rate of adaptive molecular evolution in the presence of slightly deleterious mutations and population size change. Mol Biol Evol. 2009;26:2097–108. doi: 10.1093/molbev/msp119. [DOI] [PubMed] [Google Scholar]
- 65.Charlesworth J, Eyre-Walker A. The McDonald-Kreitman test and slightly deleterious mutations. Mol Biol Evol. 2008;25:1007–15. doi: 10.1093/molbev/msn005. [DOI] [PubMed] [Google Scholar]
- 66.Nierman WC, Pain A, Anderson MJ, et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–6. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- 67.James TY, Kauff F, Schoch CL, et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature. 2006;443:818–22. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Lists of gene families in A. fumigatus used for parameter optimization of BLASTCLUST.
| Family | No of Genes | Gene ID |
|---|---|---|
| 1 | 21 | Afu8g06290, Afu2g18080, Afu8g01040, Afu8g00310, Afu6g00780, Afu3g09430, Afu4g02640, Afu4g14870, Afu6g00790, Afu6g09470, Afu6g14660, Afu7g08200, Afu4g00820, Afu5g00270, Afu6g09480, Afu6g14650, Afu3g09440, Afu4g14880, Afu3g15360, Afu4g14370, Afu7g06935 |
| 2 | 10 | Afu6g09480, Afu6g14650, Afu2g18080, Afu8g01040, Afu8g00310, Afu8g06290, Afu3g09440, Afu4g14880, Afu3g15360, Afu5g00270 |
| 3 | 9 | Afu6g03030, Afu2g01580, Afu6g03490, Afu2g15520, Afu6g11480, Afu1g13660, Afu2g00140, Afu3g02620, Afu3g03280 |
| 4 | 7 | Afu7g04180, Afu3g00680, Afu1g13440, Afu3g14590, Afu5g07360, Afu5g01470, Afu7g08470 |
| 5 | 6 | Afu7g04080, Afu4g10950, Afu1g12650, Afu2g11350, Afu6g14200, Afu8g04000 |
| 6 | 5 | Afu2g00530, Afu3g07550, Afu5g13060, Afu8g01120, Afu3g00670 |
| 7 | 5 | Afu2g11610, Afu2g11620, Afu7g06380, Afu8g07070, Afu3g07380 |
| 8 | 5 | Afu2g00710, Afu4g10130, Afu2g03230, Afu3g00900, Afu2g13460 |
| 9 | 5 | Afu8g07260, Afu2g17320, Afu3g07220, Afu3g00170, Afu6g00520 |
| 10 | 5 | Afu1g10910, Afu7g00250, Afu1g02550, Afu2g14990, Afu1g13390 |
| 11 | 4 | Afu1g11350, Afu5g09940, Afu3g03500, Afu4g01360 |
| 12 | 4 | Afu6g11890, Afu4g00540, Afu3g13580, Afu7g08580 |
| 13 | 4 | Afu1g14170, Afu3g00380, Afu1g16700, Afu6g06660 |
| 14 | 4 | Afu3g01440, Afu4g03340, Afu5g07620, Afu3g13940 |
| 15 | 4 | Afu1g10790, Afu7g04720, Afu6g13760, Afu5g10520 |
| 16 | 4 | Afu6g03890, Afu3g02270, Afu2g18030, Afu2g00200 |
| 17 | 4 | Afu5g00410, Afu3g00240, Afu2g15490, Afu5g00380 |
| 18 | 4 | Afu6g12950, Afu2g04010, Afu4g03190, Afu5g14300 |
| 19 | 4 | Afu1g12400, Afu5g03110, Afu1g12190, Afu5g02850 |
| 20 | 4 | Afu4g00730, Afu8g01530, Afu2g14320, Afu3g13130 |
| 21 | 3 | Afu2g11270, Afu3g00910, Afu1g15440 |
| 22 | 3 | Afu3g10910, Afu3g07030, Afu6g09910 |
| 23 | 3 | Afu1g17570, Afu1g04870, Afu6g03200 |
| 24 | 3 | Afu2g05880, Afu1g10930, Afu5g11020 |
| 25 | 3 | Afu8g00770, Afu7g05550, Afu2g09450 |
| 26 | 3 | Afu2g09520, Afu6g11600, Afu6g07480 |
| 27 | 3 | Afu3g01170, Afu7g01590, Afu5g13810 |
| 28 | 3 | Afu7g00420, Afu8g06100, Afu8g06590 |
| 29 | 3 | Afu2g00120, Afu2g03820, Afu2g00540 |
| 30 | 3 | Afu6g00500, Afu8g00930, Afu4g01290 |
| 31 | 2 | Afu3g07860, Afu4g14070 |
| 32 | 2 | Afu1g04780, Afu1g12910 |
| 33 | 2 | Afu2g07940, Afu6g10990 |
| 34 | 2 | Afu6g07640, Afu1g04460 |
| 35 | 2 | Afu1g11290, Afu5g14230 |
| 36 | 2 | Afu7g04800, Afu2g12640 |
| 37 | 2 | Afu8g06080, Afu4g03410 |
| 38 | 2 | Afu2g00920, Afu2g12770 |
| 39 | 2 | Afu7g05740, Afu6g05210 |
| 40 | 2 | Afu1g12050, Afu4g09540 |
| 41 | 1 | Afu1g01350 |
| 42 | 1 | Afu4g01470 |
| 43 | 1 | Afu5g04090 |
| 44 | 1 | Afu2g04580 |
| 45 | 1 | Afu1g06000 |
| 46 | 1 | Afu5g06690 |
| 47 | 1 | Afu5g08030 |
| 48 | 1 | Afu2g11880 |
| 49 | 1 | Afu3g12050 |
| 50 | 1 | Afu1g14980 |
Table S2.
