Abstract
Context:
Aerva lanata is not prescribed for long-term use in Ayurveda as it is believed to produce structural changes in the urinary tract of the patients leading to renal failure.
Aim:
To investigate the toxic effects of Aerva lanata on the structure and function of urinary tract of a rat model (Sprague-Dawley rats).
Settings and Design:
An experimental study. Thirty male and thirty female healthy rats were randomly assigned to three groups (test groups 1, 2 and control) and administered dried infusion of A. lanata 25g/200ml (low dose), 100g/200ml (high dose) and distilled water respectively, for 30 days.
Materials and Methods:
Blood and urine were collected and creatinine was measured. Creatinine clearance (Ccr) and urine flow rate (UFR) of rats were determined to assess renal function. Kidneys, ureters, and bladders of rats were harvested for light microscopic (LM) studies. Electron microscopic (EM) studies were done on kidney tissues.
Statistical Analysis Used:
Difference in mean values of Ccr and UFR between test groups and the control group were compared statistically using independent T test. LM and EM findings of the two treated groups (T1 and T2) were statistically compared with the control group (C) using standard normal distribution.
Results:
Ccr and UFR of test groups were not significantly different from that of the control group. LM studies did not show any histological changes suggesting toxicity. EM, however showed significant ultra structural changes in proximal convoluted tubular epithelial cells of rats in the two test groups.
Conclusion:
Administration of dried Aerva lanata for a period of one month did not produce significant effects on renal function of rats. However administration for the same period caused significant ultra structural changes in the proximal convoluted tubular epithelial cells.
Keywords: Aerva lanata, kidney, Sprague-Dawley rats, toxic effects
INTRODUCTION
Aerva lanata, known as polpala in Sri Lanka, is a medicinal plant belonging to the Amaranthaceae family. It is widely spread in the drier parts of the tropics and the sub tropics of the world.
Aerva lanata is commonly described in Ayurveda as a diuretic with anti-inflammatory, antihelmintic, anti-bacterial and mild analgesic effects. It is used in the treatment of lithiasis, cough, asthma, and headache and as an antidote for rat poisoning.[1–3] This herb is used as a decoction or as an herbal tea with or without other herbs in Ayurveda. It is believed that long-term ingestion of Aerva lanata is harmful, as it has adverse effects on the urinary tract.
Alkaloids, terpenoids, sterols, several flavonoid glycosides and polyphenols had been isolated from Aerva lanata by researchers in Russia and India.[4] Chemical composition of the different components of this herb is said to be different.[2,5] The compounds β-sitosterol, campesterol and chrysin have been isolated from Aerva lanata plants in Egypt and β-sitosterol, daucosterol, syringic acid, vanillic acid, feruloyltyramine and feruloylhomovanillylamine from plants in Russia.[6]
Diuretic effect of Aerva lanata has been investigated by three research groups in Sri Lanka during the last century. When it was given to humans in concentrations of 50g/200 ml and 100 g/200 ml, a significant increase in the urine volume was observed when compared with controls in one study in which the diuretic property was determined by measuring the urine output.[7]
A more comprehensive study by Goonaratna et al., measured electrolyte excretion (Na+ and K+), as most diuretics increase urine output and electrolyte excretion. They concluded that Aerva lanata does not produce diuresis, natriuresis, kaliuresis or change in urinary osmolar output.[8] However Goonaratna et al., have not mentioned the amount and the type of plant (dried or fresh) used to prepare the decoction used.
When the aqueous extracts of fresh and dried Aerva lanata were given to rats in a preliminary study, there was an increase in the urine output, urine osmolality and K+ excretion during the 4 h observation period. The study used concentrations of 50g/200ml and 100g/200ml of fresh and dried Aerva lanata (n=6 per group).[9] Effect of long-term administration of Aerva lanata on renal function and structure was also studied using a rat animal model with a limited sample size of 3 rats per group. Findings showed there was no statistically significant reduction in renal function as measured by creatinine clearance after administering two doses of dried and fresh Aerva lanata daily for a period of one month. However there was a marked reduction in creatinine clearance in rats fed with 100g/200ml of dried Aerva lanata. There were no significant histological changes detected by light microscopy in the kidneys, bladder and ureters of rats fed with Aerva lanata for one month. An ultra structural evaluation has not been performed.
Aerva lanata is not prescribed for more than a week in ayurveda medicine, in keeping with the general belief of producing structural changes in the urinary tract leading to renal failure. However as this is not scientifically proven, people continue to self medicate themselves with Aerva lanata.
To determine whether long-term administration of Aerva lanata produces toxic effects on the renal function and structure, an experimental study was designed on a rat model based on the guidelines set by the world health organization (WHO).[10]
MATERIALS AND METHODS
Animal model
Accordingly healthy, young, male and female Sprague-Dawley rats, aged three months, weighing 200±25g were obtained from an in-bred colony. Thirty males and thirty females were housed in cages in the animal house under tropical laboratory conditions and were provided with a standard laboratory diet and water.
Ethical clearance for animal experiments was obtained from the Ethics Review Committee of the Faculty of Medicine, University of Colombo and from the Ethical Review Committee of the Medical Research Institute, Colombo 08.
