Sir,
Invasive pneumococcal diseases are estimated to be responsible for about one million deaths each year globally among children less than 5 yr of age1. Colonization by Streptococcus pneumoniae is a prerequisite for invasion2. The colonizing isolates act as important reservoirs of drug resistance and disseminate by means of horizontal gene transfer to others3. Drug resistant pneumococci have been reported in both colonizing and invasive isolates from different parts of India4–8. Vaccination can be an effective way for prevention, but vaccines developed from polysaccharides and conjugates against S. pneumoniae target different serotypes. So far 919 different serotypes have been identified and their prevalence differs from one region to another and a few of these have been accounted for invasive pneumococcal diseases (IPD) globally10. Serotypes 1, 5, 6, 14, 19 and 23 are common in world including Asian and African countries and reported to be responsible for up to 68 per cent of IPD10. Studies have been carried out in India to find out the carriage rate, serotype prevalence and resistance pattern among nasopharyngeal and clinical isolates4–8,11. Since pneumococcal diseases occur after colonization of strain2, these colonizing normal flora in the nasopharynx can be considered for prediction of drug resistance patterns and serotype prevalence for treatment and vaccine formulations. It is also useful to know the locally prevalent serotypes.
The present study was carried out among rural children of Dibrugarh district of Assam, a State in the northeast India to collect information on the serotypes and drug resistance pattern of circulating S. pneumoniae in this region. During December 2009 to December 2010, a total of 811 healthy children (0-14 yr) were enrolled from 30 villages of Dibrugarh district of Assam.
The 30 cluster sampling method developed by the WHO12 was used. In the first stage, 30 villages were sampled with probability proportionate to size (PPS) of the population. In the second stage, 10 households from each of the 30 villages were selected using simple random sampling. From each household all children up to 14 yr of age were included in the study after obtaining written informed consent of the parents. Children who had symptoms of acute respiratory tract infection, had taken antibiotic in the previous two weeks or did not have the consent of their parents were excluded. In addition to the demographic data, information was collected on the number of siblings, any respiratory tract infection in the previous 3 months (excluding the last 2 wk) including ear discharge, throat infection, running nose with or without fever, cough, etc. or use of antibiotics in the previous 3 months (excluding the last 2 wk). The study protocol was approved by the Institute's ethics committee.
Univariate analyses were done to see the association between different characteristics of the children and pneumococcal carriage. Statistical package SPSS version 16 was used to carry out the analyses.
Nasopharyngeal swab were plated on blood agar plates containing 5 per cent sheep blood. The inoculated blood agar plates were incubated in a CO2 incubator at 37°C for 18-24 h. The α-haemolytic suspected pneumococcal colonies were identified by Gram staining, optochin sensitivity and bile solubility testing13. The pneumococcal isolates were stored at -70°C in brain heart infusion broth with 20 per cent glycerol. Polymerase chain reaction detection of gene encoding the pneumococcal pneumolysin (ply) was carried out using primers designed by Corless et al14. Briefly, primers F- 5’-TGCAGAGCGTCCTTTG GTCTAT-3’and R-5’- CTCTTACTCGTGGTTTCCAACTTGA-3’were used to amplify a 80 bp segment of the ply gene. The pneumococci were serotyped into group/type by slide agglutination test using Denka Kit (Denka Seiken, Tokyo, Japan) as per the manufacturer's instructions. Isolates were tested by disc diffusion as per CLSI15 guidelines for susceptibility to oxacillin (1μg), tetracycline (30 μg), erythromycin (15 μg), ciprofloxacin (5 μg) and trimethoprim-sulphamethoxazole (23.75/1.25 μg). The antibiotics were obtained from Hi-Media, Mumbai, India. The inhibitory zone diameters for isolate to be considered as resistant were: oxacillin <19 mm; ciprofloxacin <15 mm; tetracycline <18 mm; trimethoprim-sulphamethoxazole <15 mm; erythromycin <15 mm. S. pneumoniae ATCC 49619 was used as control. Pneumococcal isolates showing zone size <19 mm were tested for MIC to penicillin by E test (Hi-Comb test strips, Hi-Media, Mumbai, India) and interpreted as per CLSI criteria15 as that for non meningitis isolates. An isolate with MIC of <0.06 μg/ml was considered susceptible, 0.1 to 1.0 μg/ml as intermediate and >2 μg/ml as resistant to penicillin.
