Abstract
Background:
Herbal remedies of Adenia cissampeloides, Terminalia ivorensis, and Elaeis guineensis among others have been used in Ghana for the treatment of various ailments including malaria. However, most of these remedies have not been scientifically investigated.
Objective:
This study, therefore, seeks to investigate the anti-plasmodial activity of these plants.
Materials and Methods:
The ethanolic extracts of A. cissampeloides stem, T. ivorensis stem bark, and E. guineensis leaves were tested for in vitro anti-plasmodial activity against chloroquine-resistant strains of Plasmodium falciparum. Thin blood films were used to assess the level of parasitemia and growth inhibition of the extracts.
Results:
The IC 50 of A. cissampeloides, T. ivorensis, and E. guineensis were 8.521, 6.949, and 1.195 μg/ml, respectively, compared to artesunate with IC50 of 0.031 μg/ml.
Conclusion:
The result of this study appears to confirm the folkloric anti-malarial use these plants.
Keywords: Adenia cissampeloides, anti-plasmodial, Elaeis guineensis, herbal remedies, Terminalia ivorensis
INTRODUCTION
Malaria has been a threat to humans for many years. Despite the many advances in both preventive and curative treatments in the 20th Century, malaria is still a major killer today, and deaths from the disease have increased in the past three decades.[1] There are 350 to 500 million clinical episodes of malaria each year. It causes over 1 million deaths and is the eighth most important disease in terms of lost disability-adjusted life years.[2]
Indigenous plants play an important role in the treatment of a variety of diseases. About 80% of the population in many developing countries including Ghana, depend on plants as accessible, first line, and cost-effective therapy for malaria. However, many of these plants have not been systematically investigated as anti-malarials. Among the many species of plants employed in malaria remedies[3] in Ghana are Adenia cissampeloides (Planch.) Harms, Termina liaivorensis A. Chev., and Elaeis guineensis Jacq.[4] A. cissampeloidesis a climber. Other common uses of the plant include the treatment of dysentery, rheumatic pain relief,[5] hypertension, and numbness.[6] T. ivorensis is appreciated mainly for its timber. However,the stem bark is also employed in wound healing and as a remedy for rheumatism.[7] Other folklore uses of E. guineensis include treatment of gonorrhea, headache, rheumatism diuretic and an aphrodisiac[8] and wound healing.[9,10] The dried leaves have been reported to possess hemostatic properties.[11]
This study, therefore, seeks to verify the anti-plasmodial properties of these plants in order to justify their use in malaria remedies.
MATERIALS AND METHODS
Collection of plant materials
The plant materials were collected in February, 2011 from Kumasi in the Ashanti Region of Ghana and authenticated at the Department of Pharmacognosy, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. Voucher specimen of each plant (A. cissampeloides: FP/094/10; T. ivorensis: FP/095/10; E. guineensis: FP/096/10) have been deposited at the Herbarium of the same department.
Phytochemical screening
The presence of secondary metabolites in the powdered samples (saponins, reducing sugars, flavonoids, glycosides, steroids, terpenoids, and alkaloids) was investigated following simple qualitative methods.[14]
Preparation of plant extracts
The stem bark of T. ivorensis, whole plant of A. cissampeloides, and leaves of E. guineensis were washed and air-dried under room temperature for 7 days. The dried samples were ground to coarse powder, and each plant material (500 g) cold macerated with 70% ethanol (2 liters) at room temperature for 3 days with intermittent shaking. The percolate was evaporated into a syrupy mass under reduced pressure using a rotary evaporator and vacuum- dried for 24 hours to give a yield of 15.6%, 19.4%, and 12.8% w/w, respectively, for T. ivorensis, A. cissampeloides, and E. guineensis. The crude extracts were kept in a desicator until required for use.