Plant material and sample preparation
Whole plants of Aerva lanata were purchased from a single herb plant supplier in the Colombo city. Botanical identity of the plant was determined by the principal investigator using herbarium specimens. A. lanata was used in the dried form to prepare the infusion, as it is the commonly used form by the people. The dried form also produced more diuretic activity than the fresh form in the preliminary investigation carried out by Herath et al.[9] To obtain dried Aerva lanata, the herb was dried by keeping in an oven at 60°C for a period of 6-7 h or more, until a constant weight (no further change in weight) was obtained (wet/ dry weight ratio was 6:1). Aerva lanata drink (infusion) was prepared using dried herb in 200 ml of hot water. The herb was steeped for 15 min and then the infusion was strained.
According to WHO criteria, several doses should be used to study the long-term toxic effects of an herb.[10] Of the two different dosages used (50g/200ml and 100g/200ml) in the preliminary study by Herath et al.,[9] a greater effect was noted with 100g/200ml concentration. Therefore, 100g/200ml of polpala was used as the high dose in this detailed study with 25g/200ml as the low dose. 25g/200ml was selected as this is the amount commonly prescribed by Ayurvedic physicians (personal communication) to prepare the infusion. Dosages for rats were calculated after converting human dosage (25g/200ml) according to body surface area calculations.[11] Following two solutions were prepared.
Solution 1 - infusion of A. lanata using 25 g of dried plant in 200 ml and Solution 2 - infusion of A. lanata using 100 g of dried plant in 200 ml.
Experiment on effects of long-term use of Aerva lanata
Baseline measurements of urine output for 5 days and creatinine clearance for 3 days were obtained prior to starting the experiment by collecting 24 h urine and 0.5 to 1 ml of venous blood from the tail veins of rats kept in separate metabolic cages. Sixty rats were randomly divided into three groups. Each group consisted of an equal number of male and female rats. Rats in different groups were treated as follows:
Test group 1 (T1) - Solution 1
Test group 2 (T2) - Solution 2
Control group (C) - Distilled water
All solutions were administered orally via a metal oro-gastric tube for 30 days at the dose rate of 18 ml/kg/d body weight of rat which were comparable to that given to humans on the basis of relative weight.[11]
Effects on renal function
Each solution was administered orally to the particular group daily for a period of 30 days. On the 30th day of the experiment, rats were kept separately in metabolic cages and urine was collected for 24 h. On the 31st day, 0.5 to 1 ml of blood was drawn from the tail vein of the rats and centrifuged to separate serum. Serum and urine samples were obtained to determine the creatinine clearance (Ccr) of each rat. Creatinine levels in 20 μl of serum and urine were determined spectrophotometrically in duplicates using a creatinine reagent kit (Cat. No. 510, RANDOX Laboratories Ltd, UK). Ccr [(creatinine concentration in urine mg/ml) x (urine flow rate in ml/min)/(creatinine concentration in serum mg/ml)] was calculated to assess the renal function as it would be altered in renal toxicity. Urine flow rate (UFR = Urine output in ml/24 × 60 min) of each rat was calculated.
Effects on water consumption
Water consumption of each rat was measured on a daily basis during the baseline period and on the 30th day of the study.
Effects on renal structure
On the 31st day of the experiment, rats were anesthetized by ether. The animals were dissected through a mid-ventral laparotomy to harvest kidneys, ureters and bladder. Specimens were fixed in modified Bouin's solution (micro-anatomical fixative- picric acid 75ml: formalin 25ml: glacial acetic acid 5ml) for light microscopic studies (LM). Only part of kidney samples were preserved in 2% gluteraldehyde solution for electron microscopic studies (EM). The animals were sacrificed by cardiac puncture after harvesting the organs.
Paraffin sections were made from fixed tissues after tissue processing. Left and right kidney sections (n=120) were stained with hematoxylene and eosin (HandE) (BDH Chemicals Ltd., Poole, England), periodic acid Schiff (PAS) (LOBA CHEMIE pvt. Ltd., Mumbai, India) and silver methamine (SM) stains (3% hexamine, 5% silver nitrate and 5% borax in 2:1:2 proportions) (BDH Chemicals Ltd., Poole, England). The bladder (n=60) and the ureters (n=120) were also stained with H and E and were evaluated for toxic epithelial damage and inflammation. The kidneys were evaluated for glomerular changes, tubular epithelial damage, interstitial inflammation/fibrosis and blood vessel changes.
Resin embedded tissue sections (n=120, 2 sections per kidney) made from left and right kidney samples preserved in 2% gluteraldehyde were processed and stained and studied electron microscopically. (Only thirty animals were selected by simple random sampling for electron microscopic evaluation. Left and right kidney samples of the selected animals in each group numbered T1=12, T2=12 and C=6). Sections were examined electron microscopically in order to detect any ultra structural changes in the glomeruli (altered basement membrane, epithelial, endothelial and mesangial cells) and proximal tubular epithelial cells suggesting toxicity (brush border alterations such as haziness /damage, swollen mitochondria and disappearance of mitochondrial cristae).
Statistical analysis
Mean values of Ccr, UFR and water consumption of baseline samples and final samples (after 30 days) in each group were calculated and were expressed as mean ± SEM. Difference in mean values of Ccr and UFR between the final samples and baseline samples in test groups and the control group were considered for the statistical comparison. Statistical comparisons were done using the SPSS computer package version 15 and independent T test was used for comparison. Significance was set at P<0.05.
