Abstract
Background & objectives:
The steroidal estrogen 17α-ethynyl estradiol (EE) is an orally bio-active estrogen used in almost all modern formulations of estrogen-progestin combination preparations of oral contraceptives. Contrasting effects of treatment with combined oral contraceptives on bone mineral density of pre-, peri-, and post-menopausal women have been reported, and it has been suggested that the estrogen dose and the type of progestogen may be the main contributing factors for these contrasting results. The objective of this study was to evaluate the effects of EE on osteoprecursor cells.
Methods:
The effects of single component of oral contraceptive, EE, were tested to see the relationship between EE and osteoblast proliferation, differentiation and mineralization. Tests used included a cell viability test, alkaline phosphatase (ALP) test, alizarin red-S staining, and a Western blot analysis. The effect on cell viability was determined by MTT assay. Differentiation and mineralization were examined using an ALP test and alizarin red-S staining. Protein expressions related to bone formation, such as estrogen receptor-alpha (ER-α), estrogen receptor-beta (ER-β), bone morphogenetic protein-2 (BMP-2), osteocalcin (OCN), and osteopontin (OPN) were evaluated by using a Western blot analysis.
Results:
Cultures growing in the absence of EE presented the lowest value for the MTT value. However, there were no significant changes in viability/proliferation when EE was added in the medium. Cultures growing in the absence of EE presented the highest value for the ALP activity, and the additional presence of EE resulted in dose-dependent decrease concerning ALP activity.
Interpretation & conclusions:
Our finding showed that EE in tested dosage within MC3T3-E1 cells seem to affect the proliferation and differentiation; however, significant differences are achieved in ALP activity in early differentiation phase and further studies are needed to elucidate the mechanisms of EE on bone.
Keywords: 17-α ethynyl estradiol, differentiation, mineralization, osteoblast, proliferation
The first orally active semisynthetic steroidal estrogen, 17α-ethynyl estradiol (EE), is the 17α-ethynyl analogue of estradiol1. EE is an orally bioactive estrogen used in almost all modern formulations of the estrogen-progestin combination preparations of oral contraceptives2.
The reported positive effects of oral contraceptives (OC) regarding bone health probably are based on evidence that a combined estrogen-progestin therapy is positive for bone mineral density (BMD) in menopausal women3. However, there are relatively a few cross-sectional studies of OC use and BMD that have focused on pre-menopausal women4. Discordant results have been reported with combined oral contraceptives on bone mineral density of pre- and peri-menopausal women, and estrogen dose and the type of progestogen are thought to be the main contributing factors for these contrasting results5. Thus, a single component of the oral contraceptive, EE was tested to determine the relationship between EE and osteoblast proliferation, differentiation, and mineralization.
This in vitro study was carried out to evaluate the effects of EE on osteoprecursor cells. Murine preosteoblast cells (MC3T3-E1 cells) were cultured and different doses of EE were tested for viability, proliferation, osteoblast differentiation and mineralization.
Material & Methods
The study was carried out in The Catholic University of Korea, Seoul, Korea.
Materials: Minimum essential medium α (MEM α), foetal bovine serum (FBS), and trypsin/EDTA solution were purchased from Invitrogen (Carlsbad, USA). Unless otherwise stated, chemicals and reagents were obtained from Fisher Scientific Co. or Sigma, USA.
Cell culture: Murine preosteoblast cells (MC3T3-E1 cells) (ATCC, USA) were grown in MEM α containing 10 per cent FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Culture media were changed to differentiation medium [MEM α supplemented with 50 μg/ml ascorbic acid (AA) and 10 mM β-glycerophosphate (GP)] to induce osteogenic differentiation.