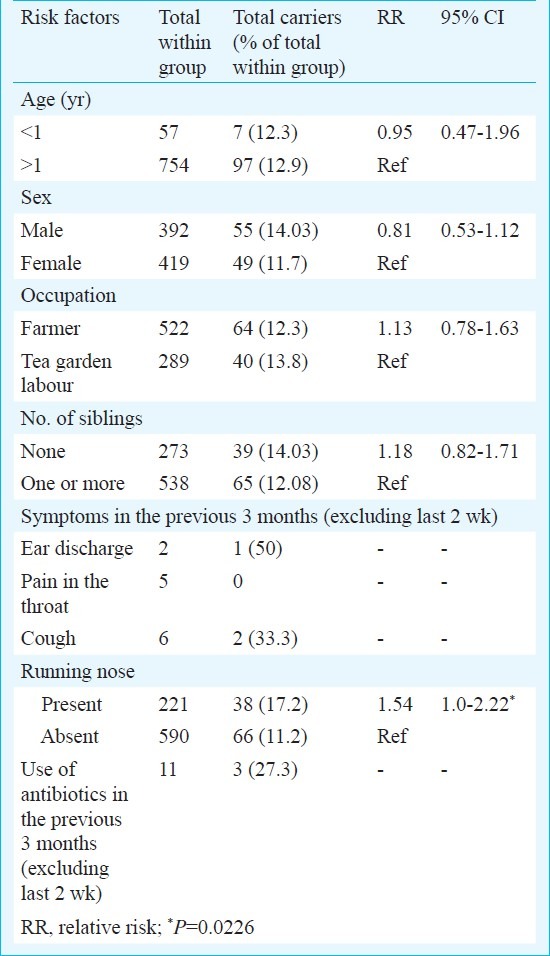
Of the 811 children studied, 392 (48.3%) were boys, and 57 (7.02%) were below 1 yr of age, 538 (66.3%) had at least one sibling, 234 (28.9%) had at least one episode of symptoms of respiratory tract infection within the last 3 months and 11 (1.4%) of them had taken antibiotics in the previous 3 months excluding the last 2 wk. A total of 104 (12.8%) children were carriers of S. pneumoniae. Univariate analysis of the possible risk factors and carriage did not show any significant association except for history of rhinitis (P<0.05) (Table I). Six households had more than one pneumococcal carriers, of whom same serotype was found among siblings of two households, namely serotypes 6 and 23.
Table I.
Risk factors associated with the S. pneumoniae (n=104) carriers of 30 villages of Dibrugarh, Assam
Serotyping of 68 isolates was done, of which 40 could be assigned a group/type [28 (41.2%) not typable]. The isolates belonged to 17 different serogroups/types. The six most common serogroups/types were type 33, 8, 1, 19, 6, and 23 which accounted for 62.5 per cent of those that could be serogrouped/typed and remaining belonged to 11 serogroups/types namely 2, 9, 11, 21, 3, 4, 7, 14, 16, 18 and 22.
Of the 104 S. pneumoniae isolates, 92 (88.5%) were sensitive to optochin.
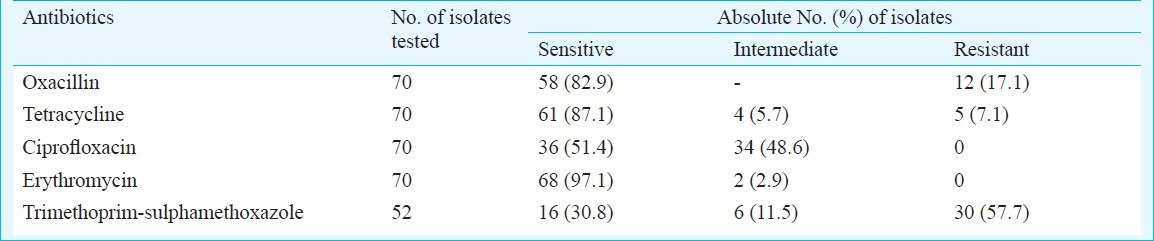
Among the 70 isolates tested, 54 (77.1%) were resistant to at least one antibiotic. Oxacillin resistance was seen among 17.1 per cent isolates (Table II). MIC of the 12 penicillin resistant isolates ranged from 2-4 μg/ml by E test. Resistance to penicillin was seen among types 8, 11, 19 and 33. The serotype most resistant to penicillin belonged to type 8 (25 %).