Lists of gene families in C. neoformans used for parameter optimization of BLASTCLUST.
| Family | No of Genes | Gene ID |
|---|---|---|
| 1 | 9 | CNAG_00792T0, CNAG_00823T0, CNAG_01575T0, CNAG_02262T0, CNAG_02430T0, CNAG_02977T0, CNAG_03450T0, CNAG_03503T0, CNAG_07781T0 |
| 2 | 7 | CNAG_02883T0, CNAG_03130T0, CNAG_03315T0, CNAG_05348T0, CNAG_05968T0, CNAG_05998T0, CNAG_06606T0 |
| 3 | 6 | CNAG_00099T0, CNAG_03341T0, CNAG_03962T0, CNAG_04052T0, CNAG_05825T0, CNAG_06182T0 |
| 4 | 6 | CNAG_00550T0, CNAG_00770T0, CNAG_01642T0, CNAG_01916T0, CNAG_02682T0, CNAG_05201T0 |
| 5 | 6 | CNAG_00859T0, CNAG_02018T0, CNAG_06944T0, CNAG_07002T0, CNAG_07753T0, CNAG_07893T0 |
| 6 | 6 | CNAG_01495T0, CNAG_05320T0, CNAG_05321T0, CNAG_05329T0, CNAG_06530T0, CNAG_07626T0 |
| 7 | 5 | CNAG_00308T0, CNAG_03420T0, CNAG_03960T0, CNAG_04141T0, CNAG_04988T0 |
| 8 | 5 | CNAG_01500T0, CNAG_01714T0, CNAG_03389T0, CNAG_06249T0, CNAG_06876T0 |
| 9 | 5 | CNAG_04474T0, CNAG_06536T0, CNAG_06537T0, CNAG_06538T0, CNAG_06539T0 |
| 10 | 5 | CNAG_05369T0, CNAG_06931T0, CNAG_06936T0, CNAG_06985T0, CNAG_07707T0 |
| 11 | 4 | CNAG_00122T0, CNAG_01940T0, CNAG_02189T0, CNAG_05264T0 |
| 12 | 4 | CNAG_00575T0, CNAG_04981T0, CNAG_05015T0, CNAG_05256T0 |
| 13 | 4 | CNAG_00789T0, CNAG_00980T0, CNAG_03477T0, CNAG_05911T0 |
| 14 | 4 | CNAG_00862T0, CNAG_05319T0, CNAG_05376T0, CNAG_05383T0 |
| 15 | 4 | CNAG_00863T0, CNAG_02475T0, CNAG_04561T0, CNAG_07389T0 |
| 16 | 4 | CNAG_01373T0, CNAG_01740T0, CNAG_02196T0, CNAG_05925T0 |
| 17 | 4 | CNAG_01968T0, CNAG_03797T0, CNAG_07447T0, CNAG_07613T0 |
| 18 | 4 | CNAG_01840T0, CNAG_03787T0, CNAG_04948T0, CNAG_06914T0 |
| 19 | 4 | CNAG_02217T0, CNAG_03326T0, CNAG_06487T0, CNAG_07499T0 |
| 20 | 4 | CNAG_05276T0, CNAG_01699T0, CNAG_05690T0, CNAG_05563T0 |
| 21 | 3 | CNAG_00063T0, CNAG_04828T0, CNAG_06745T0 |
| 22 | 3 | CNAG_00919T0, CNAG_01040T0, CNAG_02966T0 |
| 23 | 3 | CNAG_01635T0, CNAG_02016T0, CNAG_04225T0 |
| 24 | 3 | CNAG_01681T0, CNAG_04276T0, CNAG_04982T0 |
| 25 | 3 | CNAG_02552T0, CNAG_06172T0, CNAG_07445T0 |
| 26 | 3 | CNAG_02958T0, CNAG_06241T0, CNAG_07865T0 |
| 27 | 3 | CNAG_03277T0, CNAG_05316T0, CNAG_06623T0 |
| 28 | 3 | CNAG_04326T0, CNAG_06374T0, CNAG_06638T0 |
| 29 | 3 | CNAG_05937T0, CNAG_05941T0, CNAG_07703T0 |
| 30 | 3 | CNAG_06297T0, CNAG_06298T0, CNAG_06388T0 |
| 31 | 2 | CNAG_01487T0, CNAG_01511T0 |
| 32 | 2 | CNAG_00005T0, CNAG_02012T0 |
| 33 | 2 | CNAG_02086T0, CNAG_02087T0 |
| 34 | 2 | CNAG_02241T0, CNAG_05453T0 |
| 35 | 2 | CNAG_02899T0, CNAG_03007T0 |
| 36 | 2 | CNAG_02896T0, CNAG_03311T0 |
| 37 | 2 | CNAG_03355T0, CNAG_05590T0 |