MTT assay: Cells were plated at a density of 1.0 × 104 cells/ml/well in 12-well plates. The cells were incubated in the presence of EE at final concentrations ranging from 0.01 to 10 nM6,7. At the end of the incubation time, cells were incubated for 1 h with the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reagent with a final concentration of 0.5 mg/ml. Washing with phosphate-buffered saline (PBS, pH 7.4) was followed by the addition of dimethyl sulphoxide. Complete dissolution was achieved after gentle shaking. Aliquots of the resulting solutions were transferred in 96-well plates, and absorbance was recorded at 560 and 670 nm using the microplate spectrophotometer8.
Protein measurement: Cells were plated and incubated in MEM α in the presence of AA and GP for four days. Protein content was determined based on the Bradford method using the Coomassie protein assay reagent9,10. The absorbance was measured at 595 nm using the microplate spectrophotometer system, and results were presented as percentage of control values.
Alkaline phosphatase activity: Alkaline phosphatase (ALP) activity was determined by enzymatic assay9,11. MC3T3-E1 cells, grown until 70 per cent confluent, were treated with combination of AA and GP for a period of four days. After treatment, the cells were rinsed with PBS and then lysed into buffer containing 10 mM Tris-HCl pH 7.4, 0.2 per cent triton X-100. Cell lysate was centrifuged, and the soluble fraction was sonicated on ice for enzyme assay. Samples were added to glycine buffer (100 mM, pH 10.5) containing 10 mM p-nitrophenylphosphate and 1mM MgCl2 and incubated at 37°C in a water bath. Total protein content was determined in comparison with series of bovine albumin serum as internal standards. The optical density of p-nitrophenol at 405 nm was determined spectrophotometrically, and ALP activities were normalized with respect to total protein content.
Mineralization/calcium deposition assay: MC3T3-E1 cells, grown until 70 per cent confluent, were treated with combination of AA and GP for a period of 14 days. The cells were washed twice with PBS and then fixed with 70 per cent ethanol for 1 h. The cultures were stained with 40 mM alizarin red S for 30 min with gentle shaking12,13. To quantify the bound dye, the stain was solubilized with 10 per cent cetyl pyridinium chloride by shaking. The absorbance of the solubilized stain was measured at 562 nm14.
Western blot analysis: MC3T3-E1 cells were washed four times with ice cold phosphate-buffered saline and solubilized in lysis buffer (50 mM Tris-HCl, pH 8.0, with 150 mM sodium chloride, 1.0% Igepal, 0.5% odium deoxycholate, and 0.1% sodium dodecyl sulphate, SDS). The lysates were centrifuged at 21,000 g for 20 min at 4°C. The supernatants were boiled in a SDS sample buffer containing β-mercaptoethanol. These samples were separated by SDS polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and immunoblotted with the corresponding antibodies with enhancement by enhanced chemiluminescent detection kits (Pierce Biotechnology, Inc., USA). Mouse antibodies against estrogen receptor-α (ER-α), estrogen receptor-β (ER-β), bone morphogenetic protein-2 (BMP-2), osteocalcin (OCN), osteopontin (OPN), β-actin, and secondary antibodies linked with horseradish peroxidase were purchased from Santa Cruz Biotechnology (Santa Cruz, USA), Cell Signaling Technology, Inc. (Danvers, USA), and BD Biosciences (San Jose, USA).
Statistical analysis: Results were represented as means ± SD (n=3), and data analysis was performed with the commercially available program (PASW Statistics 17.0.2, SPSS, Inc., Chicago, USA). Two-way analysis of variance (ANOVA) with post-hoc was used to test for differences between groups.
Results
Viability/proliferation of the cells with MTT assay: MC3T3-E1 cell cultures growing in the absence of EE showed the lowest viablity. However, there were no significant changes in viability/proliferation when EE was added in the medium.
Viability/proliferation of the cells with protein measurement: The protein content in each culture plate was evaluated. The results showed that the protein content in the treated groups did not show any significant differences compared with the control group (Table I).
Table I.
