Abstract
Background:
Like other citrus fruits, natsumikan (Citrus natsudaidai) contains several antioxidative nutrients which occur in higher concentrations in the peel than in the pulp. A high dose of acetaminophen (APAP) generates highly reactive intermediates and causes fatal liver injury. In this study, we examined whether an extract from immature natsumikan peel prevents lethal hepatotoxicity induced by a lethal dose of APAP in mice.
Materials and Methods:
Male ICR mice were treated orally with natsumikan extract (300 and 1,000 mg/kg) 2, 26, and 50 h before single oral APAP (300 mg/kg) administration. Mice were fasted for 18 h before APAP treatment, but given tap water ad libitum. Survival was assessed for 24 h after APAP treatment.
Results:
Following administration of 300 mg/kg APAP, all mice died within 6 h. However, pretreatment with natsumikan extract (300 and 1,000 mg/kg) or silymarin (300 and 1,000 mg/kg) increased the survival rate to 16.7%, 33.3%, 16.7%, and 50%, respectively, at 24 h.
Conclusion:
The results suggest that natsumikan has a protective effect on APAP-induced lethal hepatotoxicity.
Keywords: Natsumikan, acetaminophen (APAP), lethal hepatotoxicity, hepatoprotective effect
INTRODUCTION
Acetaminophen (APAP) is one of the most commonly used drugs for reducing fever in adults and children and is generally considered a safe drug. However, a large single dose can cause potentially fatal acute liver failure in both humans and experimental animals.[1–3] APAP poisoning currently represents the most common cause of acute liver failure in North America and Europe.[4] Between 400 and 500 deaths occur annually in the United States due to APAP-related liver failure.[5] Unintentional liver injury from self-medication for pain or fever that leads to daily doses exceeding the 4 g/day package recommendation is also well recognized. However, unintentional overdosing is usually only recognized after symptoms have developed.[6]
Citrus natsudaidai (natsumikan) is a well-known citrus fruit recognized in many countries for its medicinal properties. Because natsumikan contains a large amount of antioxidative nutrients such as vitamin C and flavonoids, regular consumption of citrus fruit has been considered good for maintaining health. Natsumikan is rich in many bioactive components, such as hesperidin, neohesperidin, naringin, nobiletin, tangeretin, and aurapten, which have all been well studied for their pharmacological anti-inflammatory,[7,8] anticarcinogenic,[9] and antioxidant properties.[10]
It has been reported that some medicinal plants, such as Phyllanthus urinaria,[11,12] Dioscorea alata L,[13] and Acanthopanax koreanum[14] have protective effects with regard to APAP-induced acute liver failure. As these medicinal plants are known to have antioxidative properties, we hypothesized that natsumikan may also have a protective effect on APAP-induced hepatotoxicity.
Therefore, we designed the present study to investigate the potential usefulness of natsumikan focusing on its preventive effects against lethal hepatotoxicity induced by an overdose of APAP.
MATERIALS AND METHODS
Plant material
Immature fresh natsumikan were collected in Minamiboso city, Chiba, Japan in August 2009 and stored at 4°C prior to extraction of the active ingredients from the peel. The natsumikan peel contained the following active components per 100 g: hesperidin 11 mg, neohesperidin 370 mg, naringin 780 mg, nobiletin 1.6 mg, tangeretin 3.2 mg, and aurapten 26 mg.
Chemicals
Silymarin was obtained from Sigma Chemical (St. Louis, MO) and APAP was obtained from Wako Pure Chemical Institute (Osaka, Japan).
Extraction procedure
Natsumikan peel (1215.2 g) was cut into small pieces with scissors and air-dried at 50°C for 24 h in a mechanical air-dryer. Extraction was performed by placing the dried peel (412.4 g) in a 5-fold volume of methanol (Wako Pure Chemical Institute) at 40°C for 2 h. The resulting extract was filtered and concentrated under reduced pressure to evaporate the methanol, then lyophilized using a freeze-drying machine (Taitec, Saitama, Japan) to provide 14.2 g of dried natsumikan peel.
Animals
All experiments and procedures were approved by the Chiba University Institutional Animal Care and Use Committee. Male ICR mice, 5 weeks of age, were obtained from Japan SLC Inc. (Hamamatsu, Japan) and housed under controlled conditions of light (07:00-19:00) and temperature (24°C) with food and water available ad libitum.
Drug treatment regimens
Natsumikan extract (300 or 1,000 mg/kg) and silymarin (300 or 1,000 mg/kg) were dissolved in distilled water and administrated orally once a day for 3 days. Mice were fasted for 24 h starting at 16 h before the final administration of natsumikan or silymarin but were given tap water ad libitum. APAP (300 mg/kg) was dissolved in distilled water and administered orally once, 2 h after the last administration of natsumikan or silymarin. Control animals were treated with vehicle [Figure 1].
Figure 1.