| 38 | 2 | CNAG_05450T0, CNAG_05454T0 |
| 39 | 2 | CNAG_06010T0, CNAG_06018T0 |
| 40 | 2 | CNAG_06524T0, CNAG_06821T0 |
| 41 | 1 | CNAG_01563T0 |
| 42 | 1 | CNAG_03763T0 |
| 43 | 1 | CNAG_04049T0 |
| 44 | 1 | CNAG_04610T0 |
| 45 | 1 | CNAG_05721T0 |
| 46 | 1 | CNAG_06603T0 |
| 47 | 1 | CNAG_06620T0 |
| 48 | 1 | CNAG_06828T0 |
| 49 | 1 | CNAG_07687T0 |
| 50 | 1 | CNAG_07766T0 |
Table S3.
Gamma and 59% CIs of MKPRF results for 202 duplicate gene pairs in A. fumigatus.
|
Gene 1
|
Gene 2
|
||||
|---|---|---|---|---|---|
| Gene ID | gamma | 95% CI | Gene ID | gamma | 95% CI |
| Afu2g12020 | −4.66 | [1.64, −8.35] | Afu4g10240 | −0.09 | [3.15, −6.15] |
| Afu5g04060 | −3.80 | [2.14, −8.08] | Afu3g12850 | 1.95 | [2.25, −1.52] |
| Afu5g02180 | −3.66 | [1.54, −7.36] | Afu2g07620 | 0.41 | [1.80, −2.04] |
| Afu5g14920 | −3.39 | [0.15, −3.69] | Afu5g07980 | 1.23 | [1.84, −1.33] |
| Afu1g00350 | −3.38 | [0.25, −3.90] | Afu8g06210 | 0.05 | [0.45, −0.67] |
| Afu5g05510 | −2.88 | [1.26, −5.66] | Afu7g03750 | 3.19 | [2.02, 0.03] |
| Afu6g11710 | −2.65 | [0.28, −3.19] | Afu7g08440 | 2.37 | [2.11, −0.90] |
| Afu3g02800 | −2.55 | [0.46, −3.42] | Afu6g02480 | 0.67 | [1.82, −1.82] |
| Afu2g17900 | −2.53 | [0.41, −3.30] | Afu2g00160 | −1.43 | [0.77, −2.58] |
| Afu1g16080 | −2.53 | [0.27, −3.04] | Afu5g14990 | 2.78 | [2.06, −0.45] |
| Afu5g00280 | −2.43 | [0.27, −2.94] | Afu2g05190 | −0.61 | [1.22, −2.18] |
| Afu7g01690 | −2.41 | [0.35, −3.07] | Afu8g05220 | 2.61 | [2.09, −0.65] |
| Afu3g00670 | −2.39 | [0.59, −3.44] | Afu5g13060 | 2.60 | [2.05, −0.60] |
| Afu6g12820 | −2.37 | [2.06, −6.42] | Afu2g12200 | 0.65 | [1.79, −1.77] |
| Afu6g02420 | −2.35 | [2.02, −6.26] | Afu6g13170 | −0.25 | [1.80, −2.65] |
| Afu2g17430 | −2.33 | [0.37, −3.01] | Afu5g09600 | 1.36 | [1.89, −1.24] |
| Afu6g09410 | −2.17 | [0.16, −2.47] | Afu7g05110 | 0.38 | [0.89, −0.87] |
| Afu4g08240 | −2.17 | [0.58, −3.18] | Afu2g13270 | 1.75 | [2.26, −1.84] |
| Afu2g00920 | −2.16 | [0.49, −3.04] | Afu2g12770 | 2.10 | [2.14, −1.22] |
| Afu1g17580 | −2.15 | [0.48, −3.02] | Afu3g15040 | 0.98 | [1.85, −1.53] |
| Afu2g08910 | −2.10 | [0.44, −2.88] | Afu1g02350 | −1.82 | [0.59, −2.78] |
| Afu8g06580 | −2.10 | [0.37, −2.77] | Afu8g04910 | 0.66 | [1.80, −1.76] |
| Afu1g16030 | −2.09 | [0.14, −2.35] | Afu5g14930 | −1.39 | [0.20, −1.76] |
| Afu8g06630 | −2.07 | [0.34, −2.68] | Afu1g16115 | 3.90 | [1.96, 0.73] |
| Afu6g14200 | −2.06 | [1.04, −3.71] | Afu1g12650 | 1.87 | [2.25, −1.64] |
| Afu8g01400 | −2.05 | [0.31, −2.61] | Afu1g09980 | 2.48 | [2.12, −0.80] |
| Afu5g09580 | −2.04 | [1.04, −3.71] | Afu1g17250 | 1.71 | [2.24, −1.77] |