Table showing protein measurement, relative value of alkaline phosphatase activity and mineralization
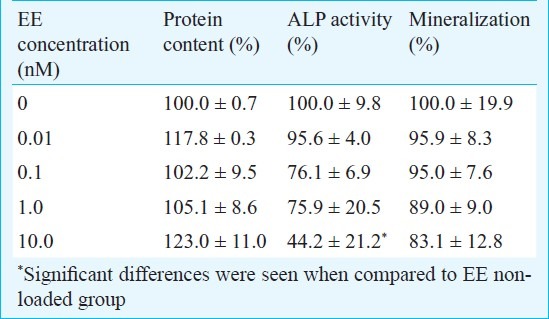
ALP activity assay: Cultures growing in the absence of EE presented the highest value for the ALP activity, and the presence of EE resulted in dose-dependent decrease in ALP activity. However, a significant difference was only seen between 0 (EE non-loaded group) and 10nM EE group (P<0.05) (Table I).
Mineralization assay: Cultures without EE showed the highest mineralized nodule formation. The cultures grown in the presence of EE showed dose-dependent reduction in mineralization. However, statistically significant differences were not achieved between the tested groups (Table I).
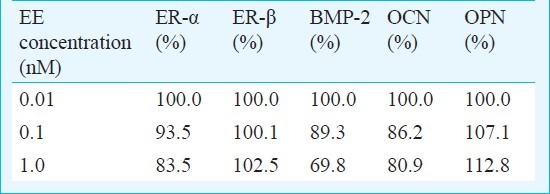
Western blot: Western blot analysis showed that the addition of EE decreased the expression of ER-α, BMP-2, and OCN after normalization by β-actin expression. The addition of EE increased the expression of OPN. The expression of ER-β was not influenced by EE (Table II).
Table II.
Quantitative analysis of protein expression of ER-α, ER-β, BMP-2, OCN and OPN after normalization with β-actin levels by densitometry
Discussion
Considerable controversy exists as to whether or not OCs possess a positive influence on bone15. Prolonged use of OCs may adversely impact young adult women's bone density16. Thus, this study was performed to evaluate short- and long-term effects of EE on proliferation, differentiation, mineralization, and protein expression in MC3T3-E1 cells.
Viability and proliferation were evaluated using the MTT and protein concentration. The MTT assay may be a more sensitive assay to assess osteoblast proliferation because it measures cell viability through the determination of mitochondrial dehydrogenase activity17. The protein assay is an indirect measurement of cell viability since it measures the protein content of viable cells that are left after washing of the treated plates18. The results show no significant effect of EE on cell number.
Osteoblast differentiation was assessed by ALP activity, an early marker of osteoblastic cell differentiation19. EE seemed to decrease osteoblastic differentiation of MC3T3-E1 cells, and a significant difference was noticed at 10nM concentration. Similar findings have been reported earlier20,21.
After the period of matrix maturation, nodule cells begin to mineralize the extracellular matrix22. In this study, the presence of calcium in cellular deposits was confirmed by alizarin red S staining with application of cetylpyridinium chloride for quantification23. The control group showed the highest mineralized nodule formation, and the addition of EE caused a dose-dependent reduction in mineralization.
Estrogens and estrogen receptor modulators bind to ER-α and/or ER-β to form discrete molecular complexes that exert pleiotropic tissue-specific effects by modulating the expression of target genes24. In the present study, EE affected ER-α more than ER-β. It was previously reported that the cellular effects of estrogens are mediated predominantly by the action of ER-α25. In the in vivo model, mice with loss of functional mutations of the ER-α gene showed only minor skeletal abnormalities with reduced longitudinal bone growth and modest reduction in bone mineral density26.
OPN is an important mediator of bone remodeling, and has been reported as a negative regulator of calcification, likely through inhibition of mineral crystal growth at a later stage of osteogenic differentiation27. An increase of OPN by overexpressing OPN mRNA shows significant decrease in BMP-2-inducible alkaline phosphatase activity, expression of ECM genes, and mineral deposition27. The Western blot data in this study showed that the expression of OPN increased with dose-dependent decrease of BMP-2.
