Abstract
Background & objectives:
Our previous study showed that cow ghee relative to soybean oil had a protective effect against carcinogen induced mammary cancer in rats. The objective of this study was to elucidate its biochemical mechanism.
Methods:
Two groups of 21 day old rats (20 each) were fed for 44 wk diet containing cow ghee or soybean oil (10%). Five animals from each group were sacrificed at 0 day and at 5, 21 and 44 wk for analysis of phase I and phase II pathways enzymes of carcinogen metabolism.
Results:
Dietary cow ghee relative to soybean oil decreased the activities of cytochrome P450 (CYP) enzymes, CYP1A1, CYP1A2, CYP1B1 and CYP2B1, responsible for activation of carcinogen in liver. Carcinogen detoxification activities of uridinediphospho-glucuronosyl transferase (UDPGT) and quinone reductase (QR) in liver, and γ-glutamyltranspeptidase (GGTP) and QR in mammary tissue were significantly higher in cow ghee fed rats than in soybean oil fed rats. The hepatic GGTP activity decreased on soybean oil diet; while in cow ghee group it remained unaffected.
Interpretation & conclusions:
Our findings show that dietary cow ghee compared to soybean oil downregulates the enzyme activities responsible for carcinogen activation in liver and upregulates carcinogen detoxification activities in liver and mammary tissues.
Keywords: Carcinogen detoxification enzymes, cow ghee, cytochrome P450 enzymes, soybean oil
The modulation of cancer by nutritional variables has been a subject of interest and controversy. Dietary fat has received considerable attention as a possible risk factor in the aetiology of breast and colon cancer. The second report by the World Cancer Research Fund and the American Institute for Cancer Research indicates that food and nutrition may affect the status of hormones that can modify breast cancer risk1. Both the quantity and quality of dietary fat influence the development of spontaneous as well as chemically-induced neoplasm in laboratory animals2,3. Our earlier studies showed that dietary cow ghee (clarified butter oil), compared with soybean oil had a protective effect against the carcinogen induced mammary cancer4, and the decreased expressions of cyclins A and D1, Bcl-2 and PKC-α mediate the mechanism of the protective effect of cow ghee5. Carcinogenesis is a complex and protracted multistage process that can be initiated due to damage of cellular macromolecule by an endogenous or exogenous agent. It is generally accepted that cancer is induced by certain chemical agents that exist in nature as inactive pro-carcinogens, the metabolism of which involves two steps. In the first step, the carcinogens are metabolized to reactive molecules by phase I enzymes. The major reactions in phase I are hydroxylation or epoxide formation catalyzed by class of liver enzymes referred to as cytochrome P450s (CYPs). In phase II, the active molecules are detoxified in liver as well as in the target tissue by their conjugation with glutathione, glucuronic acid, sulphate, acetate or certain amino acids by enzymes like glutathione-S-transferase, quinine reductase, γ-glutamyltranspeptidase or UDP-glucuronosyl transferase. Strategies for protecting cells from these initiating events include decreasing metabolic enzymes responsible for generating reactive species through phase I enzymes, and increasing activities of phase II enzymes that deactivate free radicals and electrophiles.
Most of the carcinogens metabolizing enzymes are membrane bound and require a lipid membrane for their activity. Therefore, the oxidative metabolism of carcinogens in the liver endoplasmic reticulum is markedly dependent on the composition of the dietary lipid6,7. The quantity and quality of dietary fat affect the lipid composition and physical characteristics of biological membranes and enzymatic activity of several components of the drug metabolizing enzyme system. These changes in enzymes activities have been associated with alterations in the mutagenicity and carcinogenicity of the pro-carcinogens. Keeping this in view and our earlier report on differential effects of milk fat and soybean oil on chemical induced carcinogenesis, the present study was designed to compare the effect of these two fat/oils on various phase I and phase II carcinogen metabolizing enzymes in rat liver and mammary tissue.