| Afu4g00570 | −1.99 | [0.57, −2.96] | Afu3g02060 | −1.09 | [0.46, −1.83] |
| Afu1g03280 | −1.91 | [0.58, −2.88] | Afu6g11920 | −1.18 | [0.56, −2.04] |
| Afu2g17360 | −1.70 | [0.59, −2.63] | Afu4g02750 | 1.93 | [2.18, −1.48] |
| Afu5g11080 | −1.67 | [0.45, −2.42] | Afu2g15140 | −0.57 | [0.97, −1.86] |
| Afu5g00370 | −1.66 | [0.17, −1.97] | Afu5g00930 | −1.14 | [0.42, −1.83] |
| Afu8g04110 | −1.63 | [0.75, −2.76] | Afu1g00440 | 2.58 | [2.10, −0.70] |
| Afu5g02870 | −1.60 | [1.06, −3.13] | Afu5g09290 | 1.47 | [1.83, −1.11] |
| Afu5g14230 | −1.59 | [0.30, −2.13] | Afu1g11290 | 1.77 | [1.83, −0.88] |
| Afu8g01560 | −1.55 | [0.74, −2.68] | Afu2g11250 | −1.03 | [1.17, −2.58] |
| Afu2g13020 | −1.53 | [0.78, −2.68] | Afu6g07710 | 1.77 | [2.25, −1.78] |
| Afu6g09370 | −1.52 | [0.14, −1.78] | Afu1g00150 | −1.41 | [0.13, −1.66] |
| Afu4g02720 | −1.52 | [0.45, −2.27] | Afu3g00340 | 2.45 | [2.13, −0.91] |
| Afu7g06620 | −1.50 | [0.77, −2.64] | Afu4g00600 | 1.70 | [1.86, −0.89] |
| Afu3g12770 | −1.50 | [0.98, −2.89] | Afu1g14210 | 1.78 | [2.25, −1.78] |
| Afu1g00950 | −1.49 | [0.19, −1.84] | Afu7g07090 | 4.31 | [1.93, 1.24] |
| Afu4g10000 | −1.48 | [0.52, −2.33] | Afu2g03090 | −0.37 | [1.29, −2.02] |
| Afu5g03930 | −1.46 | [0.76, −2.61] | Afu1g11020 | 3.52 | [1.66, 0.93] |
| Afu5g01230 | −1.45 | [0.62, −2.40] | Afu3g01030 | 2.26 | [2.16, −1.11] |
| Afu7g08510 | −1.42 | [0.17, −1.73] | Afu1g00650 | 4.47 | [1.85, 1.48] |
| Afu4g14510 | −1.37 | [0.63, −2.37] | Afu2g11120 | 0.81 | [1.51, −1.17] |
| Afu3g10960 | −1.37 | [0.76, −2.49] | Afu2g03670 | 2.21 | [1.83, −0.47] |
| Afu1g16040 | −1.36 | [0.15, −1.63] | Afu5g14940 | −0.33 | [0.30, −0.84] |
| Afu8g06640 | −1.34 | [0.47, −2.11] | Afu3g02400 | 1.99 | [2.23, −1.50] |
| Afu6g10820 | −1.34 | [0.81, −2.48] | Afu2g17560 | 2.58 | [2.09, −0.67] |
| Afu8g02500 | −1.33 | [0.64, −2.31] | Afu1g17010 | 1.91 | [2.22, −1.58] |
| Afu1g01670 | −1.32 | [0.47, −2.08] | Afu7g01980 | 0.11 | [1.84, −2.33] |
| Afu3g11480 | −1.31 | [1.12, −2.81] | Afu3g07560 | 2.32 | [2.15, −1.03] |
| Afu8g06930 | −1.27 | [0.26, −1.73] | Afu6g12160 | 0.88 | [1.45, −1.09] |
| Afu1g01260 | −1.24 | [0.55, −2.09] | Afu5g09470 | 1.07 | [1.82, −1.45] |
| Afu6g02790 | −1.24 | [0.66, −2.23] | Afu3g13680 | 1.55 | [1.59, −0.62] |
| Afu2g00880 | −1.24 | [0.44, −1.95] | Afu1g12450 | 1.87 | [1.84, −0.72] |
| Afu7g08250 | −1.19 | [0.21, −1.58] | Afu2g08040 | 1.79 | [1.81, −0.81] |
| Afu5g01040 | −1.18 | [1.12, −2.70] | Afu6g09970 | −0.61 | [1.25, −2.20] |
| Afu5g01160 | −1.17 | [0.65, −2.17] | Afu8g01180 | 0.57 | [1.44, −1.32] |
| Afu8g04470 | −1.16 | [0.66, −2.13] | Afu6g11320 | 1.79 | [2.31, −1.80] |
