Abstract
Background & objectives:
The discrimination between the Staphylococcus epidermidis colonizing the deep seated indwelling devices and those which are mere commensals has always been a challenge for the clinical microbiologist. This study was aimed to characterize the S. epidermidis isolates obtained from device related infection for their phenotypic and molecular markers of virulence and to see whether these markers can be used to differentiate the pathogenic S. epidermidis from the commensals.
Methods:
Fifty five S. epidermidis isolates from various device related infections such as endophthalmitis following intra-ocular lens (IOL) implantation, intravascular (IV) catheter related sepsis and orthopaedic implant infections, were studied for slime production, biotyping, antibiotic sensitivity; and mec A and ica positivity by the recommended procedures.
Results:
Twenty three (41.8%) isolates were multi-drug resistant, 26 (65.2%) were slime producers, 30 (54.5%) were adherent, 23 (41.8%) possessed the intercellular adhesin (ica) gene, and 28 (50.9%) harboured the mec A gene. Biotypes I and III were the commonest, most members of which were multi- drug resistant. Twenty two (73.3%) of the 30 adherent bacteria were slime producers as opposed to only 4 (16%) of the 25 non-adherent bacteria (P<0.001). A vast majority i.e. 21 (91.3%) of the 23 ica positive organisms were adherent to artificial surfaces in contrast to only 9 (28.1%) of the 32 non-ica positive organisms (P<0.001). Twenty (86.9%) of the 23 ica positive bacteria were slime producers, as opposed to only 6 (18.7%) of the 32 ica negative bacteria (P<0.001). Of the 23 multi-drug resistant isolates, 19 (82.6%) carried the mec A gene.
Interpretation & conclusions:
The present findings showed that ica AB and mec A were the two important virulence markers of S. epidermidis in implant infections and slime was responsible for the sessile mode of attachment on the devices.
Keywords: Adherence, Biofilms, device associated infections, implant related infections, multi drug resistance, slime, Staphylococcus epidermidis
During the last two decades, Staphylococcus epidermidis and other coagulase negative staphylococci (CoNS) have emerged as major causative agents of nosocomial infections1. These organisms, which constitute the main component of the normal skin and mucosal microflora, are particularly responsible for catheter and other medical device related infections1,2. The pathogenesis of S. epidermidis in device associated infections mostly relies on the potential of the bacterium to adhere to the device surface3,4.
Clinical and laboratory evidences support the view that significantly higher numbers of slime producing S. epidermidis isolates are adherent to artificial surfaces than the slime negative isolates5–7. Recent observations also documented that cell to cell aggregation and biofilm formation, subsequent to the bacterial attachment to the device were mediated by a component of slime i.e. the polysaccharide intercellular ahesin (PIA), which was encoded by the chromosomal ‘ica’ gene locus7. Besides the above virulence factors that are involved in the process of adherence and biofilm formation, the emergence of multidrug resistance, including methicillin resistance amongst the nosocomial S. epidermidis is a major threat to the clinician for the patient management8.
Thus, the purpose of the present study was to characterize the S. epidermidis isolates obtained from device related infections in terms of their phenotypic and molecular markers of virulence and to investigate if these markers would differentiate the S. epidermidis that cause device infections from those that are mere commensals, and to address if such characterization would eventually benefit in making clinical decisions to ascribe a particular isolate as a pathogen and not merely as a commensal.
Material & Methods
Study design: The study was conducted in the Department of Ocular Microbiology, Dr Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi. The protocol was approved by the ethics committee of the Institute. A total of 91 patients (29 females and 62 males) ranging in the age group of 29 to 67 yr, all with indwelling implant related infections were included in the study during the period from March 2008 to June 2010, after obtaining the informed consent from the subjects.
