Abstract
Background & objectives:
Dengue is an important arboviral disease. All four dengue virus serotypes are reported to be circulating in India. It is also known that different serotypes, genotypes and clades of genotype determine outbreak severity. Dengue affected children are known to have serious disease outcome. We carried out this study to give reliable diagnosis of dengue infection in children and to detect circulating serotype in central India.
Methods:
Samples collected from paediatric patients suspected to have dengue fever were subjected to IgM and IgG ELISA to determine dengue virus infection. Samples collected within 0-5 days of onset of illness and positive by IgM ELISA were tested by nested reverse transcription polymerase chain reaction (nRT-PCR). The PCR products were sequenced and analyzed.
Results:
Of the 89 samples tested, 18 and 7 were positive for dengue IgM and IgG, respectively. Dengue activity was observed in both Jabalpur city and adjoining rural settings. One sample found positive by nRT-PCR was further sequenced to confirm dengue virus 4 as aetiological agent.
Interpretation & conclusions:
Our findings demonstrated dengue virus infection in children and adolescent in central India. Because of continuous changing epidemiology, it is important to monitor dengue virus activity at both serological and molecular level in this part of the country for better patient care and management.
Keywords: Central India, dengue, dengue virus type 4, IgG IgM ELISA, paediatric patients
Dengue is one of the most important arthropod borne viral diseases caused because of infection of one or more dengue viruses (DENV). According to World Health Organization (WHO), two fifth of the world's population is at risk from dengue disease and every year 50 million DENV infections are suspected worldover1. In India, the disease is prevalent and all four serotypes are known to be circulating either singly or in combination2,3, resulting in several outbreaks over the years. The disease is more severe in children than in adults4. Classical dengue fever (DF) characterized by high grade fever and mild rashes that may lead to severe dengue haemorrhagic fever (DHF) to dengue shock syndrome (DSS) and is the major cause of hospitalization and mortality mainly among children5,6, especially in tropical and subtropical countries7. However, due to lack of any diagnostic marker and any specific clinical symptoms to identify cases who will have severe disease outcome8, early diagnosis and close monitoring with symptomatic treatment is necessary. Therefore, we undertook this study for diagnosis of dengue using IgM and IgG ELISA on serum samples of hospitalized patients suspected to have dengue infection at Jabalpur, MP, India, and to determine the circulating serotype nested reverse transcription polymerase chain reaction (nRT-PCR) was done.
Material & Methods
Sample collection: This study was carried out during the period of August - December 2010. Children (n=86) and adolescents (n=3) in and around Jabalpur city (23°9’38”N 79°56’19’’E, 1.348 ft above sea level) suspected of dengue and admitted and/or treated at government hospital (Seth Govind Das Hospital), Jabalpur and from adjoining Narsinghpur district complying WHO case definition9 were included in the study. The clinician at the hospital after thorough examination and recording symptoms collected 89 blood samples which were sent for dengue diagnosis to virology laboratory of Regional Medical Research Centre for Tribals, Jabalpur. The ethical clearance for the study was taken from center's ethical committee and data presented in this study of the participating patients is kept anonymous.
Mosquito collection: Mosquito surveys were also carried out in different parts of the city during the study period; both mature and immature stages of mosquitoes were collected and identified. The breteau index (BI), container index (CI) and for house index (HI) were calculated.
ELISA and nRT-PCR: The serum was separated from all 89 samples and was subjected to MAC-ELISA for screening DEN specific IgM and IgG antibodies using Pan Bio IgM and IgG ELISA kits (Inverness Medical Innovations Pvt. Ltd., Australia) following manufacturer's instructions. All IgM ELISA positive (n=18) and samples collected between 0-5th day of onset (n=6) were subjected to nRT-PCR for determining the serotype of the DENV10 with minor modifications. Briefly, viral RNA was isolated using QIAamp viral RNA Mini kit (QIAGEN, USA) according to the manufacturer's instructions in 40 μl extraction buffer. Human RNase P (RNP) RNA amplification11 was used as internal quality control for RNA extraction and RT-PCR amplification. Super Script III one step RT-PCR with Platinum Pfx DNA polymerase was used for the amplification according to the manufacturer's instructions (Invitrogen, USA). The amplified fragment of first RT-PCR (511 bp) was diluted in nuclease free water (1:100 v/v) and was subjected to nested-PCR for detection of serotype/s. The PCR products were analyzed in agarose gel (2%) and extracted from the gel and sequenced directly using Big Dye Terminator Cycle Sequencing kit (Applied Biosystems, USA). The resulting sequences were analyzed for their homologies using Basic Local Alignment Search Tool (BLAST)12.