Plasmodium falciparum culture and maintenance
In vitro anti-plasmodial activity of the plant extracts were assessed at the Immunology Department of the Noguchi Memorial Institute for Medical Research, Legon, Ghana. Plasmodium parasites were grown and maintained in culture using the Trager and Jensen method with some modifications[12] using P. falciparum chloroquine resistant strain, 3D7. The parasites were maintained in vitro in human O-positive red blood cells using RPMI 1640 medium supplemented with 25mM HEPES and 25 Mm NaHCO 3 and were complemented with 2% human O-positive serum and 10 mg/ml Gentamycin. The cultures were gassed with 5.5% CO2, 2% O2 , and 92.5% N2 , and incubated at 37°C. Culture flask with 3D7 parasite strains was maintained by the daily change of culture media and preparation of thin RBC smears to monitor parasite growth.
Preparation of sterile extracts
A stock solution of 100 μg/ml was prepared from the crude ethanolic extract of each plant material and filtered with millipore filter of size 0.2 μg (Carrigtwohill, Ireland) under sterile conditions. Four-fold serial dilutions were prepared from each stock solution using Complete Parasite Medium, resulting in concentrations ranging from 100 - 0.0976562 μg/ ml. Artesunate (Sigma-Aldrich, USA; 98% purity), a known standard anti-malarial drug, was used as a positive control.
Establishment of anti-plasmodial activities of extracts
The in vitro inhibition assay used was a modification of the semi-automatic microdilution technique developed by Desjardins et al.[13] The cultures were treated with the selected concentrations of the extracts in two 24-well microtitre plates (Greiner Bio-One, Belgium) and incubated for 72 hours at 37° C. Thin blood smears were prepared on properly labeled slides. The blood smears were air-dried and fixed in methanol. The dried slides were Giemsa stained and observed with 100x microscopic lens in immersion oil using a light microscope.
Statistical analysis of data
Growth inhibition due to each extract defined as the difference between the % parasitemia of each treatment group, and the corresponding positive control was calculated as follows:[14]
![]()
Where CIRBC = % parasitemia of infected RBC without extracts i.e. control
DIRBC = % parasitemia of infected RBCs incubated with extract or standard drug
Total parasitemia over the 48 hour period was calculated in arbitrary unit as the area under the curve (AUC). Differences in AUCs were analyzed by one-way analysis of variance (ANOVA) followed by Student-Newman-Keuls′ post hoc t test.
Doses and concentrations responsible for 50% of the maximal effect (IC 50) for each drug/extract were determined using an iterative computer least squares method, with the following non-linear regression (three- parameter logistic) equation.

Where, X is the logarithm of dose and Y is the response. Y starts at a (the bottom) and goes to b (the top) with a sigmoid shape.[15,16] Using regression equations of best fit of plotted growth inhibition versus concentration curves, the IC50 of each plant extract against each of the parasite strains were obtained. Graph Pad Prism for Windows version 5.0 (Graph Pad Software, San Diego, CA, USA) was used for all statistical analyzes. P < 0.05 was considered statistically significant.
RESULTS
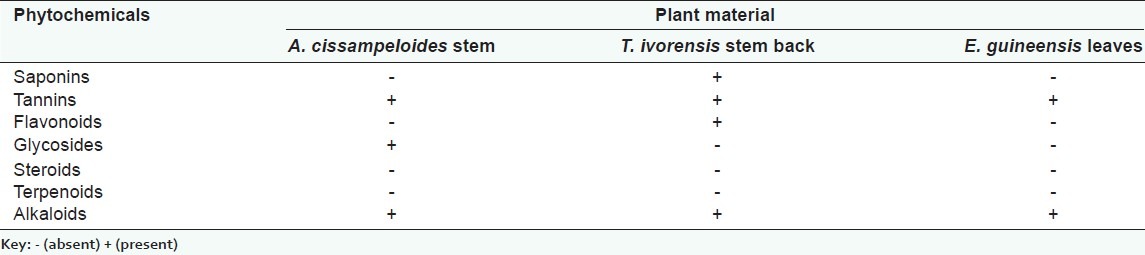
The phytochemical analysis of the extracts revealed the presence of some secondary metabolites as elaborated in [Table 1].