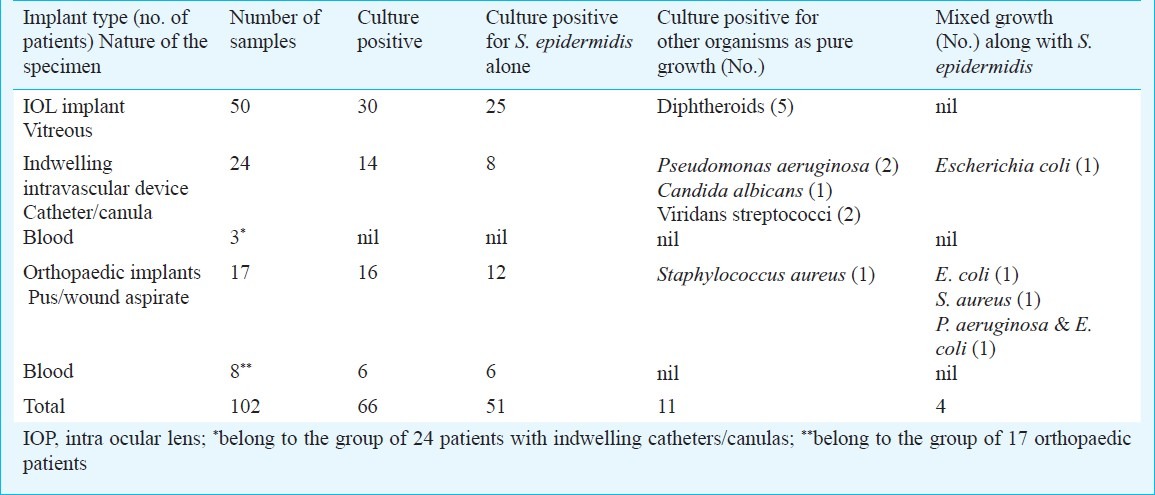
Subjects and case definitions: Vitreous samples were collected from 50 subjects with post-operative endophthalmitis following intra-ocular lens (IOL) implantation. Intravascular catheters/canulas were collected from 24 patients with catheter related sepsis, and pus/wound aspirates were taken from 17 subjects having infections due to implanted orthopaedic devices. Additionally, blood samples were collected from 3 of the 24 patients with catheter infections and from 8 of the 17 with orthopaedic implant infections amounting to a total of 102 samples from 91 patients (Table I).
Table I.
Culture findings and details of samples collected in patients with various implants
Individuals with features of endophthalmitis such as increasing pain and redness, decreasing visual acuity, flare in the anterior chamber, corneal oedema, hypopyon, and poor glow within 4-6 wk following IOL implantation were termed as the cases of late onset post-operative endophthalmitis (POE)9. The 24 patients with indwelling central venous catheters/intravascular canulas had clinical evidences of infection (catheter related sepsis) characterized by fever >38° C, pulse rate of more than 90/min, respiration rate of more than 20/min and WBC count of >12000/μl of blood. In addition, subjects with localized infection at the exit of the truncated tract were also considered as having catheter related infections10.
Those having either localized inflammatory signs at the implant site or signs of sepsis, as defined above, owing to implantation of joint prostheses, nails, plates and bone cements were designated to have orthopaedic implant associated infections.
The criteria for sample size and sample selection were based upon the number of patients available at that particular point of time, fulfilling the aforementioned parameters of the case definitions. Decision to collect pus or aspirate was in accordance with the type of the lesion. Additional blood samples were taken from patients having infections with orthopaedic or intravascular devices as and when the patients developed signs and symptoms of sepsis.
Patients with POE: About 0.1 ml of vitreous fluid was collected with the help of sterile tuberculin syringe and 26 gauge needle. The beveled tip of the needle was closed with a sterile rubber bung and was transported to the laboratory immediately. The vitreous fluid was stained and cultured according to the standard procedures described earlier11.
Patients with intravascular implants: The tips of intravascular catheters/canulas and/or central venous catheters were collected aseptically, in sterile test tubes, and were immediately inoculated onto blood agar plates according to the rolling technique improvised by Maki et al12 and subsequently onto trypticase soy broth (TSB). The blood agar plates and TSB tubes were incubated at 37°C maximum up to 48 h. For blood culture, the sample of blood was inoculated directly onto the broth.
Patients with orthopaedic implants: Aspirate was collected with sterile syringe and needle after properly disinfecting the surrounding skin. Pus or discharge was collected by rubbing the bed of the ulcer with sterile cotton tipped swab. If the material was insufficient, then wound was squeezed and the exuded purulent material was collected. Intra-operative pus, if obtained, was directly inoculated onto TSB. Blood was also collected for culture if there was indication of sepsis. Additionally, the pus, aspirate, discharge were subjected to Gram staining. The material inoculated into TSB was incubated at 37°C. Growth from TSB was subcultured onto blood agar, MacConkey's agar and Chocolate agar plates, which were incubated at 37°C.