Material & Methods
Chemicals: Benzoxyresorufin, ethoxyresorufin, methoxyresorufin, pentoxyresorufin, resorufin sodium salt, dicumarol, uridinediphosphoglucuronic acid (UDPGA), γ-glutamyl-p-nitroanilide, glycylglycine and calcium chloride were procured from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used in this study were of analytical grade and purchased from local suppliers.
Animals and diets: Female Wistar rats (21 day old) obtained from Small Animal House of the National Dairy Research Institute (NDRI), Karnal, Haryana, were housed in metal cages in a well-ventilated room and were given water and diet ad libitum. The study was conducted in the Division of Animal Biochemistry, NDRI, Karnal. Institute's Animal Ethics Committee approved all the protocols. The experimental diet comprised chick pea, 56.4 per cent; wheat, 15 per cent; groundnut cake, 10 per cent; cow ghee or soybean oil, 10 per cent; skim milk powder, 6 per cent; mineral mixture, 2.16 per cent; vitamin mix, 0.2 per cent and choline chloride, 0.2 per cent. The composition of mineral and vitamin mixtures was designed so as to provide these nutrients (including those derived from above feed ingredients) in diets in accordance to AIN-938. Overall, 35 rats were included, of which five were sacrificed to record 0 day observations. The remaining animals were tabulated in accordance to their body weight and randomly divided in to two groups of 15 each with mean body weight 22 g, and were fed on cow ghee and soybean oil diet, respectively. Five animals from each group were sacrificed at 5, 21 and 44 wk by cervical dislocation, and liver and mammary tissues were collected for enzyme analysis. Body weights were recorded biweekly. Phase I enzymes of carcinogen activation were determined at 0 day and 5 and 44 wk. The phase II activities of carcinogen detoxification were determined at 0 day and 5, 21 and 44 wk.
Preparation of liver microsomes: The liver excised immediately after sacrifice was washed with 0.9 per cent NaCl, and microsomes were prepared by differential centrifugation9. A portion of the liver was homogenized in 3 weight volume of ice cold 0.25 M sucrose in 0.01 M potassium phosphate buffer (pH 7.5), and centrifuged (12,000 × g at 4°C) for 20 min. The supernatant was mixed with CaCl2 (8 mM) and centrifuged (27,000 × g at 4°C) for 15 min. The resultant pellet was washed with equal volume of 0.15 M KCl and re-centrifuged (27,000 × g at 4°C) for 15 min.
Preparation of tissue homogenates: For glutathione-S-transferase (GST) activity, the tissue homogenate (10%) prepared in 0.1 M potassium phosphate buffer (pH 7.0) was centrifuged (10,000 × g) for 30 min, and the supernatant was filtered through Whatman filter paper to remove fat. For all other enzyme activities, the tissue homogenates (10%) were prepared in 0.25 M sucrose in potassium phosphate buffer (0.01 M, pH 7.4) and centrifuged (12,000 × g) for 20 min. The microsomal and cytosolic protein content was determined by the method of Lowry et al10.
Enzyme assays: Microsomal ethoxyresorufin-O-de-ethylase (CYP1A1), methoxyresorufin O-dealkylase (CYP1A2), benzoxyresorufin-O-dealkylase (CYP1B1) and pentoxyresorufin-O-dealkylase (CYP2B1) activities were assayed according to the procedure described by Burke et al11 and modified by Teel and Huynh12. These reactions have a common final step, the hydroxylation of the fenaxazone ring, which generates hydroxyfenaxazone (resorufin), which was measured at excitation wavelength 550 nm and emission wavelength 585 nm using fluorescence spectrophotometer (Cary1 Eclipse; Varian, California USA). The enzyme activity was expressed as pmoles of resorufin released per min per mg of microsomal protein. The CYP2E1 activity in liver microsomes was measured spectrophotometrically according to the method described by Reinke et al13. Glutathione-S-transferase activity was determined in liver and mammary tissue homogenates using 1-chloro-2, 4-divitrobenzene (CDNB) as substrate14. UDP-glucuronosyl transferase activity was determined in liver microsomes according to the method of Bock et al15 modified by Martin and Black16 using UDP-glucuronic acid and p-nitrophenol as substrates. γ-glutamyltranspeptidase was assayed in liver microsomes and mammary tissue homogenate using method described by Tate and Meister17. Quinone reductase activity was assayed according to the method of Ernster18 in liver and mammary tissue homogenates.