| Afu7g00390 | −1.13 | [0.49, −1.91] | Afu3g01120 | −0.66 | [0.98, −1.94] |
| Afu5g00980 | −1.13 | [0.82, −2.31] | Afu1g10370 | 0.97 | [1.82, −1.53] |
| Afu7g06660 | −1.11 | [0.56, −1.99] | Afu6g13800 | −0.20 | [0.76, −1.27] |
| Afu7g06750 | −1.10 | [1.13, −2.61] | Afu1g06590 | 0.83 | [1.79, −1.63] |
| Afu6g03980 | −1.06 | [0.67, −2.05] | Afu2g16330 | 0.35 | [1.37, −1.47] |
| Afu3g03740 | −1.05 | [0.30, −1.57] | Afu6g02240 | 1.17 | [1.35, −0.63] |
| Afu5g01440 | −1.01 | [1.81, −3.52] | Afu6g12500 | 1.94 | [2.22, −1.50] |
| Afu6g09140 | −1.01 | [1.82, −3.52] | Afu5g11240 | 2.80 | [2.07,−0.45] |
| Afu6g04790 | −1.00 | [1.85, −3.53] | Afu5g02150 | −0.07 | [3.18, −6.33] |
| Afu3g13650 | −0.98 | [0.69, −2.00] | Afu8g05805 | −0.94 | [0.59, −1.85] |
| Afu4g02700 | −0.97 | [0.69, −2.00] | Afu4g13750 | 1.05 | [1.50, −0.99] |
| Afu1g00810 | −0.97 | [0.31, −1.50] | Afu4g06670 | 2.54 | [2.07, −0.68] |
| Afu6g00410 | −0.94 | [0.53, −1.76] | Afu7g00440 | 0.44 | [1.20, −1.12] |
| Afu7g01920 | −0.94 | [0.71, −1.97] | Afu1g02460 | 2.15 | [2.19, −1.21] |
| Afu7g07000 | −0.90 | [0.52, −1.73] | Afu3g08140 | 2.73 | [2.05, −0.47] |
| Afu8g01190 | −0.89 | [0.48, −1.66] | Afu1g17630 | 2.05 | [1.80, −0.57] |
| Afu5g09100 | −0.88 | [0.90, −2.10] | Afu1g12940 | 1.24 | [2.43, −2.75] |
| Afu6g09850 | −0.87 | [0.87, −2.09] | Afu6g10310 | 2.37 | [2.12, −0.96] |
| Afu8g04080 | −0.87 | [1.18, −2.43] | Afu1g00470 | 2.49 | [2.10, −0.79] |
| Afu4g08410 | −0.84 | [1.22, −2.41] | Afu1g13280 | 1.86 | [2.26, −1.68] |
| Afu7g00300 | −0.83 | [0.38, −1.48] | Afu7g00260 | 0.34 | [1.14, −1.19] |
| Afu4g14830 | −0.81 | [0.90, −2.04] | Afu4g14810 | 2.53 | [2.12, −0.76] |
| Afu8g06040 | −0.81 | [0.91, −2.05] | Afu2g02390 | 2.60 | [2.01, −0.54] |
| Afu3g12790 | −0.80 | [1.21, −2.35] | Afu4g11390 | 1.75 | [2.26, −1.85] |
| Afu2g07710 | −0.80 | [1.21, −2.39] | Afu1g09240 | 2.76 | [2.08, −0.45] |
| Afu4g01400 | −0.78 | [1.20, −2.34] | Afu3g02420 | 2.03 | [1.84, −0.63] |
| Afu2g12790 | −0.75 | [1.20, −2.34] | Afu6g09880 | 3.65 | [1.70, 1.01] |
| Afu1g09930 | −0.71 | [1.79, −3.13] | Afu6g10260 | 1.96 | [2.20, −1.41] |
| Afu5g01550 | −0.69 | [0.56, −1.54] | Afu4g14720 | 0.38 | [0.80, −0.74] |
| Afu7g01470 | −0.69 | [1.81, −3.08] | Afu1g10340 | 1.25 | [2.46, −2.73] |
| Afu6g10480 | −0.63 | [1.77, −3.02] | Afu8g03890 | 1.20 | [2.43, −2.79] |
| Afu5g01210 | −0.62 | [0.75, −1.70] | Afu3g02600 | 1.39 | [0.93, 0.03] |
| Afu3g03310 | −0.62 | [0.97, −1.91] | Afu5g05640 | 4.22 | [1.95, 1.07] |
| Afu1g03150 | −0.56 | [0.96, −1.83] | Afu1g05720 | 1.93 | [1.78, −0.69] |