After overnight incubation at 37°C, those colonies showing Gram-positive cocci on smear examination were processed further. The organisms were confirmed as CoNS by their catalase positive reaction from growth on nutrient agar, ability to ferment glucose and inability to coagulate rabbit plasma both by the slide and the tube coagulase tests13. All isolates were speciated according to the Baird-Parker's modified scheme14 of the original method of Kloos and Schleifer15, and those identified as S. epidermidis were stored at -20°C as nutrient agar stab cultures until further testing. In addition, 26 commensal S. epidermidis from the hands and the conjunctival swabs of healthy volunteers isolated and identified exactly by the same procedure as mentioned above were also included. The volunteers who provided the commensal organisms did not have any contact to medical facilities or hospital settings. These commensals were also subjected to the same phenotypic and molecular characterization methods in order to look for any discrimination between the deep seated and the commensal S. epidermidis.
Biotyping and antibiotic sensitivity testing: Biotyping was done by the standard protocol using Voges-Proskauer test, phosphatase test, and fermentation of lactose, maltose and mannitol16. Antibiotic sensitivity testing was performed by the standard Kerby Bauer disc diffusion method17. The antibiotics (Hi-media, Mumbai, India) and their antimicrobial content/disc (μg) were tetracycline (30), chloramphenicol (10), genatmicin (10), cloxacillin (1), ciprofloxacin (5), gatifloxacin (5), moxifloxacin (5), tobramycin (10), vancomycin (30), cephazolin (30), and ceftazidime (30). Bacteria showing resistance to 3 or more antibiotics were labelled as multidrug resistant18.
Slime test: Test for slime production was carried out by the Congo Red Agar (CRA) plate method described earlier by Freeman et al19.
Quantitative slime test for adherence: Adherence of each isolate to smooth surfaces was determined quantitatively by the method of Christensen et al6 with only a very minor modification that quartz cuvettes were used in place of polysterine micro-titre well plates. Briefly, overnight cultures of bacteria in TSB were diluted 1 in 100 in fresh TSB and 1 ml volume of each was put into separate cuvettes. After overnight incubation at 37°C, the cuvettes were washed four times with phosphate buffered saline (PBS), fixed using Bouin's fluid (saturated solution of picric acid 75 parts, formaldehyde 25 parts and glacial acetic acid 5 parts) and then stained with Hucker's crystal violet. Excess stain was removed by decanting the cuvettes and then rinsing them very gently with tap water. The optical density (OD) of the stained bacterial biofilm was read spectrophotometrically (Spectrocolorimeter 103, Systronics, Baroda, India) at 570 nm. The cut-off OD was calculated which was three times the standard deviation (SD) above the mean OD of 10 blanks stained exactly by the same procedure.
DNA extraction: Bacterial DNA was extracted by the standard protocol improvised earlier, with some modifications1,7. Briefly, the isolates were grown on Mueller-Hinton agar (Himedia, India) at 37°C overnight. An aliquot (0.1 ml) from this culture (108 cfu) was prepared and pelleted by centrifugation (5000xg for 5 min). The bacterial pellet was resuspended in 450 μl of lysis buffer [150 μl of 0.1M Tris-HCl, pH 7.5, 30 μl of 10% SDS, 100 mM EDTA (Merck, India)] containing 30 μl of 100 μg/ml lysostaphin (Sigma, USA), 30 μl of 100 μg/ml proteinase K (Calibiochem, USA). Samples were treated with phenol-chloroform mixture (25:24) and centrifuged at 704 g for 5 min. The aqueous layer was separated and to it were added 1/10 volume of Na acetate and 0.6 volume of isopropanol, followed by washing with 70 per cent ethanol, and centrifugation at 7826 g for 5 min. The pellet containing the DNA was then suspended in 200 μl of TE buffer.
Polymerase chain reaction (PCR) for amplification of ica AB gene: The intercellular adhesin gene ‘ica’ responsible for the phenotypic character like biofilm, was amplified using the following primers; forward 5’-TTA TCA ATG CCG CAG TTG TC and reverse 5’-GTT TAA CGC GAG TGC GCT AT20. The previously employed method20 was followed after standardization as per our own laboratory conditions and with minor modifications. PCR reagent mixture consisted of 200 μM each of dATP, dTTP, dCTP and dGTP, 1.25 U of Taq polymerase and 10 pM of each PCR primer, in a reaction volume of 25 μl. A thermal amplification programme for icaA gene included the following parametrers: an initial denaturation at 94°C for 2 min, followed by 30 cycles of amplification (denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 2 min) and final extension at 72°C for 5 min. Amplification products (10 μl of each) were run on 2 per cent agarose gel electrophoresis along with molecular weight marker [50 base pair DNA ladder (Fermentas Life Sciences, Bangalore, India)].