Statistical analysis: The values were expressed as mean ± SE. Statistical analysis was done through two way analysis of variance (ANOVA), and the comparisons between means were analyzed by Tukey's multiple comparison test using PRISM 3.0 software. P<0.05 was considered significant.
Results
The growth of animals was similar in rats fed cow ghee or soybean oil (Fig.) for 44 wk, and the difference in body weight was not statistically significant. Further, there was no mortality observed in either group.
Fig.
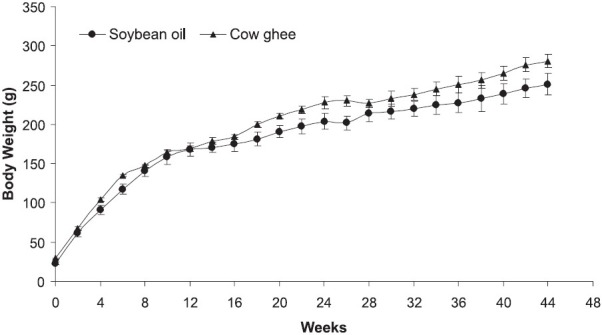
Body weight of rats fed soybean oil or cow ghee. Values are mean ± SE (n=5).
Cytochrome P450 activities of carcinogen metabolism in liver microsomes (Phase I): Cytochrome P4501A1 (CYP1A1) activity in liver increased up to 5 wk in both soybean oil and cow ghee fed rats, and the magnitude of increase was greater on soybean oil (60%) than on cow ghee (28%). The level of hepatic CYP1A1 activity in cow ghee group was lower than in soybean oil group by 20.1 and 11.4 per cent at 5 and 44 wk of dietary treatment, respectively (Table I).
Table I.
Effect of dietary fat on cytochrome P450 enzymes in rat liver
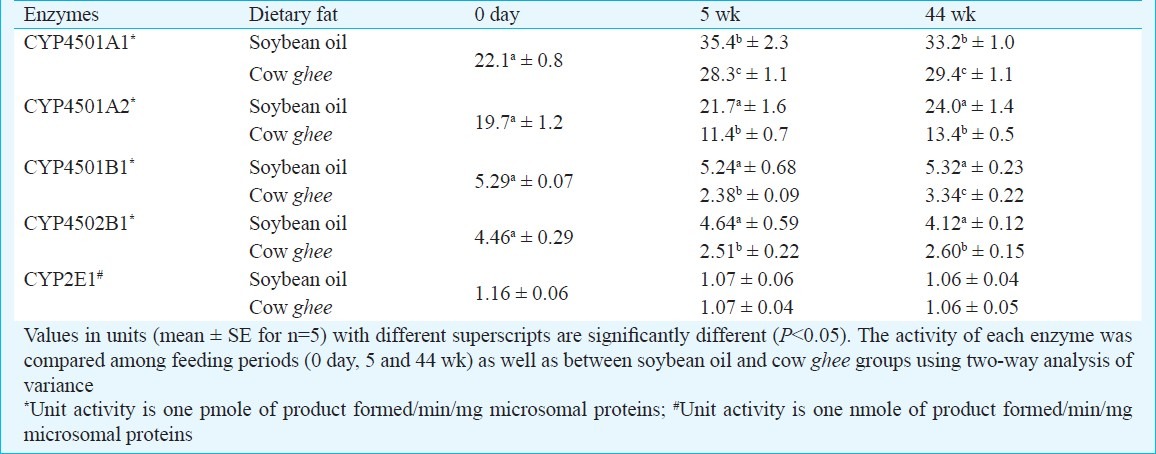
Cytochrome P4501A2 (CYP1A2) activity in liver was not significantly affected in soybean oil fed rats, while it decreased on cow ghee diet by 42.1 per cent at 5 wk and thereafter did not change significantly in either group. In cow ghee group the magnitude of hepatic CYP1A2 activity was lower than in soybean oil fed rats by 47.5 and 44.2 per cent at 5 and 44 wk of dietary treatment, respectively (Table I).