Results
Of the 18 IgM positives, seven were females. The platelet counts were available for 14 patients only (range 50 × 103 to 150 × 103/μl), and none required transfusion. Seven patients had rash and/or petechiae either on arm/limbs/chest, three had mild haemorrhage; however, no DSS case was noted. Seven patients had hepatomegaly. All patients recovered within 7-12 days of onset and were discharged.
In all, 47 samples received from rural areas adjoining Jabalpur. Out of these 10 samples were found IgM positive whereas out of 28 samples received from Jabalpur city, 7 were IgM positive. One IgM positive sample was from adjoining Narsinghpur district.
The mosquito collection done in the area demonstrated presence of the vector mosquito Ae. aegypti. The water storage containers such as cement tanks, mud pots and metal drums were main source of vector breeding sites. Breteau index (26.8) container index (9.0) house index (25.5) were high.
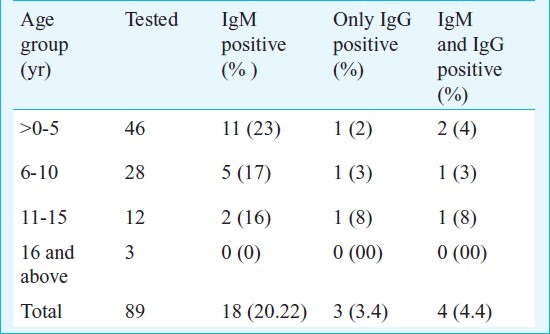
ELISA and nRT-PCR: Of the 89 screened samples, 18 (20.22%) were positive for DEN IgM and 7 were positive for IgG, of which 4 were positive for IgM as well (Table).
Table.
Age distribution of the suspected and confirmed dengue cases
Twenty four samples subjected to nRT-PCR showed RNP amplification, and one sample showed presence of DENV, in which the desired product of 511 bp was observed. On nested PCR for identification of serotype, the DENV 4 was identified (Fig.). Sequencing and BLAST analyses confirmed the identity of DENV 4 (Gen bank accession no. JF929180). When analyzed further, using blast tree/distance tree (Neighbor Joining method)13, this virus showed 99 per cent nucleotide homology and 100 per cent protein homology with the virus isolated from Andhra Pradesh, India in 2007 (ND 110) belonging to clade C of South East Asian genotype I14,15.
Fig.
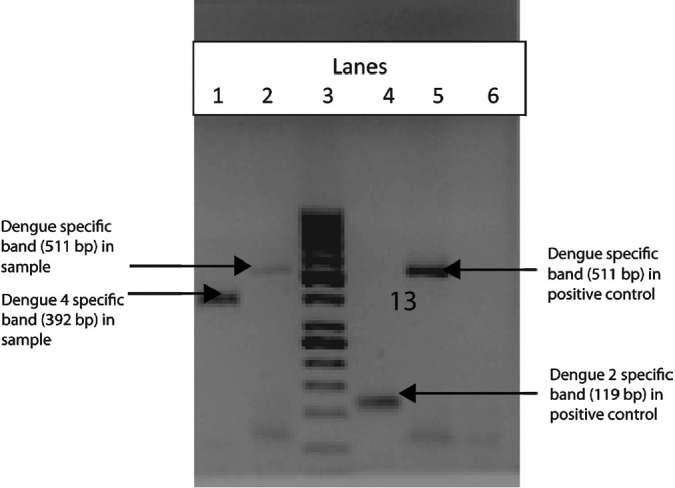
nRT-PCR of sample and positive control. Lane 1: nested PCR product of sample showing dengue 4 specific band (392 bp). Lane 2: 1st PCR product of the sample showing dengue specific band (511 bp). Lane 3: Molecular weight marker 1 kb. Lane 4: nested PCR product of positive control showing dengue 2 specific band (119 bp). Lane 5: 1st PCR product of the positive control showing dengue specific band (510 bp). Lane 6: Negative control (NTC).