PCR for amplification of mec A gene20: In order to verify the presence of the mec A gene, PCR was carried out using the following primers: mec A - F; GTA GAA ATG ACT GAA CGT CCG ATA A mec A- R ; CCA ATT CCA CAT TGT TTC GGT CTA A
The assay was performed in a final volume of 25μl reaction mixture, which consisted of 200 μM each of dATP, dTTP, dCTP and dGTP, 10 pM of mec A forward and reverse primers and 1.25 U of Taq polymerase. PCR reaction mixture was subjected to the following thermal cycling programme: initial denaturation for 4 min at 94°C, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, extension at 72°C for 1 min and with a final extension for 5 min. 10 μl of the amplified products were run on 2 per cent agarose gel electrophoresis along with the molecular marker (50 bp DNA ladder).
Statistical analysis: Chi-square test was employed to analyze the data to find out the significant associations amongst the various phenotypic and molecular characteristics of the isolates.
Results
Overall culture positivity was found in 66 (64.7%) samples, out of which 55 grew S. epidermidis (as pure growth in 51 samples and as mixed growth in 4; Table I). Other organisms recovered as pure growth were, 5 isolates of Diphtheroids from the vitreous fluid; 2 Pseudomonas aeruginosa, 1 Candida albicans and 2 Viridans Streptococci, all from intravascular catheters/canulas; and a single isolate of Staphylococcus aureus from a pus sample in a patient with orthopaedic implant. All three blood cultures in patients with intravascular devices were sterile. Of the 8 blood samples received from patients with orthopaedic implants, 6 yielded S. epidermidis.
Other organisms isolated as mixed growth along with S. epidermidis were Escherichia coli in two specimens (one from catheter and the other from pus), S. aureus in one pus specimen and P. aeruginosa plus E. coli in another pus specimen (Table I).
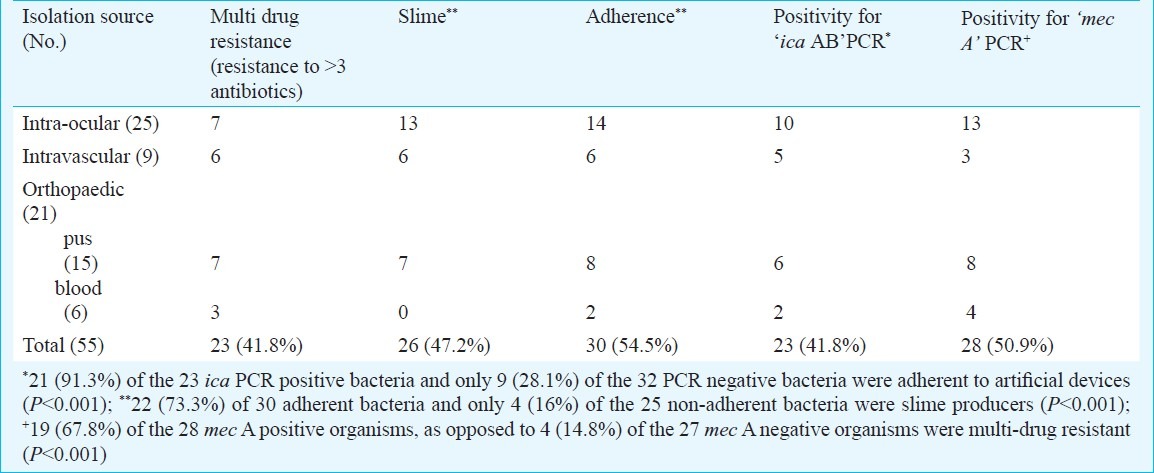
Phenotypic characteristics of S. epidermidis: Production of slime by all the isolates under study was assessed by culture on CRA plates. Slime producing organisms exhibited black shiny colonies with a metallic tinge, whereas nonslime producers looked pink. Of the total of 55 S. epidermidis isolates studied, 26 (47.2%) were found to be slime positive (Table II). A total of 30 (54.5%) isolates were adherent to artificial surfaces, as determined by our quantitative slime test, and 23 (41.8%) isolates were multi-drug resistant. A majority (22 of 30 i.e. 73.3%) of the adherent bacteria were slime producers, as compared to the non-adherent bacteria (only 4 out of 25; 16% being slime producers), and this difference was statistically significant (P<0.001).