Cytochrome P4501B1 (CYP1B1) activity in liver remained unaffected on soybean oil diet throughout the experimental period, while on cow ghee it decreased by 55 and 37 per cent at 5 and 44 wk, respectively. The level of hepatic CYP1B1 activity was lower in cow ghee group than in soybean oil fed rats by 55 and 37 per cent at 5 and 44 wk of dietary treatment, respectively (Table I). Cytochrome P4502B1 (CYP2B1) activity in liver was not affected on soybean oil diet, while on cow ghee it decreased by 44 per cent at 5 wk. The magnitude of hepatic CYP2B1 activity was lower in cow ghee fed rats than in soybean oil group by 46 and 37 per cent at 5 and 44 wk of dietary treatment, respectively (Table I).
Cytochrome P4502E1 activity in liver microsomes was not affected by feeding either cow ghee or soybean oil, and no difference was observed between the two dietary treatments throughout the experimental period (Table I).
Carcinogen detoxification activities in liver and mammary tissues (Phase II): Glutathione-S-transferase (GST) activity in liver equally increased in soybean oil and cow ghee groups during first 5 wk. Thereafter the rise in hepatic GST activity was not significant in soybean oil group, while in cow ghee group, it increased significantly at 44 wk (Table II). In mammary tissue the difference in GST activity between two dietary groups was not statistically significant at any stage (Table III).
Table II.
Effect of dietary fat on carcinogen detoxification activities in rat liver
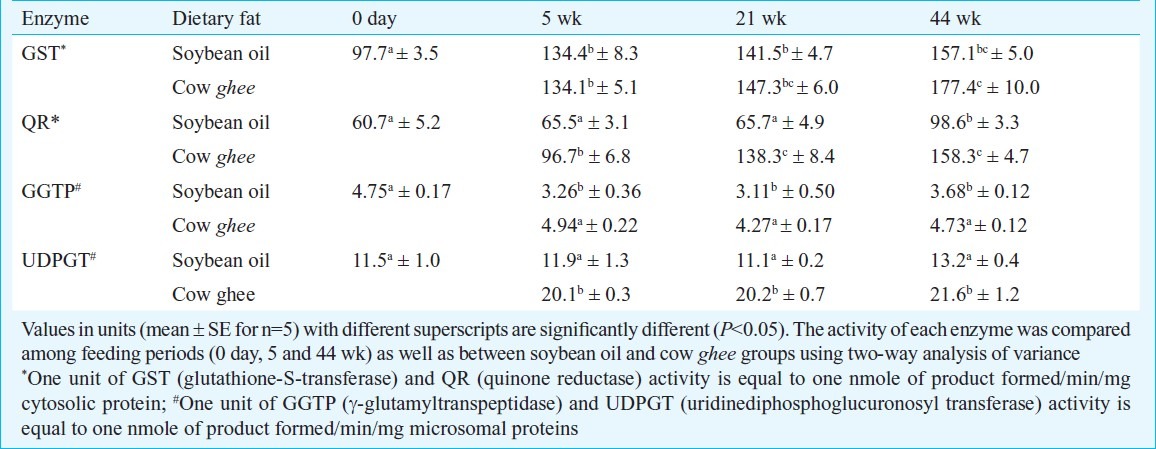
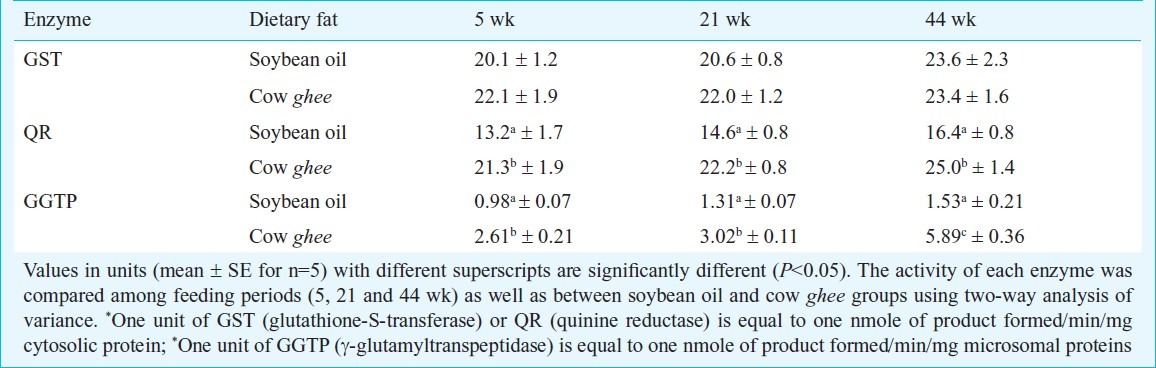
Table III.