Table 1.
Phytochemical constituents of the plants extracts
The ethanol extracts of A. cissampeloides, T. ivorensis, and E. guineensis manifested significant activities against the chloroquine-resistant strain of P. falciparum. The percentage growth inhibition was determined using different concentrations. Maximum growth inhibition of 73.95% was obtained for A. cissampeloides at a concentration of 1.562 μg/ml. The concentrations above this (25 and 6.25μg/ ml) exhibited similar percentage inhibitions. T. ivorensis also exhibited a maximum percentage inhibition of 83.34% at a concentration of 25 μg/ml while E. guineensis showed a maximum percentage inhibition of 83.72% at a concentration of 25 μg/ml. The IC 50 values of these plants were calculated and found to be 8.52, 6.95, and 1.20 μg/ ml for A. cissampeloides, T. ivorensis, and E. guineensis, respectively. That of artesunate control was found to be 0.03 μg/ml. The respective IC50 values are shown in Table 2.
Table 2.
IC50 values for the effect of plant extracts on chloroquine resistant strain of P. falciparum

DISCUSSION
Malaria still poses as a major health problem in the developing world. It is the number one cause of morbidity, accounting for over 40% of outpatient attendance in public health facilities.[17] Pregnant women and children are the most vulnerable to the disease.[18] When malaria is not properly treated in pregnant women, it can cause anemia and can also lead to miscarriages, stillbirths, underweight babies, and maternal death.[17] Malaria is caused by the unicellular protozoan of the genus Plasmodium. Four species are infectious to man, with P. falciparum being the deadliest[19] and accounts for up to 80% of all malaria cases in the world.[20]
In many parts of the world, the parasites have developed resistance to a number of anti-malarials such as chloroquine, sulphadoxine-pyrimethamine, and other conventional drugs.[21] Resistance to these drugs has been reported to be as high as 40 to 60% in some African and Asian countries.[22] Further studies indicate that resistance to chloroquine, which was the most widely used treatment for malaria, may be responsible for the increase in malaria-specific mortality in many malaria-endemic countries.[23,24] There is, therefore, an urgent need to discover new compounds with different modes of action. However, the use of plant-derived drugs for the treatment of malaria has a long and successful tradition. For example, quinine isolated from Cinchona succirubra and artemisinin from Artemisia annua illustrate the potential value of investigating traditionally used malaria herbal remedies for anti-malarial drugs or lead compounds for drug development agenda.[25]
The present study assessed the anti-plasmodial activity of A. cissampeloides, T. ivorensis, and E. guineensis. E. guineensis exhibited the highest activity with an IC 50 value 1.195 μg/ ml.This was followed T. ivorensis (IC 50: 6.949 μg/ml) and A. cissampeloides showed the least activity (IC50: 8.521 μg/ml). However, artesunate, a known anti-malarial used as a positive control in this experiment, exhibited a much higher activity (IC50: 0.031 μg/ml) than the plant extracts under. According to Zofou et al.,[26] , an anti-plasmodial agent is very active when its IC 50 value is less than 10 μg/ml, moderately active when its IC 50 falls between 10 and 25 μg/ml, weakly active when its IC 50 falls between 25 and 50 μg/ml, and very weakly active when the IC 50 exceeds 50 μg/ml. This indicates that all 3 plants were highly active against P. falciparum.