| Afu8g02310 | −0.54 | [0.96, −1.85] | Afu4g01550 | 2.63 | [1.22, 0.78] |
| Afu3g01610 | −0.52 | [1.29, −2.15] | Afu4g09300 | 1.02 | [1.83, −1.46] |
| Afu1g15120 | −0.50 | [0.67, −1.48] | Afu6g12910 | 1.98 | [1.81, −0.62] |
| Afu3g00450 | −0.49 | [0.80, −1.61] | Afu1g12460 | 2.33 | [2.14, −1.01] |
| Afu2g07550 | −0.47 | [1.79, −2.85] | Afu1g13600 | −0.27 | [1.31, −1.95] |
| Afu6g09440 | −0.44 | [1.29, −2.06] | Afu8g04160 | 1.79 | [1.84, −0.82] |
| Afu5g02410 | −0.36 | [1.28, −2.02] | Afu3g07200 | 1.96 | [1.83, −0.67] |
| Afu2g16320 | −0.36 | [0.82, −1.5] | Afu4g06050 | 2.59 | [2.09, −0.65] |
| Afu7g08290 | −0.31 | [0.35, −0.89] | Afu7g07030 | 1.28 | [0.87, 0.02] |
| Afu5g09950 | −0.30 | [1.01, −1.66] | Afu1g10900 | 0.87 | [1.83, −1.59] |
| Afu7g05650 | −0.30 | [1.32, −1.99] | Afu7g04870 | 1.06 | [1.85, −1.46] |
| Afu8g00680 | −0.26 | [1.02, −1.64] | Afu3g03620 | 2.51 | [2.16, −0.81] |
| Afu6g09300 | −0.24 | [0.26, −0.69] | Afu4g14400 | 5.15 | [1.75, 2.31] |
| Afu6g00270 | −0.22 | [1.33, −1.91] | Afu1g11260 | 2.14 | [2.18, −1.23] |
| Afu5g01340 | −0.21 | [1.02, −1.57] | Afu4g08720 | 1.35 | [1.83, −1.23] |
| Afu5g00460 | −0.19 | [0.41, −0.86] | Afu6g13390 | 0.96 | [1.50, −1.06] |
| Afu4g03550 | −0.19 | [1.04, −1.58] | Afu6g04370 | 2.28 | [2.20, −1.14] |
| Afu8g02530 | −0.17 | [1.30, −1.86] | Afu4g04010 | 2.68 | [2.09, −0.58] |
| Afu4g04810 | −0.13 | [3.17, −6.33] | Afu2g15570 | −0.02 | [3.17, −6.24] |
| Afu1g13780 | −0.09 | [3.16, −6.21] | Afu2g13860 | −0.07 | [3.16, −6.21] |
| Afu1g04950 | −0.08 | [3.15, −6.19] | Afu5g06700 | 2.59 | [2.12, −0.68] |
| Afu5g06580 | −0.07 | [1.32, −1.79] | Afu4g11780 | 2.39 | [2.11, −0.89] |
| Afu7g05950 | −0.06 | [3.17, −6.21] | Afu6g14240 | 0.85 | [2.64, −3.79] |
| Afu6g03520 | −0.05 | [3.19, −6.20] | Afu2g01400 | 0.67 | [1.84, −1.79] |
| Afu5g09210 | −0.04 | [1.82, −2.44] | Afu7g04930 | 2.66 | [2.14, −0.66] |
| Afu5g11430 | 0.02 | [1.81, −2.36] | Afu1g02090 | 1.71 | [2.32, −1.93] |
| Afu3g08960 | 0.07 | [1.31, −1.68] | Afu4g03260 | 2.60 | [1.61, 0.19] |
| Afu4g01040 | 0.09 | [1.41, −1.70] | Afu6g02990 | 0.22 | [1.82, −2.22] |
| Afu3g11280 | 0.10 | [1.83, −2.33] | Afu8g01410 | 0.73 | [1.84, −1.77] |
| Afu3g02780 | 0.12 | [1.36, −1.66] | Afu5g00420 | 0.95 | [0.70, −0.10] |
| Afu2g11420 | 0.12 | [1.34, −1.64] | Afu2g12500 | 1.25 | [1.83, −1.29] |
| Afu4g08230 | 0.14 | [0.57, −0.74] | Afu2g02950 | 2.37 | [1.81, −0.32] |
| Afu4g11060 | 0.15 | [1.37, −1.61] | Afu3g15380 | 2.44 | [1.24, 0.62] |
| Afu5g00760 | 0.16 | [1.37, −1.59] | Afu4g04180 | 2.67 | [2.07, −0.60] |
| Afu8g00410 | 0.17 | [1.43, −1.66] | Afu2g01750 | 0.26 | [1.81, −2.17] |