Discussion
Dengue virus infection is known to be endemic in India3. It is now well established that different genotypes belonging to four DENV serotypes are circulating, either singly or in combinations2,3,16. These serotypes and genotypes are known to replace each other17,18. Rodrigues et al19 established the aetiology of outbreak of febrile illness of 1966 as DENV at Jabalpur, Madhya Pradesh. During the same period, Sehgal and colleagues18 reported entomological evidences for the presence of DENV vectors20. Although a few other studies have demonstrated presence of DENV vectors21 and serological evidences of dengue infection22,23, yet for a long period dengue remained a neglected disease in the central province of India and virus detection or serotyping was not done.
Of the samples tested, 20.2 per cent yielded IgM positivity, which is noteworthy, since the samples were not collected during any epidemic or outbreak situation. Further, seven samples were positive for dengue IgG, of which 4 were positive for IgM indicating secondary infection24 and regular circulation of dengue virus in the area. The study although based on small sample size, reconfirms the presence of DENV activity in and around Jabalpur city after a long lull.
In this study, we observed that patients from both urban and rural settings were admitted with suspicion of dengue infection. Out of 89 samples, about 50 per cent and out of 18 DEN IgM positive samples 10 (55%) were from rural settings clearly indicating establishment of DEN in the rural area neighboring Jabalpur. The serological studies on the samples collected by door-to-door surveys in 2006-2007 also have demonstrated high (37%) IgM positivity23. The entomological surveys conducted in the wards having DENV activity in the city and in the rural areas have demonstrated that, the water storage containers were the main sources of vector breeding sites.
Detection of DENV 4 in this study corroborates active DENV circulation in this area. The DENV 2 activity has been reported from central India earlier25. It was noted that in Delhi DENV 2 and 3 are replaced by DENV 117. The emergence of DENV 4 has been reported from Andhra Pradesh, south India also14, during the outbreak of 2007, recently Cecilia and others15 also reported detection of DENV 4 from Maharashtra, western India, however, this is perhaps the first report of detection of DENV 4 from central India. South East Asian genotype I of DENV 4 is known to be circulating in southern and western India, and the DENV 4 grouped in this cluster are highly diverse15. The clinical manifestations of the DENV 4 from Jabalpur were not as severe as seen at Pune15, however, it will be worthwhile to further characterize this DENV 4 virus presently circulating in the central India so as to understand the epidemiology of the disease and allocate it to a particular clade.
One of the aims of this study was to give IgM based diagnosis of dengue infection, hence samples were preferably collected after 7th day of onset of illness. That could be a reason for getting only one RT-PCR positive as it is well known that virus can be detected up to 5th day of illness9.
Presence of vectors and mosquitogenic conditions in the area are alarming and call for rigorous surveillance of both vector mosquitoes and patients, so that large outbreaks can be prevented. As different serotypes, genotypes and clades of genotype determine outbreak severity13, it is important to monitor dengue virus activity at both serological and molecular level in this part of the country.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for financial support and thank the staff of Virology Department of RMRCT, Jabalpur, for technical support.
References
- 1.Would Health Organization. [accessed on November 9, 2010]. Available from: http://www.who.int/mediacentre/factsheets/fs117/en/
- 2.Angel B, Joshi V. Distribution of dengue virus types in Aedes aegypti in dengue endemic districts of Rajasthan, India. Indian J Med Res. 2009;129:665–8. [PubMed] [Google Scholar]
- 3.Gupta E, Dar L, Broor S. Concurrent infection by two dengue virus serotypes among dengue patients. Indian J Med Microbiol. 2008;26:402–3. [PubMed] [Google Scholar]
- 4.Hammond SN, Balmaseda A, Pérez L, Tellez Y, Saborío SI, Mercado JC, et al. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg. 2005;73:1063–70. [PubMed] [Google Scholar]
- 5.Monath TP. Dengue: The risk to developed and developing countries. Proc Natl Acad Sci USA. 1994;91:2395–400. doi: 10.1073/pnas.91.7.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubler DJ. Dengue and dengue hemorrhagic fever in the Americas. In: Thongcharoen P, editor. Dengue hemorrhagic fever. WHO Monograph. New Delhi, India: World Health Organization; 1993. pp. 1–63. [Google Scholar]
- 7.Halstead SB. More dengue, more questions. Emerg Infect Dis. 2005;1:740–1. [Google Scholar]
- 8.Basu M, Dasgupta MK, Kundu TK, Sengupta B, De GK, Roy BN. Profile of pediatric dengue cases from a tertiary care hospital in Kolkata. Indian J Public Health. 2007;51:234–6. [PubMed] [Google Scholar]
- 9.Dengue guidelines for diagnosis, treatment, prevention and control, World Health Organization. Geneva, Switzerland: WHO Press; 2009. World Health Organization (WHO); Special Programme for Research and Training in Tropical Diseases (TDR) pp. 25–4. [Google Scholar]
- 10.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. CDC protocol of realtime RTPCR for influenza A (H1N1) [accessed on November 9, 2010]. Available from: http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRT-PCRprotocol.pdf .