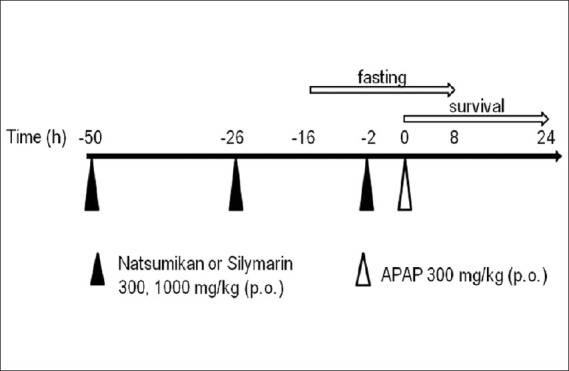
Schedule for the elicitation of APAP-induced hepatotoxicity showing administration of reagents
Survival experiments
Mice were administered as mentioned above and received the final administration of natsumikan or silymarin 2 h before 300 mg/kg (lethal dose) of APAP intoxication [Figure 1]. Mortality was observed for 24 h after administration of APAP. Each group contained 6 mice.
RESULTS AND DISCUSSION
The aim of this study was to investigate the protective effect of natsumikan on APAP-induced hepatotoxicity. When mice were administered the lethal dose of APAP (300 mg/kg), all died within 6 h. However, in mice pre- treated with natsumikan extract (300 and 1,000 mg/ kg) before APAP dosing, the survival rate was 16.7% and 33.3%, respectively, at 24 h after APAP. Similarly, silymarin (300 and 1,000 mg/kg) increased the survival rate to 16.7% and 50%, respectively, at 24 h [Figure 2].
Figure 2.
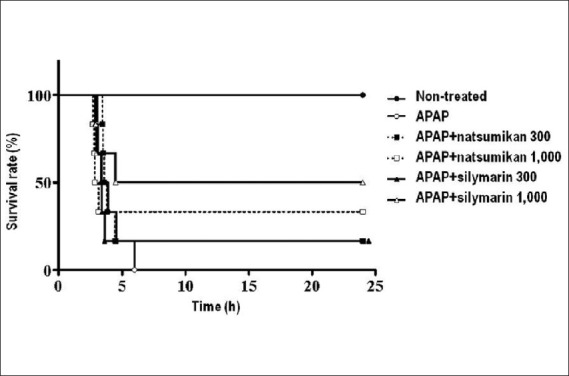
The effect of natsumikan treatment on APAP-induced mortality. Survival rate in control group treated with distilled water (●; n=6) and mice treated with APAP alone (○; n=6), or APAP plus natsumikan 300 mg/kg (■; n=6), natsumikan 1,000 mg/kg (□; n=6), silymarin 300 mg/kg (▲; n=6), or silymarin 1,000 mg/kg (Δ; n=6)
Silymarin is known to exhibit a hepatoprotective effect through antioxidant and cell-regenerating functions,[12] and is often used as a positive control for studies of hepatic injury. Moreover, many types of herbal extracts that can prevent APAP-induced acute liver failure via mediation of antioxidant effects.[15,16,17] Recently, we reported that the extract of immature natsumikan peel is effective for the treatment of chronic allergic contact dermatitis, and this effect might be mediated by its antioxidative properties.[18] Therefore, it can be assumed that the inhibitory effect of natsumikan on APAP-induced hepatotoxicity in the present study is mediated, at least in part, by its antioxidative properties. We hypothesized that the antioxidative elements of natsumikan may be hesperidin and naringin, which have been reported to exhibit antioxidative effects,[19,20] but further studies are required to confirm which component has the greatest protective effect.
High doses of APAP generate the highly reactive intermediate N-acetyl-p-benzoquinone imine (NAPQI) via cytochrome P450 (CYP) enzymes, which results in acute liver failure. In particular, CYP2E1 is an important isoform that generates NAPQI. Recently, it was reported that some herbal extracts attenuate APAP-induced acute liver failure through inhibition of CYP2E1.[21,22] Furthermore, it is possible that natumikan inhibits APAP-induced acute liver failure via inhibition of CYP2E1 activity, but further studies are required to confirm this.
To our knowledge, this is the first study to show the protective effects of immature natsumikan peel extract on APAP-induced lethal hepatotoxicity in mice. If APAP is taken to reduce fever caused by illnesses such as the common cold or flu, prior ingestion of immature natsumikan peel as a dietary supplement may be useful for preventing unintentional overdosing and consequent APAP-induced acute liver failure.
