Abstract
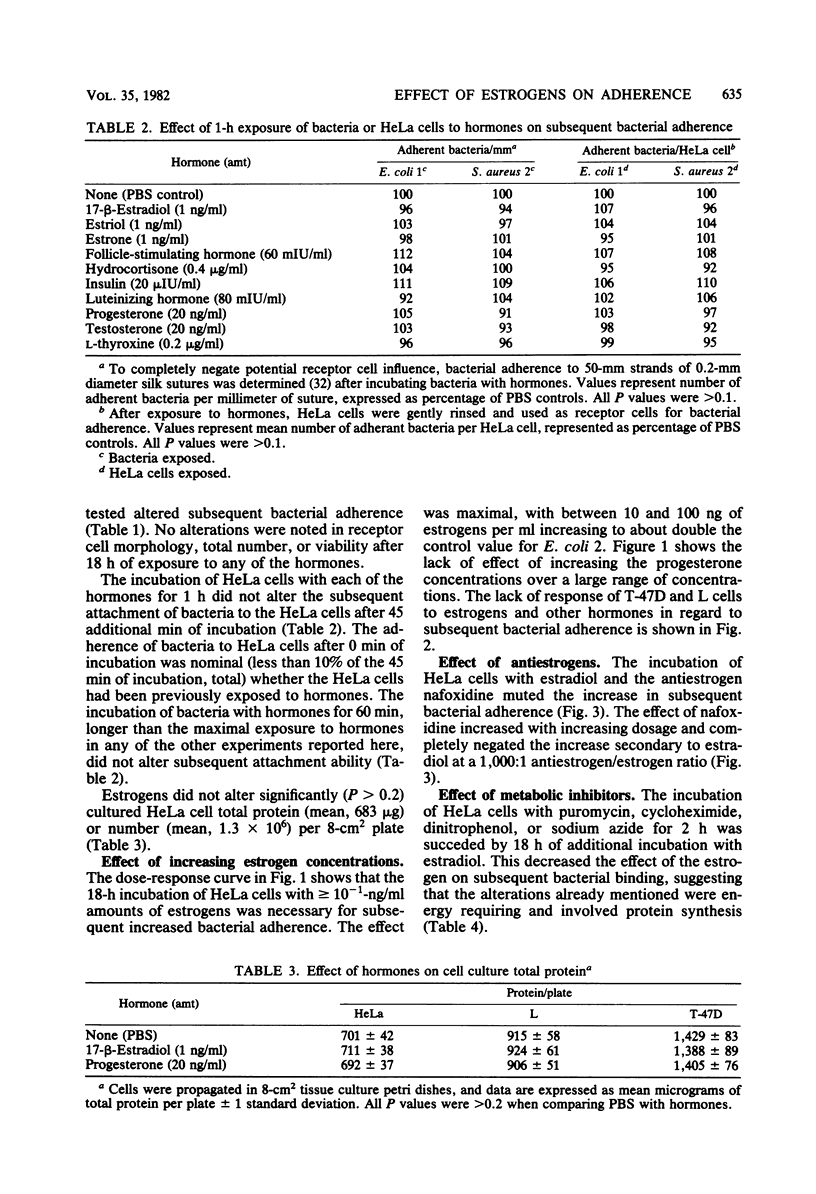
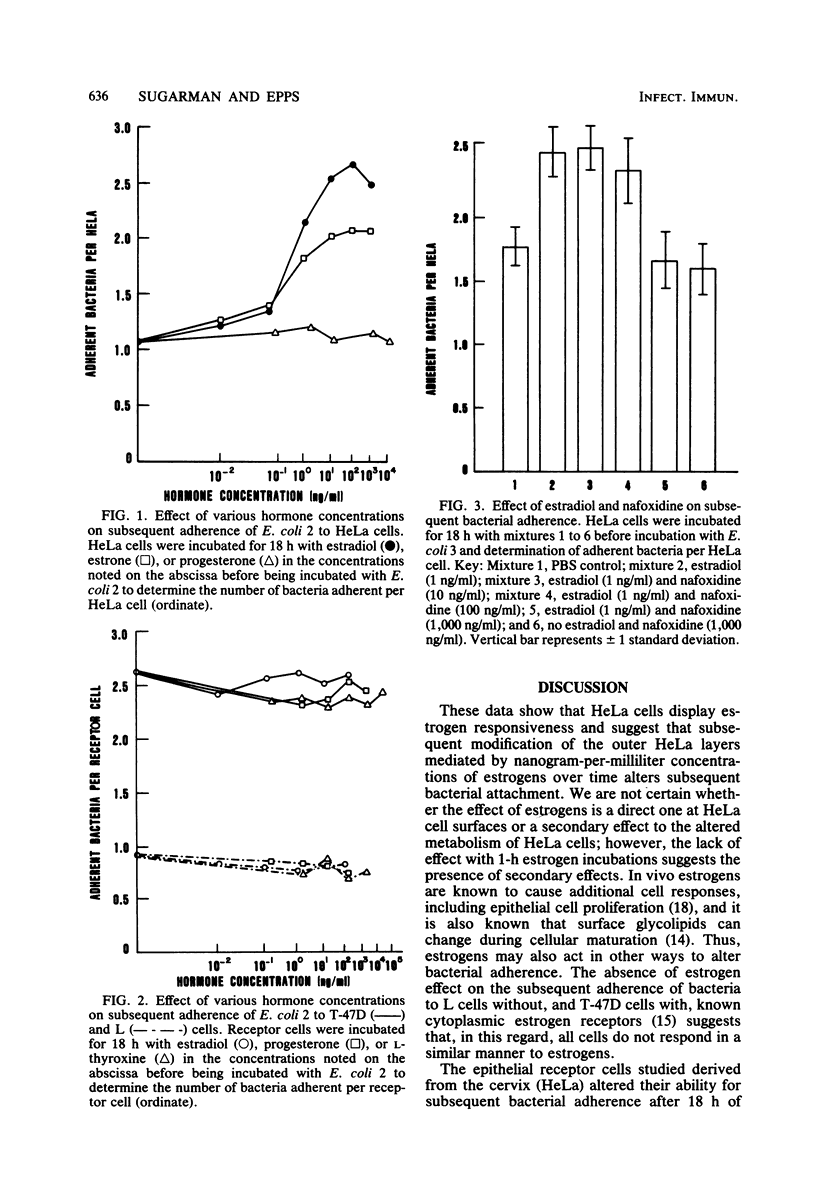
Incubating confluent cell culture HeLa cells for 18 h with increasing concentrations of estrogens progressively enhanced the subsequent attachment of a variety of radiolabeled bacteria to the HeLa cells. This effect was not caused by other hormones and was not produced by 1-h incubations of HeLa cells or bacteria with hormones. Estrogens did not similarly affect two other receptor cell lines studied. The addition of metabolic inhibitors showed that this effect of estrogens on HeLa cells was energy dependent and involved protein synthesis. Concurrent incubation of the HeLa cells and estrogens with the antiestrogen nafoxidine blocked the subsequent increase in adherence. These data suggest that estrogen receptors are present in HeLa cells and that hormonally-induced alterations in the synthesis of bacterial receptor sites may modify the capacity of certain cells to bind bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aly R., Shinefield H. R., Litz C., Maibach H. I. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J Infect Dis. 1980 Apr;141(4):463–465. doi: 10.1093/infdis/141.4.463. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Goroff D. K., Alpert S., Crockett V. A., Zinner S. H., Evrard J. R., Rosner B., McCormack W. M. Vaginal colonization with group B streptococcus: a study in college women. J Infect Dis. 1977 Mar;135(3):392–397. doi: 10.1093/infdis/135.3.392. [DOI] [PubMed] [Google Scholar]
- Banai M., Razin S., Bredt W., Kahane I. Isolation of binding sites to glycophorin from Mycoplasma pneumoniae membranes. Infect Immun. 1980 Dec;30(3):628–634. doi: 10.1128/iai.30.3.628-634.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Binding of group A streptococci to human oral mucosal cells by lipoteichoic acid. Trans Assoc Am Physicians. 1975;88:285–292. [PubMed] [Google Scholar]
- Botta G. A. Hormonal and type-dependent adhesion of group B streptococci to human vaginal cells. Infect Immun. 1979 Sep;25(3):1084–1086. doi: 10.1128/iai.25.3.1084-1086.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Larsson P., Lomberg H. Attachment of Proteus mirabilis to human urinary sediment epithelial cells in vitro is different from that of Escherichia coli. Infect Immun. 1980 Mar;27(3):804–807. doi: 10.1128/iai.27.3.804-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Leffler H. Glycosphingolipids of human urinary tract epithelial cells as possible receptors for adhering Escherichia coli bacteria. Scand J Infect Dis Suppl. 1980;Suppl 24:144–147. [PubMed] [Google Scholar]