Corresponding LM findings of kidney samples of the rats randomly selected for EM studies and the EM findings were expressed as percentages in each group. The two treated groups (T1 and T2) were then statistically compared with the control group (C) using standard normal distribution (SND). Significance was set at P <0.05.
RESULTS
No changes in animal behavior were observed during feeding time.
Effects on renal function
Mean values of Ccr, UFR and water consumption in baseline and final samples are shown in Figures 1–3. Difference in the mean values of Ccr, UFR and water consumption is summarized in Table 1. Difference in the mean value of Ccr after oral administration of dried Aerva lanata was not significant compared to the values in the control group (P= 0.062 for T1 and C, P= 0.487 for T2 and C). However, there is a marked reduction in the Ccr in T1 compared to the control. There is an increase in UFR of Group 1 and 2 treated with 25 g/200ml and 100 g /200ml of dried Aerva lanata compared to the control group. However, this difference is also not statistically significant (P= 0.949 for T1 and C, P= 0.548 for T2 and C).
Figure 1.
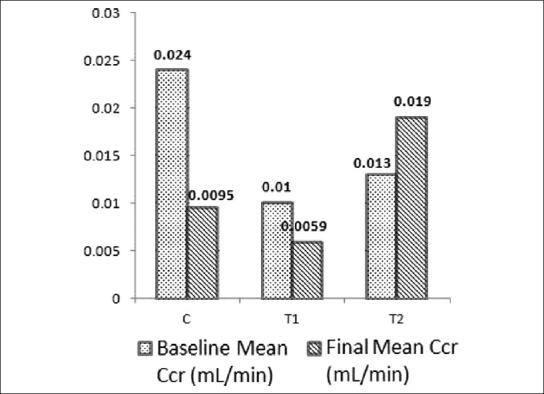
Mean values of creatinine clearance (Ccr) in baseline and final (after 30 days) samples of rats in the control group, C and test groups, T1 and T2
Figure 3.
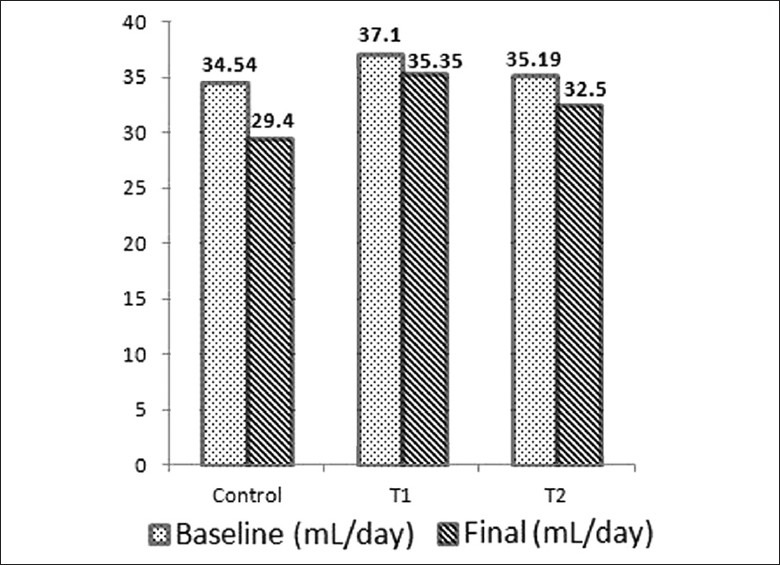
Mean values of water consumption of rats during baseline period and on the final day (after 30 days) in the control group, C and test groups, T1 and T2
Table 1.
Differences in the mean values of creatinine clearance, urine flow rate and water consumption in rats after feeding of dried infusion of Aerva lanata for 30 day period (Mean ± SEM)
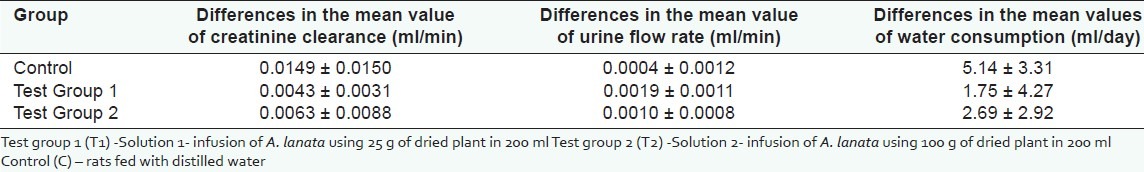
Figure 2.