Different classes of compounds in plants such as alkaloids[27] , quassinoids, sesquiterpene lactones[23] , and flavonoids[28] have been found to exhibit anti-plasmodial activity through several mechanisms. For instance, some alkaloids exhibit their action by binding with the haem within the erythrocytes, thus preventing its detoxification into its polymeric form (hemozoin) and thus preventing consumption by the parasite,[29,30] while some flavonoids have also been known to chelate metals such as iron and copper as part of their antioxidant effects and that iron chelating therapies have been recommended for malaria patients.[31] Further studies to determine the stage-specific anti-plasmodial activities of the alkaloids and flavonoids from our plant samples are on-going in our laboratories.
CONCLUSION
The results of this study show that the crude extracts of A. cissampeloides, T. ivorensis, and E. guineensis possess in vitro anti-plasmodial activity against P. falciparum. These findings put them in the limelight as potential candidates for further studies.
ACKNOWLEDGEMENTS
The authors would like to thank the technical staff of the Department of Immunology, Noguchi Memorial Institute for Medical Research, Legon, Accra, Ghana for helping in the laboratory work outlined in this manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev. 2002;15:564–94. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breman J, Mills A, Snow R, Steketee R, White N, Mendis K. Conquering malaria. In: Jamison DT, Breman JG, Maesham AR, editors. Disease Control Priorities in Developing Countries. New York: Oxford University Press; 2006. p. 413. [Google Scholar]
- 3.Asase A, Oteng-Yeboah AA, Odamtten GT, Simmonds MS. Ethnobotanical study of some Ghanaian anti-malarial plants. J Ethnopharmacol. 2005;99:273–9. doi: 10.1016/j.jep.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Asase A, Akwetey GA, Achel DG. Ethnopharmacological use of herbal remedies for the treatment of malaria in the Dangme West District of Ghana. J Ethnopharmacol. 2010;129:367–76. doi: 10.1016/j.jep.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Schmelzer G.H, Gurib-Fakim A, editors. Netherlands / CTA, Wageningen: Backhuys Publishers, Leiden; 2008. Plant Resources of Tropical Africa 11(1). Medicinal plants 1. PROTA Foundation; pp. 34–37. [Google Scholar]
- 6.Nyarko AA, Addy ME. Effect of aqueous extract of Adenia cissampeoides on blood pressure and serum analytes of hypertensive patients. Phytother. Res. 2006;4:25–8. Available at: http://onlinelibrary.wiley.com/doi/10.1002/ptr.2650040107/abstract . [Google Scholar]
- 7.Burkhill HM. Vol. 1. A-D. Kew: Royal Botanic Gardens; 1985. The useful plants of West Tropical Africa. [Google Scholar]
- 8.Irvine TT. Wound Healing. Arch Emerg Med. 1985;2:3–10. doi: 10.1136/emj.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mshana NR, Abbiw DK, Addae-Mensah I, Adjanouhoun E, Ahyi RA, Ekpere JA, et al. Contribution to the revision of ethnobotanic and floristic studies in Ghana. Traditional Medicine and Pharmacopoeia. Organisation of African Unity/ Scientific, Technical and Research Commission. 2000:465–466. [Google Scholar]
- 10.Keay RWJ. London: Oxford Science publishers; 1989. Trees in nigeria; p. 476. [Google Scholar]
- 11.Idu M, Onyibe HI. Medicinal Plants of Edo State, Nigeria. Res. J. Med. Plants. 2007;1(2):32–41. Available at: http://www.scialert.net/abstract/?doi=rjmp.2007.32.41 . [Google Scholar]