| Afu2g01170 | 0.23 | [1.80, −2.19] | Afu2g05340 | 1.41 | [1.80, −1.14] |
| Afu3g10690 | 0.25 | [1.14, −1.26] | Afu1g10880 | 1.74 | [1.83, −0.88] |
| Afu8g05750 | 0.29 | [1.00, −1.05] | Afu3g01040 | 2.51 | [2.14, −0.84] |
| Afu4g03460 | 0.31 | [1.81, −2.11] | Afu1g17060 | 0.92 | [1.56, −1.11] |
| Afu6g03320 | 0.33 | [1.42, −1.52] | Afu1g12620 | 2.60 | [2.07, −0.61] |
| Afu6g11560 | 0.44 | [1.82, −2.01] | Afu2g15440 | 2.24 | [2.18, −1.18] |
| Afu2g04070 | 0.49 | [1.02, −0.91] | Afu6g03720 | 3.75 | [1.81, 0.91] |
| Afu4g00860 | 0.50 | [1.45, −1.40] | Afu6g12180 | 1.26 | [1.78, −1.29] |
| Afu2g04380 | 0.51 | [1.81, −1.90] | Afu3g15280 | 1.08 | [1.81, −1.43] |
| Afu3g00920 | 0.55 | [1.44, −1.33] | Afu6g14140 | 2.13 | [2.20, −1.28] |
| Afu4g03321 | 0.66 | [1.82, −1.82] | Afu7g06080 | 2.10 | [2.18, −1.29] |
| Afu2g10140 | 0.72 | [1.46, −1.23] | Afu3g14010 | 2.18 | [2.16, −1.22] |
| Afu4g00800 | 0.74 | [1.80, −1.71] | Afu4g00990 | 2.37 | [2.12, −0.95] |
| Afu5g06230 | 0.76 | [1.82, −1.74] | Afu4g03370 | 1.32 | [1.81, −1.21] |
| Afu2g11600 | 0.82 | [1.82, −1.65] | Afu1g13320 | 0.99 | [1.83, −1.53] |
| Afu5g13290 | 0.82 | [1.82, −1.68] | Afu2g15730 | 2.52 | [1.86, −0.18] |
| Afu3g11560 | 0.88 | [1.79, −1.58] | Afu2g13050 | 2.12 | [2.21, −1.32] |
| Afu6g02630 | 0.89 | [2.64, −3.68] | Afu1g15620 | 3.14 | [2.00, 0.06] |
| Afu6g07320 | 0.95 | [1.47, −1.04] | Afu6g09110 | 2.39 | [2.13, −0.91] |
| Afu6g08550 | 0.95 | [1.49, −1.05] | Afu6g00120 | 4.37 | [1.50, 1.96] |
| Afu4g11890 | 0.99 | [1.82, −1.47] | Afu6g02840 | 2.62 | [2.11, −0.68] |
| Afu8g07150 | 0.99 | [1.84, −1.52] | Afu1g16120 | 3.28 | [2.01, 0.14] |
| Afu8g05740 | 1.04 | [1.01, −0.39] | Afu2g00420 | 1.48 | [1.84, −1.07] |
| Afu5g10180 | 1.05 | [1.79, −1.46] | Afu4g01530 | 3.34 | [1.52, 1.07] |
| Afu4g01340 | 1.06 | [1.81, −1.38] | Afu1g11830 | 2.33 | [2.15, −1.05] |
| Afu8g02350 | 1.07 | [0.95, −0.29] | Afu3g02570 | 9.08 | [1.86, 5.79] |
| Afu5g05480 | 1.20 | [2.41, −2.77] | Afu3g10740 | 1.99 | [2.22, −1.47] |
| Afu2g10100 | 1.21 | [2.46, −2.83] | Afu1g06830 | 1.36 | [2.36, −2.45] |
| Afu3g01340 | 1.24 | [2.45, −2.71] | Afu2g15290 | 1.44 | [2.37, −2.37] |
| Afu5g11230 | 1.25 | [2.46, −2.74] | Afu5g12130 | 1.83 | [2.25, −1.71] |
| Afu8g04650 | 1.27 | [2.43, −2.75] | Afu2g17450 | 2.14 | [2.17, −1.22] |
| Afu8g06160 | 1.35 | [0.89, 0.06] | Afu1g01050 | 5.42 | [1.96, 2.18] |
| Afu6g05040 | 1.41 | [2.34, −2.37] | Afu5g01870 | 2.05 | [2.19, −1.37] |
| Afu1g14380 | 1.45 | [2.37, −2.34] | Afu7g00840 | 2.58 | [2.11, −0.66] |
| Afu4g12010 | 1.46 | [1.53, −0.66] | Afu1g00490 | 4.07 | [1.97, 0.91] |
| Afu4g13310 | 1.49 | [1.57, −0.69] | Afu5g12770 | 2.20 | [2.17, −1.20] |