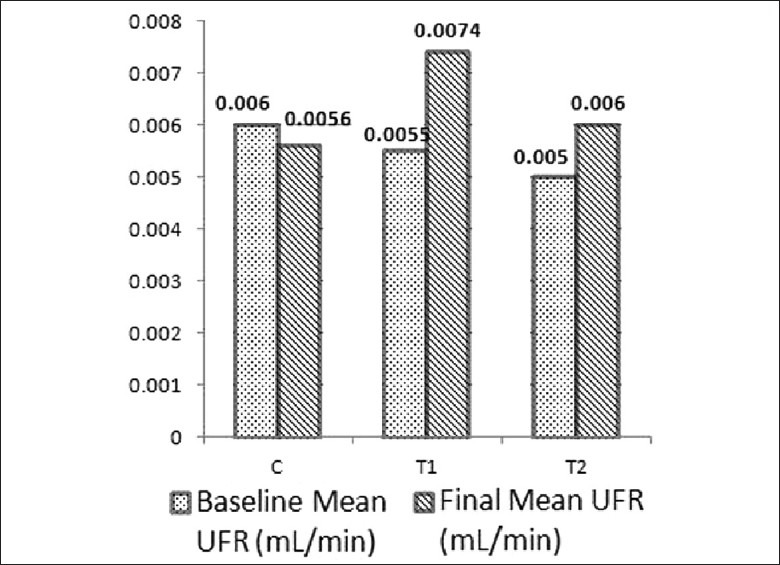
Mean values of urine flow rate (UFR) in baseline and final (after 30 days) samples of rats in the control group, C and test groups, T1 and T2
Effects on renal structure
Light microscopic studies
Light microscopically visible changes were not seen in the glomeruli of kidney specimens stained with HandE, PAS and SM. Hydropic swelling was observed in the tubular epithelial cells of rats in the test groups as well as in the control group. No toxic epithelial damage and inflammation were observed in the bladder and ureter samples in both the test groups and the control group.
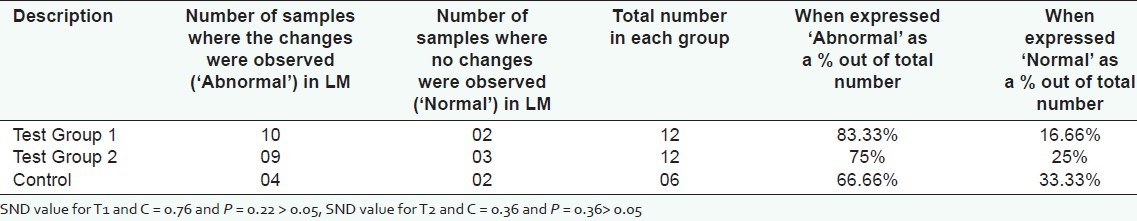
When LM findings of kidney samples of the rats randomly selected for EM studies were compared statistically, we could not find any statistical significance between test groups and the control group (P = 0.22 for T1 and C and P = 0.35 for T2 and C). Number of samples with and without tubular epithelial swelling are expressed as percentages after considering the total number in each group (T1 = 12, T2 = 12 and C = 6) and shown in Figure 4. Results are given with SND values in Table 2. (Though an equal number of animals were recruited to control and test groups, the sample size of each group was limited for electron microscopic studies by simple random sampling and only 30 samples were selected (T1=12, T2=12 and C=6). Swelling of renal tubular epithelial cells was observed in 28 (left and right kidneys of 14 animals) out of 40 samples (left and right kidneys of 20 animals) in the control group. When expressed ′abnormal′ as a % out of total number it is only 70%).
Figure 4.
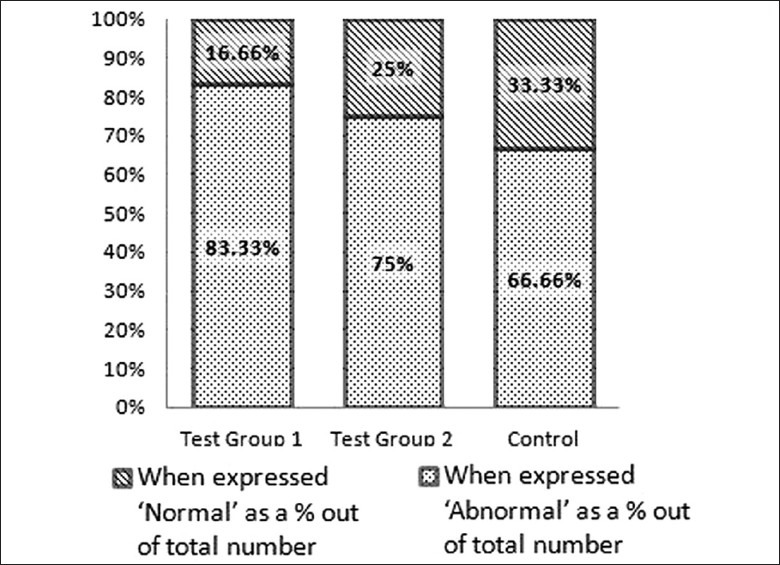
Light microscopic findings when expressed as percentages after comparing number of samples with and without any abnormalities in each group
Table 2.
Light microscopic changes in the tubular epithelium of kidney samples of randomly selected 30 rats after feeding with dried infusion of Aerva lanata for thirty days
Electron microscopic studies
Electron microscopic examination, however, showed statistically significant ultra structurally detectable changes in the proximal convoluted tubular epithelial cells (PCT) in the test groups, compared to the control group. The glomeruli in all three groups did not show any ultra structural changes. The PCT epithelial cells were considered normal when the brush border was intact, crisp and clear, and the cell organelles were intact in the epithelial cells. When the brush border was hazy or disrupted and/or cell organelles were altered (swollen mitochondria and hazy or absent cristae) in the PCT, it was considered as abnormal. Number of samples with and without abnormalities are expressed as percentages after considering the total number in each group (T1 = 12, T2 = 12 and C = 6) and shown in Figure 5. Results are given with SND values in Table 3. Figures 6–8 show EM appearance of normal glomeruli, proximal convoluted tubular epithelial cells and brush border. Electron microscopic changes observed in the kidney specimens of rats in the test group 2 are shown in 9 (a), 9 (b) and 9 (c).