- 12.Trager W, Jensen JB. Human malaria parasites in continuous culture.1976. J Parasitol. 2005;91:484–6. doi: 10.1645/0022-3395(2005)091[0484:HMPICC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–8. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ene AC, Atawodi SE, Ameh DA, Ndukwe GI, Kwanashie HO. Bioassay-guided fractionation and in vivo antiplasmodial effect of fractions of chloroform extract of Artemisia maciverae Linn. Acta Trop. 2009;112:288–94. doi: 10.1016/j.actatropica.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Miller JR. San Diego, CAA: Graphpad Software Inc; 2003. Graphpad prism version 4.0, step by step examples. [Google Scholar]
- 16.Motulski H. San Diego, CA, USA: Statistical analysis for laboratory and clinical researchers; 2003. Prism for statistical guide. [Google Scholar]
- 17.Asenso-Okyere K, Asante FA. Economic burden of malaria in Ghana. Ghana: WHO/AFRO. 2003 Technical report. [Google Scholar]
- 18.Bloom DE, Bloom RL, Weston M. Business and Malaria: A neglected? World Economic Forum, Global Health Initiative, Geneva, Switzerland. 2006:1–53. Available at: http://www.weforum.org/pdf/MalariaReport.pdf . [Google Scholar]
- 19.Ashley E, McGready R, Proux S, Nosten F. Malaria. Travel Med Infect Dis. 2006;4:159–73. doi: 10.1016/j.tmaid.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Wongsrichanalai C, Pickard AL, Wernsdofer WH, Meshnick SR. Epidemiology of Drug-Resistant Malaria. Lancet infectious disease. 2002;2:209–18. doi: 10.1016/s1473-3099(02)00239-6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11937421 . [DOI] [PubMed] [Google Scholar]
- 21.Kassim OO, Loyevsky M, Amonoo H, Lashley L, Ako-Nai KA, Gordeuk VR. Inhibition of in vitro growth of Plasmodium falciparum by Pseudocedrela kotschyi extract alone and in combination with Fagara zanthoxyloides extract. Trans R Soc Trop Med Hyg. 2009;103:698–702. doi: 10.1016/j.trstmh.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Trape JF, Pison G, Spiegel A, Enel C, Rogier C. Combating malaria in Africa. Trends Parasitol. 2002;18:224–30. doi: 10.1016/s1471-4922(02)02249-3. [DOI] [PubMed] [Google Scholar]
- 23.Bero J, Frederich M, Quetin-Leclerq J. Antimalarial compounds isolated from plants used in traditional medicine. J. Pharm. and Pharmacol. 2009;61:1401–33. doi: 10.1211/jpp/61.11.0001. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19903367 . [DOI] [PubMed] [Google Scholar]
- 24.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64:12–7. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 25.Srisilam K, Veersham C. Role of Biotechnology in Medicinal and aromatic plants. In: Khanum A, Khan IA, editors. Antimalarials of plant origin. Vol. 7. Hyderabad India: Ukaaz Publishers; 2003. pp. 17–47. [Google Scholar]
- 26.Zofou D, Tene M, Ngemenya MN, Tane P, Titanji VP. In vitro antiplasmodial activity and cytotoxicity of extracts of selected medicinal plants used by traditional healers of Western cameroon. Malar Res Treat. 2011;2011:561342. doi: 10.4061/2011/561342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena S, Pant N, Jain DC, Bhakuni RS. Antimalarial agents from plant sources. Current Sci. 2003;85(9):1315–29. Article available at: http://www.iisc.ernet.in/currsci/nov102003/1314.pdf . [Google Scholar]
- 28.Kaur K, Jain M, Kaur J, Jain R. Antimalarials from nature. Bioorg Med Chem. 2009;17:3229–56. doi: 10.1016/j.bmc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 29.Paulo A, Gomes ET, Steele J, Warhurst DC, Houghton PJ. Antiplasmodial activity of Cryptolepis sanguinolenta from leaves and roots. Planta Medica. 2000;66:30–34. doi: 10.1055/s-2000-11106. http://www.ff.ul.pt/FCT/PTDC/SAU-FAR/114864/2009/ref_8.pdf . [DOI] [PubMed] [Google Scholar]
- 30.Wright CW. Trease and Evans Pharmacognosy. In: Evans WC, Evans D, editors. Antiprotozoal natural products. India: Elsevier; 2009. pp. 408–13. [Google Scholar]
- 31.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment beta-haematin. Nature. 2000;404:307–10. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]