- Engelhardt J. A., Gabridge M. G. Effect of squamous metaplasia on infection of hamster trachea organ cultures with Mycoplasma pneumoniae. Infect Immun. 1977 Feb;15(2):647–655. doi: 10.1128/iai.15.2.647-655.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Avots-Avotins A. E., Davis C. P. Evidence for pili-mediated adherence of Klebsiella pneumoniae to rat bladder epithelial cells in vitro. Infect Immun. 1979 Aug;25(2):729–737. doi: 10.1128/iai.25.2.729-737.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslin L., Danielsson D., Falk V. Variations in attachment of Neisseria gonorrhoeae to vaginal epithelial cells during the menstrual cycle and early pregnancy. Med Microbiol Immunol. 1979;167(4):231–238. doi: 10.1007/BF02120808. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Taylor-Robinson D. Interaction of Mycoplasma pneumoniae with human lung fibroblasts: role of receptor sites. Infect Immun. 1979 Jul;25(1):455–459. doi: 10.1128/iai.25.1.455-459.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. K., Counts G. W., Beaty H. N. Disseminated gonococcal infection. Ann Intern Med. 1971 Jun;74(6):979–993. doi: 10.7326/0003-4819-74-6-979. [DOI] [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Demonstration of specific binding sites for human serum albumin in group C and G streptococci. Infect Immun. 1980 Jan;27(1):6–14. doi: 10.1128/iai.27.1.6-14.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson-Rees W. A. Hormones and breast cancer in vitro. Cancer Res. 1977 Sep;37(9):3464–3465. [PubMed] [Google Scholar]
- Parsons C. L., Mulholland S. G., Anwar H. Antibacterial activity of bladder surface mucin duplicated by exogenous glycosaminoglycan (heparin). Infect Immun. 1979 May;24(2):552–557. doi: 10.1128/iai.24.2.552-557.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons C. L., Pollen J. J., Anwar H., Stauffer C., Schmidt J. D. Antibacterial activity of bladder surface mucin duplicated in the rabbit bladder by exogenous glycosaminoglycan (sodium pentosanpolysulfate). Infect Immun. 