Effect of dietary fat on carcinogen detoxification activities (units*/mg cytosolic proteins) in rat mammary tissue
The UDP-glucuronosyl transferase (UDPGT) activity in liver was not significantly affected on soybean oil diet, while on cow ghee diet, it increased by about 75 per cent at 5 wk, and thereafter remained stable. The UDPGT activity in ghee group was 63-69 per cent higher than in soybean oil group during 5 to 44 wk of dietary treatment (Table II). The γ- glutamyl transpeptidase (GGTP) activity in liver was not affected by dietary treatment with cow ghee, while it decreased significantly (P<0.01) on soybean oil diet. The hepatic GGTP activity in soybean oil fed rats was decreased by 34, 27 and 22 per cent at 5, 21 and 44 wk, respectively, compared to age-matched cow ghee fed rats (Table II).
In mammary tissue the GGTP activity though increased in both soybean oil and cow ghee fed rats, the magnitude of increase was not statistically significant in soybean oil group. The quinone reductase (QR) activity in liver was not affected up to 21 wk, and increased by 63 per cent at 44 wk in soybean oil group, while in cow ghee group it increased by 59, 127 and 161 per cent at 5, 21 and 44 wk, respectively (Table II), relative to 0 day value. The QR activity in cow ghee group compared to soybean oil group, increased by 48, 110 and 61 per cent at 5, 21 and 44 wk, respectively (Table II). The QR activity in mammary tissue increased in cow ghee fed rats by over 50 per cent, compared to age-matched soybean oil fed rats (Table III).
Discussion
The metabolism of chemical carcinogen involves two steps; in the first step carcinogen is metabolized to a reactive molecule by phase-I enzymes and in the second step, active metabolite gets detoxified by several phase-II enzymes. Thus the relative activity of phase I and phase II enzymes would determine the extent of tumorigenesis. The phase I cytochrome P450 enzymes are membrane bound and their activities are influenced by the lipid environment. Therefore, altering membrane lipid composition by feeding animals on singular source of fat might affect carcinogen metabolism7. Diet rich in polyunsaturated fatty acids (fish and sunflower oil) increases the level of microsomal cytochrome P-450 in rat liver as compared to that rich in saturated (partially and highly hydrogenated fish oil) fatty acids19. Saito et al20 also observed low levels of cytochrome P-450 and aminopyrine N-demethylase activity in the lard fed rats as compared to soybean oil fed ones, and suggested that increased proportion of saturated fatty acids in microsomal membrane phospholipids might have altered the membrane conformation and fluidity leading to a low activity of these enzymes.
Cow ghee, which contains a large amount of saturated and monounsaturated fatty acids can modify the degree of unsaturation in lipids and thereby change the physicochemical environment of the microsomal membranes, which may be responsible for the decrease CYP1A1, CYP1A2 and CYP1B1 activities observed in cow ghee fed rats. The diminution of these phase I enzymes might have contributed to decreased incidence of mammary tumours in cow ghee fed rats compared to soybean oil ones4. Resveratrol inhibits CYP1A1 activity, and also prevents tumour development in 7,12-dimethylbenz(a) anthracene (DMBA) treated mice21,22.