| Afu1g00540 | 1.54 | [1.78, −1.00] | Afu8g04060 | 1.74 | [1.85, −0.86] |
| Afu2g10920 | 1.57 | [2.32, −2.09] | Afu3g03410 | 1.95 | [2.24, −1.52] |
| Afu8g04070 | 1.66 | [1.85, −0.92] | Afu1g00480 | 2.98 | [2.01, −0.19] |
| Afu3g07830 | 1.67 | [2.27, −1.88] | Afu1g06710 | 2.55 | [2.08, −0.69] |
| Afu2g10840 | 1.81 | [1.29, 0.01] | Afu6g11810 | 2.47 | [1.61, 0.10] |
| Afu4g12050 | 1.84 | [2.22, −1.62] | Afu1g00530 | 3.55 | [1.99, 0.39] |
| Afu4g09700 | 1.89 | [2.21, −1.61] | Afu6g07000 | 3.57 | [1.84, 0.71] |
| Afu8g04090 | 1.91 | [2.22, −1.58] | Afu1g00460 | 2.36 | [2.16, −0.99] |
| Afu8g04020 | 1.93 | [1.85, −0.71] | Afu1g00610 | 3.99 | [1.95, 0.88] |
| Afu3g01560 | 1.93 | [2.22, −1.55] | Afu8g02200 | 5.98 | [1.94, 2.69] |
| Afu2g01230 | 1.97 | [2.22, −1.46] | Afu8g02270 | 4.73 | [1.84, 1.78] |
| Afu3g13130 | 2.00 | [2.19, −1.45] | Afu8g01530 | 2.15 | [2.21, −1.29] |
| Afu8g04100 | 2.00 | [2.19, −1.45] | Afu1g00450 | 2.27 | [2.12, −1.05] |
| Afu4g07330 | 2.01 | [2.19, −1.46] | Afu2g11750 | 2.43 | [2.14, −0.87] |
| Afu1g05270 | 2.02 | [2.21, −1.44] | Afu4g09890 | 2.72 | [1.87, −0.07] |
| Afu1g01990 | 2.10 | [2.19, −1.37] | Afu6g12970 | 2.11 | [2.16, −1.27] |
| Afu1g01610 | 2.22 | [1.83, −0.41] | Afu4g02880 | 2.80 | [2.04, −0.42] |
| Afu6g07070 | 2.24 | [2.13, −1.11] | Afu6g11610 | 2.61 | [2.07, −0.63] |
| Afu1g00310 | 2.26 | [1.61, −0.07] | Afu3g07160 | 2.51 | [2.18, −0.85] |
| Afu2g14590 | 2.26 | [2.17, −1.13] | Afu2g02110 | 2.53 | [1.87, −0.19] |
| Afu7g06400 | 2.29 | [2.16, −1.08] | Afu8g05910 | 2.38 | [2.08, −0.87] |
| Afu3g03080 | 2.29 | [2.12, −1.05] | Afu6g14540 | 2.57 | [2.12, −0.76] |
| Afu1g11560 | 2.34 | [2.15, −1.04] | Afu3g14820 | 4.04 | [1.80, 1.19] |
| Afu8g04130 | 2.39 | [2.12, −0.88] | Afu1g00410 | 2.92 | [2.04, −0.26] |
| Afu1g16090 | 2.41 | [2.08, −0.83] | Afu5g15000 | 2.58 | [2.06, −0.63] |
| Afu8g04120 | 2.43 | [2.13, −0.86] | Afu1g00420 | 2.94 | [2.03, −0.26] |
| Afu8g04040 | 2.46 | [1.81, −0.25] | Afu1g00590 | 3.69 | [1.95, 0.59] |
| Afu7g00280 | 2.48 | [1.66, 0.04] | Afu8g05090 | 3.76 | [1.99, 0.62] |
| Afu5g07560 | 2.68 | [1.84, −0.06] | Afu4g14360 | 3.45 | [1.99, 0.31] |
| Afu2g17160 | 2.73 | [1.81, −0.03] | Afu3g13630 | 4.11 | [1.80, 1.25] |
| Afu5g15010 | 2.78 | [2.04, −0.39] | Afu1g16100 | 2.88 | [2.02, −0.28] |
| Afu6g14450 | 2.84 | [1.78, 0.11] | Afu3g01970 | 3.24 | [1.54, 0.89] |
| Afu5 g15020 | 2.86 | [2.09, −0.37] | Afu1g16110 | 3.05 | [2.03, −0.14] |
| Afu8 g06380 | 3.10 | [2.01, −0.04] | Afu7g08610 | 3.50 | [1.92, 0.39] |
| Afu5 g15040 | 3.14 | [2.06, −0.04] | Afu1g16170 | 3.16 | [2.04, −0.03] |