- 12.Basic Local Alignment Search Tool web based application. [accessed on February 19, 2011]. Available from: http://blast.ncbi.nlm.gov/Blast.cgi?CMD=Web&PAGE_Type=BlastHome .
- 13.Blast Tree/distance tree web based application. [accessed on February 19, 2011]. Available from: http://www.ncbi.nlm.gov/blast/treeview/treeView.cgi?request=page&blastRID .
- 14.Dash PK, Sharma S, Srivastava A, Santhosh SR, Parida MM, Neeraja M, et al. Emergence of dengue virus type 4 (genotype I) in India. Epidemiol Infect. 2011;139:857–61. doi: 10.1017/S0950268810001706. [DOI] [PubMed] [Google Scholar]
- 15.Cecilia D, Kakade MB, Bhagat AB, Vallentyne J, Singh A, Patil JA, et al. Detection of dengue-4 virus in Pune, western India after an absence of 30 years - its association with two severe cases. Virol J. 2011;1:46–8. doi: 10.1186/1743-422X-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi UC, Nagar RJ. Dengue and dengue haemorrhagic fever: Indian perspective. J Biosci. 2008;33:429–41. doi: 10.1007/s12038-008-0062-3. [DOI] [PubMed] [Google Scholar]
- 17.Chakravarti A, Kumar A, Matlani M. Displacement of dengue virus type 3 and type 2 by dengue virus type 1 in Delhi during 2008. Indian J Med Microbiol. 2010;28:412. doi: 10.4103/0255-0857.71806. [DOI] [PubMed] [Google Scholar]
- 18.Kumar SR, Patil JA, Cecilia D, Cherian SS, Barde PV, Walimbe AM, et al. Evolution, dispersal and replacement of American genotype dengue type 2 viruses in India (1956-2005): selection pressure and molecular clock analyses. J Gen Virol. 2010;91:707–20. doi: 10.1099/vir.0.017954-0. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues FM, Pavri KM, Dandawate CN, Banerjee K, Bhatt PN. An investigation of the aetiology of the 1966 outbreak of febrile illness in Jabalpur, Madhya Pradesh. Indian J Med Res. 1973;61:1462–70. [PubMed] [Google Scholar]
- 20.Sehgal PN, Kalra NL, Pattanayak S, Wattal BL, Srivastav JB. A study of an outbreak of dengue epidemic in Jabalpur, Madhya Pradesh. Bull Indian Soc Mal Commun Dis. 1967;4:91–108. [Google Scholar]
- 21.Singh N, Mishra AK, Singh OP. Preliminary observations on mosquito collection by light traps in tribal village of Madhya Pradesh. Indian J Malariol. 1993;30:101–7. [PubMed] [Google Scholar]
- 22.Ukey P, Bondade S, Paunipagar P, Powar R, Akulwar S. Study of seroprevalence of dengue fever in central India. Indian J Community Med. 2010;35:517–9. doi: 10.4103/0970-0218.74366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annual Report 2006-07. Vectore borne diseases; 2008. Regional Medical Research Centre for Tribals (ICMR) Jabalpur; pp. 10–2. [Google Scholar]
- 24.Gunasekaran P, Kaveri K, Mohana S, Arunagiri K, Suresh Babu BV, Padma Priya P, et al. Dengue disease status in Chennai (2006-2008): A retrospective analysis. Indian J Med Res. 2011;133:322–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Mahadev PV, Prasad SR, Ilkal MA, Mavale MS, Bedekar SS, Banerjee K. Activity of dengue-2 virus and prevalence of Aedes aegypti in the Chirimiri colliery area, Madhya Pradesh, India. Southeast Asian J Trop Med Public Health. 1997;28:126–37. [PubMed] [Google Scholar]