Table II.
Virulence markers of the 55 implant associated S. epidermidis isolates
Our observation on the distribution of S. epidermidis biotypes colonizing the various implants revealed that biotypes I and III isolates were the commonest (47.2 and 32.7%, respectively), and majority of these [15 of 26 (57.7%) biotype I , and 10 of 18 (55.5%) biotype III] were multi-drug resistant (data not shown).
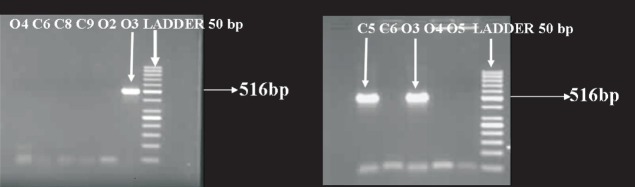
Molecular characterization of S. epidermidis: The ica AB amplified product was a 516 bp fragment (Fig. 1) and 23 (41.8%) of our isolates were positive in the ica PCR assay (Table II). On analyzing retrospectively, it was found that 20 (76.9%) of the 26 slime producing organisms were “ ica “positive. Contrary to this, there were only in 3 (10.3%) of the 29 non-slime producers which were “ica” positive. This difference was statistically significant (P<0.001), suggesting thereby that a vast majority of the bacteria carrying the ica genes possessed the capability to produce slime on colonization to the indwelling devices. It was also noted that majority of the bacteria positive for the ica AB PCR were adherent to artificial surfaces as compared to the PCR negative strains (Table II, P<0.001).
Fig. 1.
ica gene PCR; Lane C5: Isolate from IV catheter +ve; Lane C6: isolate from IV catheter -ve; Lane 3: isolate from pus +ve; Lane 4 & 5: isolates from pus −ve; Ladder 50 bp.
The mec A gene product was amplified as a 310 bp fragment (Fig. 2). A total of 28 (50.9%) isolates were mec A positive (Table II) and as would be expected most (19 of 28; 67.8%) mec A positive isolates were multi-drug resistant as compared to the mec A negatives (Table II, P<0.001).
Fig. 2.
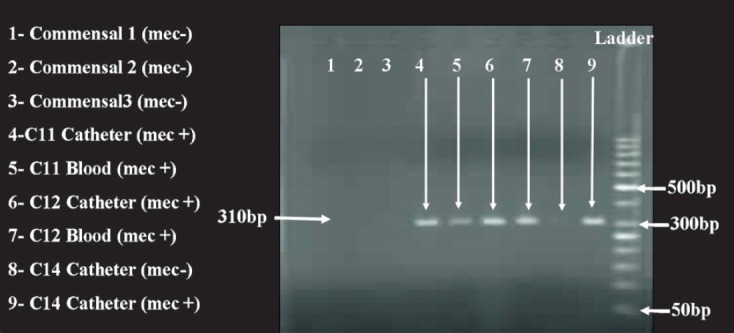
mecA gene PCR showing amplification of 310bp fragments in lanes 4, 5, 6, 7 and 9. Lanes 1,2 and 3 denote commensal S. epidermidis. Ladder 50bp
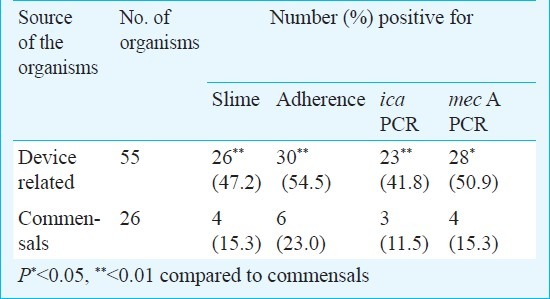
Virulence markers amongst the deep vs. commensal isolates: There were statistically significant differences in the ica, mec A gene positivities and in the presence of the phenotypic markers like slime and adherence; between the device associated i.e. the deep and the commensal organisms (Table III). Multi-drug resistance, however, was not found to be significantly higher amongst the device associated bacteria as compared to the commensal bacteria.