Figure 5.
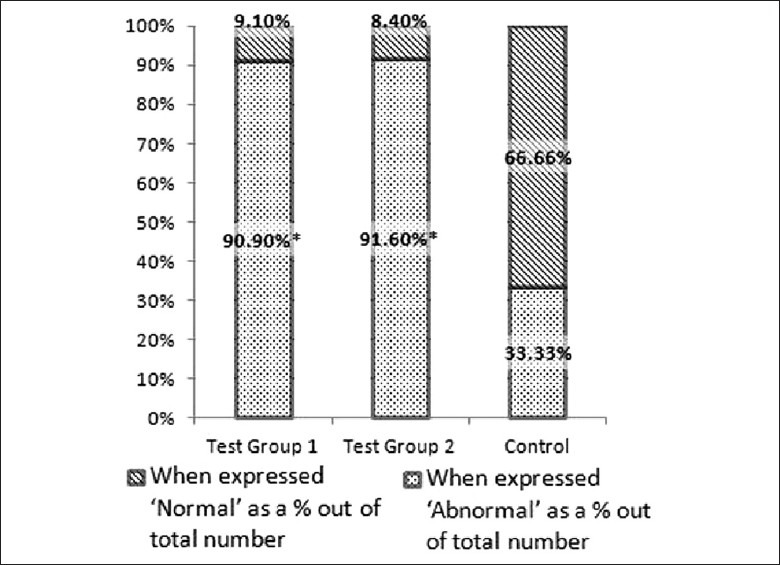
Electron microscopic findings when expressed as percentages after comparing number of samples with abnormalities and without any abnormalities in each group
Table 3.
Electron microscopic changes in proximal convoluted tubular epithelial cells in randomly selected 30 rats, after feeding with dried infusion of Aerva lanata for thirty days
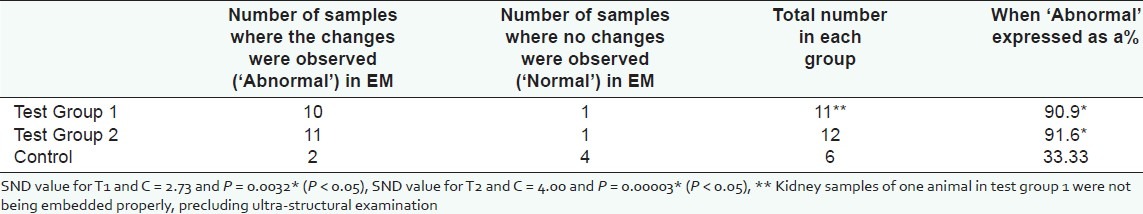
Figure 6.
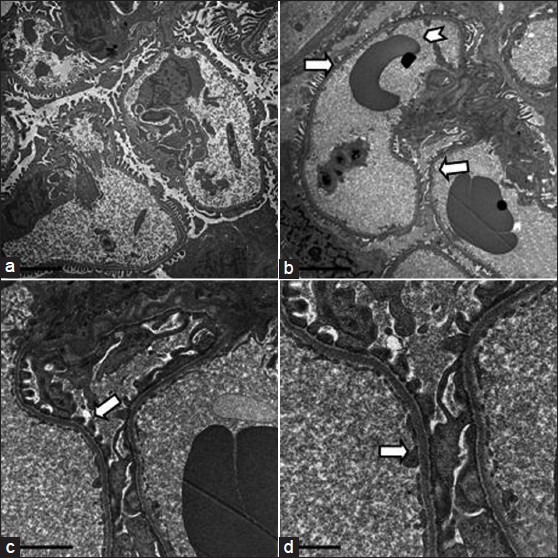
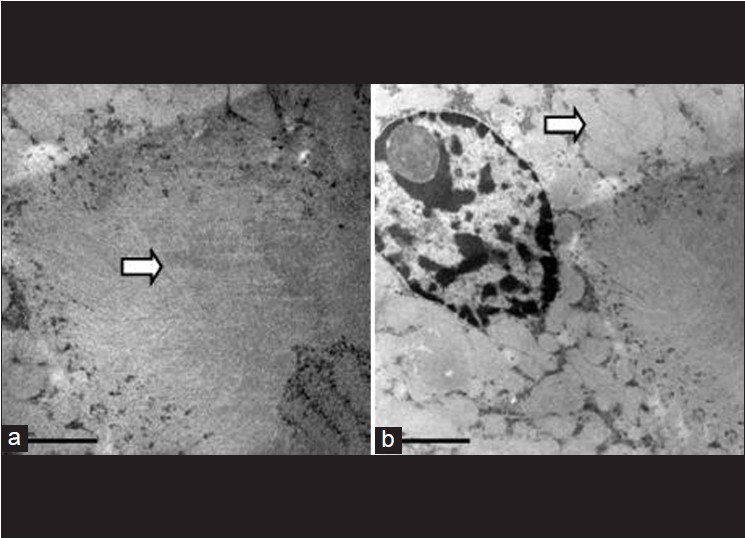
Electron microscopic appearance of a normal glomerulus. a. magnification -5000X b. intact basement membrane (arrow) without any immune complex deposits and red blood cells within capillaries (arrow head)- 6000X c. note foot processes of podocytes (arrow) - 15000X d. endothelial cells (arrow)- 25000X
Figure 8.