ACKNOWLEDGEMENTS
We thank the Minamiboso Yoi-shoku Entrepreneurs Association for providing the natsumikan used in this study. This work was supported by a grant-in-aid from Chiba University, Japan.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Black M. Acetaminophen hepatotoxicity. Annu Rev Med. 1984;35:577–93. doi: 10.1146/annurev.me.35.020184.003045. [DOI] [PubMed] [Google Scholar]
- 2.Hinson JA. Reactive metabolites of phenacetin and acetaminophen: A review. Environ Health Perspect. 1983;49:71–9. doi: 10.1289/ehp.834971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott LF, Roscoe P, Wright N, Brown SS. Plasma-paracetamol half-life and hepatic necrosis in patients with paracetamol overdosage. Lancet. 1971;1:519–22. doi: 10.1016/s0140-6736(71)91125-1. [DOI] [PubMed] [Google Scholar]
- 4.Larsen FS, Kirkegaard P, Rasmussen A, Hansen BA. The Danish liver transplantation program and patients with serious acetaminophen intoxication. Transplant Proc. 1995;27:3519–20. [PubMed] [Google Scholar]
- 5.Lee WM, Squires RH, Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008;47:1401–15. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe S, Kinuta Y, Yasumatsu H, Takayanagi M, Kobayashi S, Takido N, et al. Effects of Citrus unshiu powder on the cytokine balance in peripheral blood mononuclear cells of patients with seasonal allergic rhinitis to pollen. Biosci Biotechnol Biochem. 2007;71:2852–5. doi: 10.1271/bbb.70397. [DOI] [PubMed] [Google Scholar]
- 8.Lee NK, Choi SH, Park SH, Park EK, Kim DH. Antiallergic activity of hesperidin is activated by intestinal microflora. Pharmacology. 2004;71:174–80. doi: 10.1159/000078083. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Kawabata K, Kakumoto M, Makita H, Hara A, Mori H, et al. Citrus auraptene inhibits chemically induced colonic aberrant crypt foci in male F344 rats. Carcinogenesis. 1997;18:2155–61. doi: 10.1093/carcin/18.11.2155. [DOI] [PubMed] [Google Scholar]
- 10.Sugiura M, Ohshima M, Ogawa K, Yano M. Chronic administration of Satsuma mandarin fruit (Citrus unshiu Marc.) improves oxidative stress in streptozotocin-induced diabetic rat liver. Biol Pharm Bull. 2006;29:588–91. doi: 10.1248/bpb.29.588. [DOI] [PubMed] [Google Scholar]
- 11.Hau DK, Wong RS, Cheng GY, Wong WY, Tong SW, Chan KW, et al. Novel use of silymarin as delayed therapy for acetaminophen-induced acute hepatic injury. Forsch Komplementmed. 2010;17:209–13. doi: 10.1159/000319317. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- 13.Lee SC, Tsai CC, Chen JC, Lin JG, Lin CC, Hu ML, et al. Effects of “Chinese yam” on hepato-nephrotoxicity of acetaminophen in rats. Acta Pharmacol Sin. 2002;23:503–8. [PubMed] [Google Scholar]
- 14.Wu YL, Jiang YZ, Jin XJ, Lian LH, Piao JY, Wan Y, et al. Acanthoic acid, a diterpene in Acanthopanax koreanum, protects acetaminophen-induced hepatic toxicity in mice. Phytomedicine. 2010;17:475–9. doi: 10.1016/j.phymed.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Kim ST, Kim JD, Ahn SH, Ahn GS, Lee YI, Jeong YS. Hepatoprotective and antioxidant effects of Alnus japonica extracts on acetaminophen-induced hepatotoxicity in rats. Phytother Res. 2004;18:971–5. doi: 10.1002/ptr.1540. [DOI] [PubMed] [Google Scholar]
- 16.Yen FL, Wu TH, Lin LT, Lin CC. Hepatoprotective and antioxidant effects of Cuscuta chinensis against acetaminophen-induced hepatotoxicity in rats. J Ethnopharmacol. 2007;111:123–8. doi: 10.1016/j.jep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Ajith TA, Hema U, Aswathy MS. Zingiber officinale Roscoe prevents acetaminophen-induced acute hepatotoxicity by enhancing hepatic antioxidant status. Food Chem Toxicol. 2007;45:2267–72. doi: 10.1016/j.fct.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama N, Yamaura K, Shimada M, Ueno K. Extract from peel of Citrus natsudaidai alleviates experimental chronic allergic dermatitis in mice. Pharmacognosy Res. 2011;3:155–9. doi: 10.4103/0974-8490.84999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain M, Parmar HS. Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm Res. 2011;60:483–91. doi: 10.1007/s00011-010-0295-0. [DOI] [PubMed] [Google Scholar]
- 20.Jeon SM, Bok SH, Jang MK, Lee MK, Nam KT, Park YB, et al. Antioxidative activity of naringin and lovastatin in high cholesterol-fed rabbits. Life Sci. 2001;69:2855–66. doi: 10.1016/s0024-3205(01)01363-7. [DOI] [PubMed] [Google Scholar]
- 21.Yamaura K, Shimada M, Nakayama N, Ueno K. Protective effects of goldenseal (Hydrastis canadensis L.) on acetaminophen-induced hepatotoxicity through inhibition of CYP2E1 in rats. Pharmacognosy Res. 2011;3:250–5. doi: 10.4103/0974-8490.89745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hau DK, Gambari R, Wong RS, Yuen MC, Cheng GY, Tong CS, et al. Phyllanthus urinaria extract attenuates acetaminophen induced hepatotoxicity: Involvement of cytochrome P450 CYP2E1. Phytomedicine. 2009;16:751–60. doi: 10.1016/j.phymed.2009.01.008. [DOI] [PubMed] [Google Scholar]