Conjugated linoleic acid (CLA), a well documented anticarcinogenic agent23 present in dairy products inhibits 2-amino-3-methylimidazo[4,5f]quinoline (IQ) induced colon cancer by decreasing the activity of CYP1A1 and CYP1A224. In an in vitro study CLA (0.5 μM) was reported to inhibit CYP1A1, CYP1A2, and CYP1B1 activities in hamster liver microsome12. Cow ghee used in our study contained rich amount of CLA (5.47 mg/g), and a 12-fold increase in CLA content in rat mammary tissue by feeding cow ghee was earlier reported25.
The GST activity in liver and mammary tissue did not vary between soybean oil and cow ghee fed rats. The γ-glutamyltranspeptidase (GGTP) play an important role in detoxification of compounds such as carcinogens by formation of their mercapturic acid derivatives26. The increased GGTP activity, observed in present study, in liver and mammary tissue of rats on cow ghee compared to soybean oil diet, might be one of the contributing factors for protective effect of cow ghee on tumorigenesis4. Feeding rats with cow ghee also increased UDPGT activity in liver, which might have caused increased rate of glucuronidation of DMBA dihydrodiols leading to carcinogen detoxification. Elegbede et al27 reported that the anticarcinogenic activity of monotrepene was mediated through induction of hepatic detoxification enzymes GST and UDPGT.
To conclude, cow ghee opposed to soybean oil upregulated carcinogen detoxification activities of GGTP, UDPGT and QR in liver and GGTP and QR in mammary tissue, and downregulated the phase I activities of carcinogen activation, CYP1A1, CYP1A2, CYP1B1 and CYP2B1 in liver. These metabolic changes might have contributed to the decrease in DMBA induced incidence of mammary tumours observed in cow ghee fed rats compared to soybean oil fed ones4.
References
- 1.Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007. World Cancer Research Fund/American Institute for Cancer Research. [Google Scholar]
- 2.Fay MP, Freedman LS, Clifford CK, Midthune DN. Effect of different types and amounts of fat on the development of mammary tumors in rodents: A review. Cancer Res. 1997;57:3979–88. [PubMed] [Google Scholar]
- 3.MacLennan M, Ma DWL. Role of dietary fatty acids in mammary gland development and breast cancer. Breast Cancer Res. 2010;12:211–31. doi: 10.1186/bcr2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rani R, Kansal VK. Dietary intervention of cow Ghee versus soybean oil on 7, 12-dimethylbenz(a)anthracene induced mammary carcinogenesis and expression of cyclooxygenase-2 and peroxisome proliferators activated receptor- γ in rats. Indian J Med Res. 2010;133:497–503. [PMC free article] [PubMed] [Google Scholar]
- 5.Rani R, Kansal VK, Kaushal D, De S. Influence of dairy fat on expression of gene involved in cell cycle and apoptosis in carcinogen induced rat mammary carcinogenesis. Mol Bio Rep. 2011;38:3299–307. doi: 10.1007/s11033-010-0435-1. [DOI] [PubMed] [Google Scholar]
- 6.Morgado N, Sanhueza J, Nieto S, Valenzuela A. Effect of the degree of hydrogenation of fish oil on the enzymatic activity and on the fatty acid composition of hepatic microsomes from young and aged rats. Ann Nutr Metab. 2003;47:124–131. doi: 10.1159/000070034. [DOI] [PubMed] [Google Scholar]
- 7.Talaska G, Warshawsky D, Heffelfinger S, Gear R, Schnieder J, Schumann B, et al. 3rd Annual BCERC Early Environmental Exposures Conference. Berkeley, CA: 2006. Nov 2-3, Dietary Fat composition and intake affects DMBA metabolism and DNA adduct formation in breast organoids. [Google Scholar]
- 8.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad-hoc committee on the reformation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 9.Cinti DL, Moldeus P, Schenkman JB. Kinetic parameters of drug-metabolizing enzymes in Ca2+- sedimented microsomes from rat liver. Biochem Pharmacol. 1972;21:3249–56. doi: 10.1016/0006-2952(72)90089-5. [DOI] [PubMed] [Google Scholar]