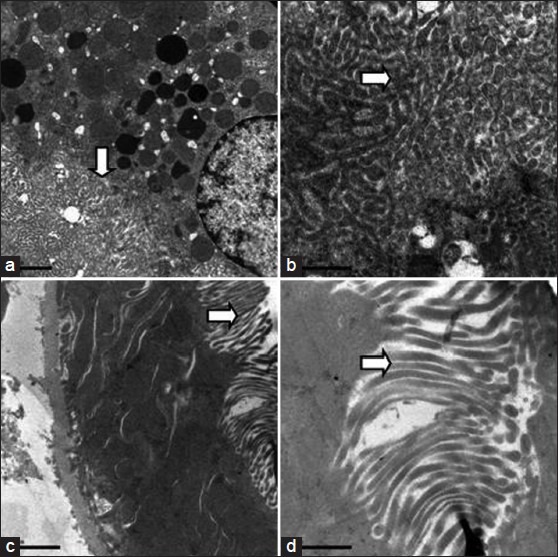
Electron microscopic appearance of normal brush border (arrow) in proximal convoluted epithelial cells at different magnifications a. 10000X b. 30000X c. 12000X d. 30000X
Figure 9 (a).
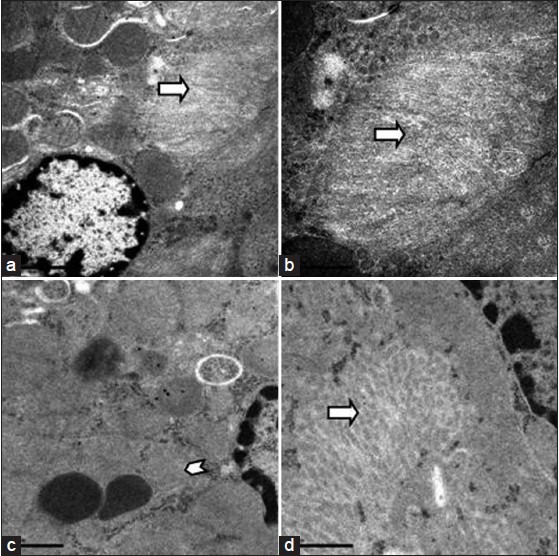
Electron microscopic appearance of damaged brush border (arrow) in the proximal convoluted epithelial cells in a kidney specimen of a rat in the test group a.15000X b. 30000X d. 30000X c. Disappearance of cristae of mitochondria (arrow head) in PCT epithelial cells 25000X
Figure 9 (b).
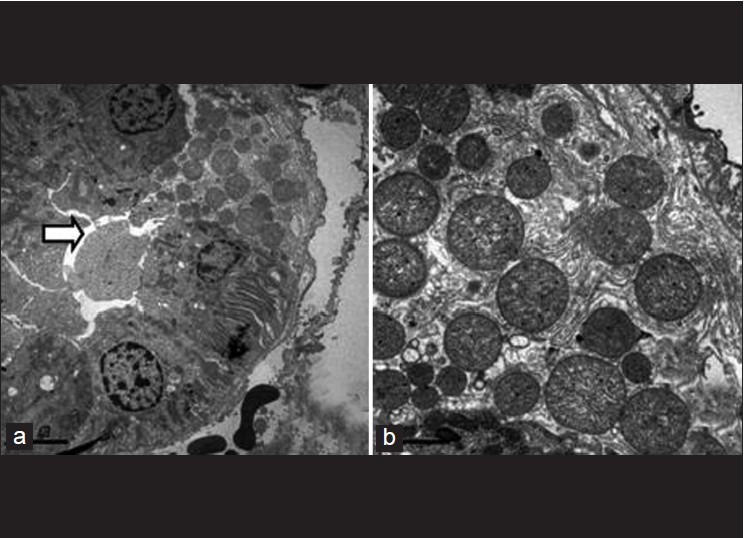
Completely degenerated brush border (arrow). a. 4000X Same field at a higher magnification b. 12000X -Epithelial cells appear normal with well preserved organelles despite brush border damage
Figure 9 (c).
The hazy appearance of the brush border in (a) and swollen mitochondria of PCT cells in (b). 15000X
Figure 7.