Table III.
Phenotypic and molecular markers amongst device associated and commensal bacteria
Discussion
During the last two decades, S. epidermidis has emerged as an important cause of infections in patients with indwelling medical devices21. Slime as a virulence factor of S. epidermidis in medical implant/device related infections was documented in the past1,4,22. It was also shown that comparatively higher number of slime producing isolates of S. epidermidis from the keratitic lesions were adherent to artificial surfaces as compared to the commensal isolates from the eye5. In addition, the gene responsible for biofilm production i.e. the ica operon was identified in the majority of S. epidermidis isolates from catheter related sepsis, whereas none of the commensal isolates from skin and mucosa of healthy volunteers possessed this intercellular adhesin gene7. This however, was an isolated report on catheter related infections only, and not on any other device associated conditions.
The present study showed that 47.2 per cent of the isolates were slime positive. This is in good agreement with the reports of Arciola et al7 who noted that 48.5 per cent of their clinical isolates of S. epidermidis were slime positive. We also observed previously that approximately 43 per cent of the corneal ulcer isolates of S. epidermidis were slime producers5. The marginally higher rate of slime positivity amongst our present isolates as compared to those in our previous studies5,23 on keratitis, suggests that S. epidermidis colonizing the indwelling device may be more virulent than those colonizing the corneal surface. Whereas Arciola et al7 studied slime production on intravascular catheter isolates only; ours were not only from vascular devices, but also from other sources such as IOLs and orthopaedic implants.
Our results indicated that bacteria carrying ‘ica’ operon mostly possessed the quality of slime production. These organisms, upon colonization to indwelling devices having the potential to adhere, could trigger the intriguing pathway of slime production, intercellular adhesion leading eventually to biofilm formation that is very crucial for the rigid sessile form of attached bacteria onto the device1,24.
Adherence of coagulase negative Staphylococci was earlier analysed by Muller et al25 who observed that more number of polysaccharide adhesin (PS/A) positive organisms bound to 1.5 cm segments of silicon-elastomer catheters after 15 min of exposure, than did the PS/A negative isolates. Zmantar and colleagues26 reported the association between slime production and the presence of ica A/D genes by PCR assay in 46 clinical isolates of S. aureus. The gene profile of all the clinical isolates of S. epidermidis from orthopaedic prostheses associated infections was compared with the phenotypic biofilm forming ability evaluated by CRA method27 and it was noted that 57 per cent of the biofilm producing isolates turned out to be ica positive. Duggirala and colleagues28 reported that ica AB was positive in 69.64 per cent of their clinical isolates of S. epidermidis from cases of keratitis and endophthalmitis. But they did not compare ica positivity of their isolates with biofilm forming ability, though biofilm production was studied as a separate entity. Unlike others27, we noticed that 70 per cent of the biofilm forming (adherent) organisms in our study were ica positive, all these isolates being from various clinical implants, and not merely from orthopaedic prostheses. This might suggest that in different in vivo conditions and in the presence of varying colonizing surfaces, the organism may behave differently with varying capabilities for the expression of phenotypic and genotypic characters22,24.
S. epidermidis biotypes I and III were the most common and virulent forms, exhibiting multi drug resistance patterns associated with the deep seated device related organisms. This is in contrast to corneal ulcer isolates most of which belonged to biotype II and I5 and no definite multi drug resistance pattern could be ascribed to these. If such predisposition between certain S. epidermidis strain types and particular device colonization can be established, in future, by analyzing a large number of invasive clinical isolates, this will have an impact on the patient management.
The present study showed significantly higher number of device associated organisms to be slime producers and adherent as compared to the commensals. In addition, the amplification of ica AB and mec A genes revealed striking differences between the two groups. These results are in agreement with those of other investigators29, who demonstrated that S. epidermidis recovered from clinical materials behaved differently from the commensal S. epidermidis not only by the presence of the ica A and the ica B genes, but also by their tendency towards phase variation, adherence to polymer surfaces, and capabilities for slime production. Another study documented that majority of the infecting strains (sepsis and catheter related infections) of S. epidermidis possessed the ica and mecA genes as compared to the contaminating strains20.