1980 Mar;27(3):876–881. doi: 10.1128/iai.27.3.876-881.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ronda C. H. Adherence of glucan-positive and glucan-negative streptococcal strains to normal and damaged heart valves. J Clin Invest. 1978 Oct;62(4):805–814. doi: 10.1172/JCI109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., McNiece M. T., Polack F. M. Adherence of Pseudomonas aeruginosa to the injured cornea: a step in the pathogenesis of corneal infections. Ann Ophthalmol. 1981 Apr;13(4):421–425. [PubMed] [Google Scholar]
- Ramphal R., Small P. M., Shands J. W., Jr, Fischlschweiger W., Small P. A., Jr Adherence of Pseudomonas aeruginosa to tracheal cells injured by influenza infection or by endotracheal intubation. Infect Immun. 1980 Feb;27(2):614–619. doi: 10.1128/iai.27.2.614-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer A. J., Amundsen S. K., Schmidt L. N. Adherence of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1979 Jun;24(3):753–759. doi: 10.1128/iai.24.3.753-759.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer A. J., Jones J. M., Dunn J. K. Association of vitro Escherichia coli adherence to vaginal and buccal epithelial cells with susceptibility of women to recurrent urinary-tract infections. N Engl J Med. 1981 Apr 30;304(18):1062–1066. doi: 10.1056/NEJM198104303041802. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Ofek I., Beachey E. H. Fatty acid binding sites of serum albumin as membrane receptor analogs for streptococcal lipoteichoic acid. Infect Immun. 1980 Jul;29(1):119–122. doi: 10.1128/iai.29.1.119-122.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Schneider J., Kaye D., Levison M. E. Adherence of bacteria to vaginal epithelial cells at various times in the menstrual cycle. Infect Immun. 1981 Apr;32(1):194–197. doi: 10.1128/iai.32.1.194-197.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B., Donta S. T. Specificity of attachment of certain enterobacteriaceae to mammalian cells. J Gen Microbiol. 1979 Dec;115(2):509–512. doi: 10.1099/00221287-115-2-509. [DOI] [PubMed] [Google Scholar]
- Sugarman B., Musher D. Adherence of bacteria to suture materials. Proc Soc Exp Biol Med. 1981 Jun;167(2):156–160. doi: 10.3181/00379727-167-41141. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