In conclusion, the present data show that EE in tested dosage on MC3T3-E1 cells affect the proliferation and differentiation; however, significant differences are achieved in ALP activity in early differentiation phase. This suggests that the effect of estrogen on osteoblasts depends on multiple factors such as differentiation stage, species differences, and cell system21. Thus, the bone-preserving action of estrogen in in vivo model might be mediated predominantly through effects on osteoclast number and activity rather than osteoblast number and activity26. It should be also considered that the effects of estrogen and other circulating hormones on bone remodeling are mediated not only by their direct actions on osteoclasts and osteoblasts but also by their interaction with this complex network28, and there are limitations in extrapolating in vitro data directly to in vivo situation.
References
- 1.Djerassi C. Chemical birth of the pill. 1992. Am J Obstet Gynecol. 2006;194:290–8. doi: 10.1016/j.ajog.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Pinter B, Kocijancic A, Marc J, Andolsek-Jeras L, Prezelj J. Vitamin D receptor gene polymorphism and bone metabolism during low-dose oral contraceptive use in young women. Contraception. 2003;67:33–7. doi: 10.1016/s0010-7824(02)00432-8. [DOI] [PubMed] [Google Scholar]
- 3.Prior JC, Kirkland SA, Joseph L, Kreiger N, Murray TM, Hanley DA, et al. Oral contraceptive use and bone mineral density in premenopausal women: cross-sectional, population-based data from the Canadian Multicentre Osteoporosis Study. CMAJ. 2001;165:1023–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Allali F, El Mansouri L, Abourazzak F, Ichchou L, Khazzani H, Bennani L, et al. The effect of past use of oral contraceptive on bone mineral density, bone biochemical markers and muscle strength in healthy pre and post menopausal women. BMC Womens Health. 2009;9:31. doi: 10.1186/1472-6874-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nappi C, Di Spiezio Sardo A, Greco E, Tommaselli GA, Giordano E, Guida M. Effects of an oral contraceptive containing drospirenone on bone turnover and bone mineral density. Obstet Gynecol. 2005;105:53–60. doi: 10.1097/01.AOG.0000148344.26475.fc. [DOI] [PubMed] [Google Scholar]
- 6.Naciff JM, Khambatta ZS, Thomason RG, Carr GJ, Tiesman JP, Singleton DW, et al. The genomic response of a human uterine endometrial adenocarcinoma cell line to 17 alpha-ethynyl estradiol. Toxicol Sci. 2009;107:40–55. doi: 10.1093/toxsci/kfn219. [DOI] [PubMed] [Google Scholar]
- 7.Park JB. Effects of low dose of estrone on the proliferation, differentiation, and mineralization of the osteoprecursor cells. Exp Ther Med. 2012;4:681–4. doi: 10.3892/etm.2012.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JB. The effects of dexamethasone, ascorbic acid, and beta-glycerophosphate on osteoblastic differentiation by regulating estrogen receptor and osteopontin expression. J Surg Res. 2012;173:99–104. doi: 10.1016/j.jss.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Park JB, Zhang H, Lin CY, Chung CP, Byun Y, Park YS, et al. Simvastatin maintains osteoblastic viability while promoting differentiation by partially regulating the expressions of estrogen receptors alpha. J Surg Res. 2012;174:278–83. doi: 10.1016/j.jss.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Park JB. Low dose of doxycyline promotes early differentiation of preosteoblasts by partially regulating the expression of estrogen receptors. J Surg Res. 2012 doi: 10.1016/j.jss.2012.03.072. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Park JB. Effects of fibroblast growth 2 on osteoblastic proliferation and differentiation by regulating bone morphogenetic protein receptor expression. J Craniofac Surg. 2011;22:1880–2. doi: 10.1097/SCS.0b013e31822e8434. [DOI] [PubMed] [Google Scholar]