- 10.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 11.Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer T. Ethoxy-,pentoxy-, and benzyloxyphenoxazones and homologues: a series of substances to distinguish between different induced cytochrome P450. Biochem Pharmacol. 1985;34:337–45. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 12.Teel RW, Huynh H. Modulation by phytochemicals of cytochrome P450- linked enzyme activity. Cancer Lett. 1998;133:135–41. doi: 10.1016/s0304-3835(98)00218-3. [DOI] [PubMed] [Google Scholar]
- 13.Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation: a microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985;13:548–52. [PubMed] [Google Scholar]
- 14.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase: The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 15.Bock KW, Burcell B, Dutton GJ, Hanninen O, Mulder GJ, Owens IS, et al. UDP-glucuronosyltransferase activities.Guidelines for consistent interim terminology and assay conditions. Biochem Pharmacol. 1983;32:953–5. doi: 10.1016/0006-2952(83)90610-x. [DOI] [PubMed] [Google Scholar]
- 16.Martin ST, Black SD. Detergent effects in rabbit liver microsomal UDP-glucuronosyltransferase studied by means of a continuous spectrophotometric assay with p-nitrophenol. Biochem Biophys Res Commun. 1994;200:1093–8. doi: 10.1006/bbrc.1994.1562. [DOI] [PubMed] [Google Scholar]
- 17.Tate SS, Meister A. Interaction of gamma-glutamyl transpeptidase with amino acids, dipeptides and derivatives and analogs of glutathione. J Biol Chem. 1974;249:7593–602. [PubMed] [Google Scholar]
- 18.Ernster L. DT Diaphrose. Methods Enzymol. 1967;10:309–17. [Google Scholar]
- 19.Morgado N, Galleguillos A, Sanhueza J, Garrido A, Nieto S, Valenzuela A. Effect of the degree of hydrogenation of dietary fish oil on the trans fatty acid content and enzymatic activity of rat hepatic microsomes. Lipid. 1998;33:669–73. doi: 10.1007/s11745-998-0255-1. [DOI] [PubMed] [Google Scholar]
- 20.Satio BM, Oh-Hashi A, Kubota M, Nishide E, Yamaguchi M. Mixed function oxidases in response to different types of dietary lipids in rats. Br J Nutr. 1990;63:249–57. doi: 10.1079/bjn19900112. [DOI] [PubMed] [Google Scholar]
- 21.Chun YJ, Kim MF, Guengerich FP. Reserveratol is a selective human cytochrome P450 1A1 inhibitor. Biochem Biophys Res Commun. 1999;262:20–4. doi: 10.1006/bbrc.1999.1152. [DOI] [PubMed] [Google Scholar]
- 22.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of reseveratol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 23.Ip C, Briggs SP, Haegele AD, Thompson HJ, Storkson J, Scimeca JA. The efficacy of conjugated linoleic acid in mammary cancer prevention is independent of the level or type of fat in the diet. Carcinogenesis. 1996;17:1045–50. doi: 10.1093/carcin/17.5.1045. [DOI] [PubMed] [Google Scholar]
- 24.Liew C, Schut HAJ, Chin SF, Pariza MW, Dashwood RH. Protection of conjugated linoleic acids against 2-amino-3-methylimidazo[4,5f] quinoline-induced colon carcinogenesis in the F344 rat: A study of inhibitory mechanisms. Carcinogenesis. 1995;16:3037–43. doi: 10.1093/carcin/16.12.3037. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia E. Karnal, India: National Dairy Research Institute; 2005. Comparative studies on bioprotective properties of cow and buffalo Ghee. Ph.D thesis. [Google Scholar]
- 26.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 27.Elegbede JA, Maltzman TH, Elson CE, Gould MN. Effects of anticarcinogenic monoterpenes on phase II hepatic metabolizing enzymes. Carcinogenesis. 1993;14:1221–5. doi: 10.1093/carcin/14.6.1221. [DOI] [PubMed] [Google Scholar]