Although phenotypic markers such as slime and adherence reflect the potential for virulence5,6 and may be easier to be demonstrated than the molecular markers, the determination of such phenotypes is often hampered by phase variation exhibited by the organism, reliability in interpreting the test, and subjective variation in recording the results. Therefore, while guiding clinical decisions, detection of the ica and mec A genes may be more reliable tools than the phenotypic characters, to discriminate between virulent and avirulent/commensal bacteria.
In the present study, mec A was found in almost half of the deep isolates, as compared to the commensals. Most of mec A positive bacteria in our study were multi drug resistant, which was a true reflection of the nosocomial isolates8. However, in 15.3 per cent of our commensal organisms, we detected the mec A gene, which could suggest the presence of methicillin resistant Staphylococcus epidermidis (MRSE) in the general population. Whether the presence of the mec A gene in S. epidermidis from the healthy individuals is a reflection of the dissemination of hospital strains to the community or the role of antibiotics in food remains to be elucidated20.
In conclusion, our study results showed that the distinction between colonizing and infecting S. epidermidis with the help of molecular markers ica AB and mec A may help in clinical judgment in the overall management of patients with indwelling device associated infections.
Acknowledgment
The authors acknowledge the Indian Council of Medical Research, New Delhi, for financial support.
References
- 1.Perazzi B, Fermepin MR, Malimovka A, Garcia SD, Orgambide M, Vay CA, et al. Accuracy of cefoxitin disc testing for characterization of oxacillin resistance mediated by penicillin binding protein 2 a in coagulase negative Staphylococci. J Clin Microbiol. 2006;44:3634–9. doi: 10.1128/JCM.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diazo-Mitoma F, Harding GKM, Hoban DJ, Roberts RS, Low DE. Clinical significance of a test of slime production in ventriculoperitoneal shunt infections caused by coagulase negative staphylococci. J Infect Dis. 1987;156:555–60. doi: 10.1093/infdis/156.4.555. [DOI] [PubMed] [Google Scholar]
- 3.Christensen GD, Simpson WA, Bisno AL, Beachey EH. Adherence of slime producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–26. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother. 2010;65:1955–8. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 5.Nayak N, Satpathy G, Vajpayee RB, Mrudula S. Phenotypic and plasmid pattern analysis of Staphylcoccus epidermidis in bacterial keratitis. Indian J Ophthalmol. 2007;55:9–13. doi: 10.4103/0301-4738.29488. [DOI] [PubMed] [Google Scholar]
- 6.Christensen GD, Simpson WA, Younger JJ, Baddour LM, Barett FF, Malton DW, et al. Adherence of coagulase negative Staphylococci to plastic tissue culture plates: a quantitative model to the adherence of Staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arciola CR, Baldassarri L, Montanaro L. Presence of icaA and icaD genes and slime production in a collection of staphylococcal strains from catheter-associated infections. J Clin Microbiol. 2001;39:2151–6. doi: 10.1128/JCM.39.6.2151-2156.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez LR, d’Azevedo PA. Evaluation of the accuracy of various phenotypic tests to detect oxacillin resistance in coagulase negative staphylococci. Braz J Infectious Dis. 2008;12:2010–2. doi: 10.1590/s1413-86702008000300009. [DOI] [PubMed] [Google Scholar]
- 9.Mandelbaum S, Meisler DM. Postoperative chronic microbial endophthalmitis. Int Oph Clinic. 1993;33:71–9. doi: 10.1097/00004397-199303310-00007. [DOI] [PubMed] [Google Scholar]
- 10.Mermel LA, Farr BM, Sherertz RJ, Raad II, O’Grady N, Harris JA, et al. Guidelines for the management of intravascular cathter related infections. Clin Infect Dis. 2001;32:1249–72. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 11.Das T, Dogra MR, Gopal L, Jalali S, Kumar A, Malpani A, et al. Postsurgical endophthalmitis: Diagnosis and management. Indian J Ophthalmol. 1995;43:103–16. [PubMed] [Google Scholar]