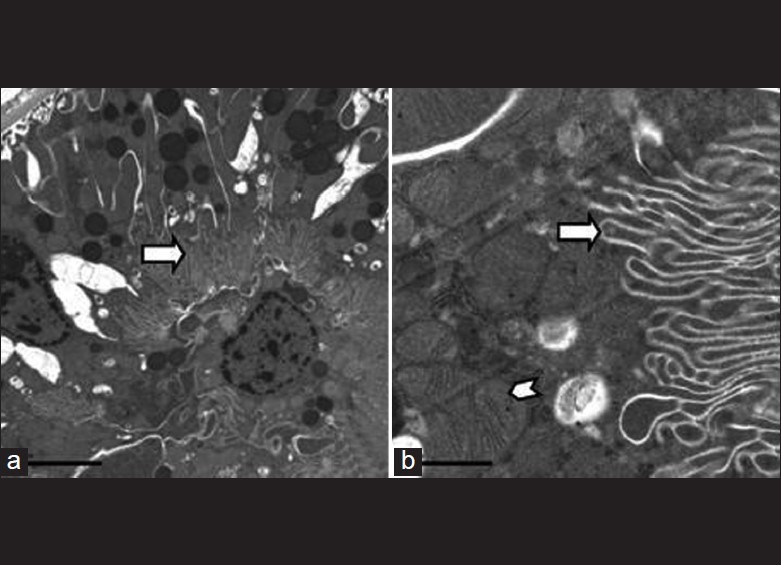
Electron microscopic appearance of normal proximal convoluted tubular epithelial cells with intact brush border (arrow) and preserved cell organelles (arrow head) a. 6000X b. 30000X
DISCUSSION
This is the first detailed experimental study investigating the effect of Aerva lanata on the structure and function of the urinary tract. The study using in-bred Sprague-Dawley rats show that long-term administration of dried infusion of Aerva lanata has no statistically significant effect on the renal function of rats, assessed by calculating the Ccr. However, Ccr was markedly reduced in T1 treated with dried infusion of Aerva lanata (25g/200ml). This result is similar to the results of the preliminary study conducted by Herath et al.[9] In the present study, however, we used dried infusion of Aerva lanata in contrast to the preliminary study, where an aqueous extract of Aerva lanata was used. Ccr could be reduced with an increase in serum creatinine concentration or with a reduction in the UFR. Marked reduction in Ccr that was observed is possibly not due to the reduction in the UFR, as increased UFR values were seen in the test groups in this study. Serum creatinine could be increased when there is a glomerular damage or with a reduction in the renal blood flow with reduced filtration of substances. Both these possibilities are unlikely in this case as the serum creatinine values are not significantly changed in the test groups (P for T1 and C = 0.75 and P for T2 and C= 0.40). This is further supported by the histopathological and ultra structural findings of normal glomeruli in the study. Reason for the marked reduction in Ccr in the test groups is unclear. During the study period, the behavior of rats was not observed continuously for 24 h except during the feeding time. If the behavior of the animal is altered (inactive or aggressive) due to the toxic effect of Aerva lanata after absorption into the system, this could have altered the amount of creatinine (decreased or increased) produced in the body due to muscle metabolism. However this is also unlikely as we did not find a significant difference among the serum creatinine values of test and control groups. The reduction in water consumption observed in test groups could also be related to alterations (reduction) in the behavior of rats for 24 h. We cannot be certain that this is the cause for the reduction in Ccr in the test groups. Dehydration also leads to a reduction in urinary creatinine. Thus the reason for the reduction in Ccr in the test groups is unclear. However as there was also no statistically significant difference in Ccr among the test and the control groups, we could conclude that Aerva lanata has no statistically significant effect on the renal function of rats based on the Ccr.
The marked increase in the UFR in test groups however could be related to the significant ultra-structural changes observed at the level of proximal convoluted tubular (PCT) epithelium. PCT is responsible for reabsorption of 60-65% of filtered water from the tubular lumen. Disrupted brush border and altered mitochondria may affect the reabsorption of solutes from the tubular lumen leading to a reduction in the passive reabsorption of water at the level of PCT. The ultra structural changes that we observed are unlikely to be due to ischemia. In toxicity, damage occurs to cell organelles without damaging the basement membrane as observed in this study.[12] The marked increase in the UFR may also be related to a reduction in the anti-diuretic hormone (ADH) secretion from the posterior pituitary, reducing ADH dependant water reabsorption from the collecting tubules. Reduction in the ADH secretion could occur with an increase in water consumption or in the presence of substances like alcohol directly affecting ADH secretion. Water consumption could not be a cause in this case as there is no significant increase in water consumption in the test groups compared to the control. Without analyzing chemically, it is not possible to comment about the presence of alcoholic compounds in the Sri Lankan variety of Aerva lanata. Reduction in the water consumption of rats in the test groups compared to the control group may also be related to alterations in the behavior of rats. When animals are less active or lethargic, their water consumption is reduced.
Though light microscopic examination showed hydropic swelling of proximal tubular epithelial cells in the two treated groups, the results were not significant in comparison to the control group. Therefore the hydropic swelling observed in renal tubular epithelial cells may be due to passive reabsorption of water by the renal tubules of animals in all three groups. The presence of significant ultra structural changes in the test groups of rats is important as these changes are expected to occur prior to light microscopically detected histological changes. The findings are also clinically useful as the changes are seen in both test groups (low dose and high dose) including the test group that consumed a similar dose (25g/200ml) to that commonly prescribed in indigenous medicine, however, for a longer period time (30 days).