- 13.Park JB. Effects of doxycycline, minocycline, and tetracycline on cell proliferation, differentiation, and protein expression in osteoprecursor cells. J Craniofac Surg. 2011;22:1839–42. doi: 10.1097/SCS.0b013e31822e8216. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Gupta D, Panetta NJ, Levi B, James AW, Wan D, et al. Elucidating mechanisms of osteogenesis in human adipose-derived stromal cells via microarray analysis. J Craniofac Surg. 2010;21:1136–41. doi: 10.1097/SCS.0b013e3181e488d6. [DOI] [PubMed] [Google Scholar]
- 15.Warner KE, Jenkins JJ. Effects of 17alpha-ethinylestradiol and bisphenol A on vertebral development in the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2007;26:732–7. doi: 10.1897/06-482r.1. [DOI] [PubMed] [Google Scholar]
- 16.Scholes D, Hubbard RA, Ichikawa LE, LaCroix AZ, Spangler L, Beasley JM, et al. Oral contraceptive use and bone density change in adolescent and young adult women: a prospective study of age, hormone dose, and discontinuation. J Clin Endocrinol Metab. 2011;96:E1380–7. doi: 10.1210/jc.2010-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JB. Combination of simvastatin and bone morphogenetic protein-2 enhances differentiation of osteoblastic cells by regulating the expressions of phospho-Smad1/5/8. Exp Ther Med. 2012;4:303–6. doi: 10.3892/etm.2012.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160:171–7. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Shen Q, Zhu S, Hu J, Geng N, Zou S. Recombinant human bone morphogenetic protein-4 (BMP-4)-stimulated cell differentiation and bone formation within the expanding calvarial suture in rats. J Craniofac Surg. 2009;20:1561–5. doi: 10.1097/SCS.0b013e3181b09cc1. [DOI] [PubMed] [Google Scholar]
- 20.Keeting PE, Scott RE, Colvard DS, Han IK, Spelsberg TC, Riggs BL. Lack of a direct effect of estrogen on proliferation and differentiation of normal human osteoblast-like cells. J Bone Miner Res. 1991;6:297–304. doi: 10.1002/jbmr.5650060312. [DOI] [PubMed] [Google Scholar]
- 21.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–96. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 22.Porter RM, Huckle WR, Goldstein AS. Effect of dexamethasone withdrawal on osteoblastic differentiation of bone marrow stromal cells. J Cell Biochem. 2003;90:13–22. doi: 10.1002/jcb.10592. [DOI] [PubMed] [Google Scholar]
- 23.Guo X, Gough JE, Xiao P, Liu J, Shen Z. Fabrication of nanostructured hydroxyapatite and analysis of human osteoblastic cellular response. J Biomed Mater Res A. 2007;82:1022–32. doi: 10.1002/jbm.a.31200. [DOI] [PubMed] [Google Scholar]
- 24.Pinzone JJ, Stevenson H, Strobl JS, Berg PE. Molecular and cellular determinants of estrogen receptor alpha expression. Mol Cell Biol. 2004;24:4605–12. doi: 10.1128/MCB.24.11.4605-4612.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai Y, Youn MY, Kondoh S, Nakamura T, Kouzmenko A, Matsumoto T, et al. Estrogens maintain bone mass by regulating expression of genes controlling function and life span in mature osteoclasts. Ann N Y Acad Sci. 2009;1173(Suppl 1):E31–9. doi: 10.1111/j.1749-6632.2009.04954.x. [DOI] [PubMed] [Google Scholar]
- 26.Compston JE. Sex steroids and bone. Physiol Rev. 2001;81:419–47. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, Carlsen B, Rudkin G, Berry M, Ishida K, Yamaguchi DT, et al. Osteopontin is a negative regulator of proliferation and differentiation in MC3T3-E1 pre-osteoblastic cells. Bone. 2004;34:799–808. doi: 10.1016/j.bone.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Lemaire V, Tobin FL, Greller LD, Cho CR, Suva LJ. Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J Theor Biol. 2004;229:293–309. doi: 10.1016/j.jtbi.2004.03.023. [DOI] [PubMed] [Google Scholar]