- 12.Maki DG, Weise CE, Sarafin HW. A semiquantitative culture method for identifying intravenous catheter related infections. N Eng J Med. 1977;296:1305–9. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- 13.Dugoid JP. Staphylococcus: cluster forming Gram positive cocci. In: Collee JG, Dugoid JP, Fraser AG, Marmion BP, editors. Practical medical microbiology. 13th ed. II. Edinburgh: Churchill Livingstone; 1989. pp. 303–16. [Google Scholar]
- 14.Baird-Parker AC. Methods for identifying Staphylococci and Micrococci. In: Skinner FA, Lovelock DW, editors. Identification methods for microbiologists, The Society for Applied Microbiology, Technical Series No.14. London: Academic Press; 1979. pp. 201–10. [Google Scholar]
- 15.Kloos WE, Schleiffer KH. Simplified scheme for routine identification of human Staphylococcus species. J Clin Microbiol. 1975;1:82–8. doi: 10.1128/jcm.1.1.82-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker MT. Staphylococcus and Micrococcus; the anaerobic Gram positive cocci. In: Parker MT, editor. Topley and Wilson's principles of bacteriology, virology and immunity, Systemic bacteriology. 7th ed. Vol. 2. London: Edward Arnold Ltd; 1983. pp. 218–45. [Google Scholar]
- 17.Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardised single disc method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 18.Landman D, Mobarakai NK, Quale JM. Novel antibiotic regimens against Enterococcus faecium resistant to ampicillin, vancomycin and gentamicin. Antimicrob Agents Chemother. 1993;37:1904–8. doi: 10.1128/aac.37.9.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman DJ, Falkiner FR, Keane CT. New method for detecting slime production by coagulase negative Staphylococci. J Clin Pathol. 1989;42:872–4. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frebourg NB, Lefebvre S, Baert S, Lemeland JF. PCR-based assay for discrimination between invasive and contaminating Staphylococcus epidermidis strains. J Clin Microbiol. 2000;38:877–80. doi: 10.1128/jcm.38.2.877-880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aparna MS, Yadav S. Biofilms: Microbes and disease. Braz J Infect Dis. 2008;12:526–30. doi: 10.1590/s1413-86702008000600016. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhury A, Nagaraja M, Kumar AG. Potential of biofilm formation by Staphylococci on polymer surface and its correlation with methicillin susceptibility. Indian J Med Microbiol. 2009;27:377–8. doi: 10.4103/0255-0857.55450. [DOI] [PubMed] [Google Scholar]
- 23.Nayak N, Nag TC, Satpathy G, Ray SB. Ultrastructural analysis of slime positive and slime negative Staphylococcus epidermidis isolates in infectious keratitis. Indian J Med Res. 2007;125:767–71. [PubMed] [Google Scholar]
- 24.Schroeder AC, Schmidbauer JM, Sobke H, Seitz B, Ruprecht KW, Herrmann M. Inflence of fibronectin on the adherence of Staphylococcus epidermidis to coated and uncoated intra ocular lenses. J Catract Refr Surg. 2008;34:497–504. doi: 10.1016/j.jcrs.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 25.Muller E, Takeda S, Shiro H, Goldmann D, Pier GB. Occurance of capsular polysaccharide/adhesin among clinical isolates of coagulase negative staphylococci. J Infect Dis. 1993;168:1211–8. doi: 10.1093/infdis/168.5.1211. [DOI] [PubMed] [Google Scholar]
- 26.Zmantar T, Chaieb K, Makni H, Miladi H, Abdallah FB, Mahdouani K, et al. Detection by PCR of adhesins genes and slime production in clinical Staphylococcus aureus. J Basic Microbiol. 2008;48:308–14. doi: 10.1002/jobm.200700289. [DOI] [PubMed] [Google Scholar]
- 27.Arciola CR, Gamberini S, Campoccia D, Visai L, Speziale P, Bladassarri L, et al. A multiplex PCR method for the detection of all five individual genes of ica locus in Staphylococcus epidermidis. A survey on 400 clinical isolates from prosthesis associated infections. J Biomed Mater Res A. 2005;75:408–13. doi: 10.1002/jbm.a.30445. [DOI] [PubMed] [Google Scholar]
- 28.Duggirala A, Kenchappa P, Sharma S, Peeters JK, Ahmed N, Garg P, et al. High- resolution genome profiling differentiated Staphylococcus epidermidis isolated from patients with ocular infections and normal individuals. Invest Ophthalmol Vis Sci. 2007;48:3239–45. doi: 10.1167/iovs.06-1365. [DOI] [PubMed] [Google Scholar]
- 29.Ziebuhr W, Heilmann C, Gotz F, Meyer P, Wilms K, Straube E, et al. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect Immun. 1997;65:890–6. doi: 10.1128/iai.65.3.890-896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]