As this study was carried out in a rodent species, the findings cannot be directly applied to humans. A human study of similar nature is also not feasible. However our findings on this animal model support the belief of the Ayurvedic physicians of not prescribing it for more than a week in the belief that it can damage the renal structure leading to renal failure in humans. Those people who tend to self medicate with Aerva lanata should be cautious in view of these findings, in a setting where many people still depend on Ayurvedic and traditional systems of health care. It would also be interesting and appropriate to study whether patients who present with chronic kidney disease of unknown etiology in the Sri Lankan setting have a history of prolonged Aerva lanata ingestion.
CONCLUSION
This study demonstrates that the administration of dried Aerva lanata at a concentration of 25g/200 ml and 100g/200ml for a period of one month has no statistically significant effects on the renal function of rats. However, consumption ofAerva lanata at similar concentrations for thirty days caused ultra structural changes in the proximal convoluted tubular epithelial cells of Sprague-Dawley rats.
Note: Mr S M C Senanayake (BSc, MSc) who was employed as the research assistant under the research grant RG/2004/TM/02, carried out the animal experiments. Preparation of Aerva lanata infusions, feeding of animals, sample collection, harvesting of organs, determination of creatinine in urine and serum and calculation of Ccr were done by him.
ACKNOWLEDGEMENTS
We wish to acknowledge the staff of University of Colombo namely, Mr. Kamal Perera of Animal House, for the assistance given to carry out animal studies, Dr. Nalika Gunawardana and Ms. Kantha Lankathilaka, Senior lecturers in the Department of Community Medicine for providing statistical advice and Mr. S. M. Priyantha Seneviratne, Technical Officer, Department of Microbiology for the support extended towards the study. Dr. L Rasheed, former Director and Dr. Nishali Ekanayake, Head, Department of Pathology of Medical Research Institute, Colombo 08 are acknowledged for providing the opportunity to develop kidney samples for electron microscopic studies in the EM lab. We thank Ms. Kowther Al Adawi and Mr. Mohammed Al Kindi, the biomedical scientists at the Electron Microscopy unit, Sultan Qaboos University, Muscat, Oman for the technical assistance for electron microscopy and Mr. Issa Al Amri the Head, Electron Microscopy unit, at the Sultan Qaboos University, Muscat, Oman for the support extended towards this study.
Footnotes
Source of Support: We gratefully acknowledge the financial assistance provided by the National Science Foundation (Grant number RG/2004/TM/02) of Sri Lanka and Sultan Qaboos University, Oman (SQU grant number MREC# 287 in 2009) by way of research grants.
Conflict of Interest: No.
REFERENCES
- 1.Jayaweera DM. Colombo: The National Science Council of Sri Lanka; 1981. Medicinal plants (indigenous and exotic) used in Ceylon-Part I; pp. 40–1. [Google Scholar]
- 2.Prasad KV, Sankarasubramanian S, Guruswamy MN. Pharmacognostical studies on the roots of Aerva lanata. Arogya. 1986;12:6–13. [Google Scholar]
- 3.Rajesh R, Chitra K, Padmaa M, Paarakh In vitro anthelmintic activity of aerial parts of Aerva lanata Linn Juss. Int J Pharm Sci Drug Res. 2010;2:269–71. [Google Scholar]
- 4.Jayasingha P, Warnasuriya D, Dissanayake H. Colombo: Industrial Technology Institute; 1999. Aerva lanata: A literature Survey-Medicinal and aromatic plant series; pp. 1–7. [Google Scholar]
- 5.Zapesochnaya GG, Pervykh LN, Kurkin VA. A study of the herb Aerva lanata III.Alkaloids. Chem Nat Compd. 1991;27:336–40. [Google Scholar]
- 6.Pervykh LN, Karasartov BS, Zapesochnaya GG. A study of the herb Aerva lanata IV.Flavonoid glycosides. Chem Nat Compd. 1992;28:509–10. [Google Scholar]
- 7.Udupihille M, Jiffry MT. Diuretic Effect of Aerua lanata with water, normal saline and coriander as Controls. Indian J Physiol Pharmacol. 1986;30:91–7. [PubMed] [Google Scholar]
- 8.Goonaratna C, Thabrew I, Wijewardena K. Does Aerua lanata have diuretic properties? Indian J Physiol Pharmacol. 1993;37:135–7. [PubMed] [Google Scholar]
- 9.Herath HM, Gunatilake M, Lokuhetty D, Wijayabandara J. A preliminary investigation on the effects of polpala (Aerva lanata) on the structure and function of urinary tract of rats. Ceylon J Med Sci. 2005;48:33–41. [Google Scholar]
- 10.Research guidelines for evaluating the safety and efficacy of herbal medicines. Geneva: World Health Organization; 2000. In, General guidelines for methodologies on research and evaluation of traditional medicine; pp. 27–31. [Google Scholar]
- 11.Paget GE, Barnes JM. Evaluation of Drug activities-Pharmacometrics. In: Laurence DR, Bacharach AL, editors. Toxicity tests. London: Academic Press; 1964. p. 135. [Google Scholar]
- 12.Mitchell J. Basic Pathology. In: Kumar V, Cotran RS, Robbins SL, editors. The kidney and its collecting system. 7th ed. New York: WB Saunders Company; 2002. pp. 509–42. [Google Scholar]