Table II.
Susceptibility pattern of the S. pneumoniae isolates from children of Dibrugarh district of Assam, India
The ply gene encoding pneumolysin was present in all the 70 isolates tested. A total of 28 (41.17%) isolates were non typeable with the commercially available kit. These were confirmed as S. pneumoniae by their staining character and shape, optochin sensitivity at 37°C in presence of CO2 and positivity for pneumolysin (ply) gene.
The isolation rate of S. pneumoniae in our study was 12.8 per cent. Different hospital, school and day care centers based studies from different parts of India have reported a prevalence rate of nasopharyngeal colonization from 6.5 to 70.2 per cent among healthy children5,7,11 indicating geographical variation.
All of the isolated serogroups/types in the present study are reported to cause systemic and other infections like conjunctivitis infections4,8,10. Serotype 33 was the most prevalent serotype among the children in this region as has also been reported previously from South Indian infants5.
In our study 41.17 per cent of the pneumococcal isolates were not typeable with the commercially available kit. Non typeable pneumococci have been isolated from different parts of the world from both carriage and diseases like acute conjunctivitis7,16,17.
All the 70 isolates tested were positive for pneumolysin gene. Studies have been conducted to detect this gene in invasive as well as non invasive pneumococcal diseases18,19. The highest resistance was observed against trimethoprim-sulphamethoxazole. Similar results were observed among studies from India4–7. Penicillin resistance was seen among 17.1 per cent isolates in our study which was similar to a study carried out in northern India6 showing 18.3 per cent clinical isolates and 16 per cent nasopharyngeal isolates with decreased susceptibility to penicillin. Penicillin resistance varying from 7.3 to 34 per cent has been reported from south India4,5. It is, therefore, necessary to have a continuous monitoring of the resistance pattern of the pneumococcal isolates in a particular geographic region.
In conclusion, S. pneumoniae was detected among the rural children of Dibrugarh, Assam, and among the 17 different serogroups/types identified, type 33 was the commonest and had shown reduced susceptibility to antibiotics. Penicillin and trimethoprim-sulphamethoxazole resistance was prevalent among the carriage isolates as seen in other parts of the country.
Acknowledgment
The authors thank the ASHA workers, parents and the children of the different villages of Dibrugarh district for their participation in the study. The study was funded by the Indian Council of Medical Research, New Delhi.
References
- 1.Pneumococcal conjugate vaccine for childhood immunization-WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- 2.Bogaert D, De Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–54. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 3.Tomasz A. Antibiotic resistance in Streptococcus pneumoniae. Clin Infect Dis. 1997;24(Suppl 1):585–8. doi: 10.1093/clinids/24.supplement_1.s85. [DOI] [PubMed] [Google Scholar]
- 4.Kanungo R, Rajalakshmi B. Serotype distribution and antimicrobial resistance in Streptococcus pneumoniae causing invasive and other infections in South India. Indian J Med Res. 2001;114:127–32. [PubMed] [Google Scholar]
- 5.Coles CL, Rahmathullah L, Kanungo R, Thulasiraj RD, Katz J, Santosham M, et al. Nasopharyngeal carriage of resistant pneumococci in young south Indian infants. Epidemiol Infect. 2002;129:491–7. doi: 10.1017/s0950268802007586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goyal R, Singh NP, Kaur M, Talwar V. Antimicrobial resistance in invasive and colonising Streptococcus pneumoniae in North India. Indian J Med Microbiol. 2007;25:256–9. doi: 10.4103/0255-0857.34770. [DOI] [PubMed] [Google Scholar]
- 7.Wattal C, Oberoi JK, Pruthi PK, Gupta S. Nasopharyngeal carriage of Streptococcus pneumoniae. Indian J Pediatr. 2007;74:905–7. doi: 10.1007/s12098-007-0166-z. [DOI] [PubMed] [Google Scholar]
- 8.Kar UK, Satpathy G, Nayak N, Das BK, Panda SK. Serotype distribution of Streptococcus pneumoniae isolates from ophthalmic and systemic infections and of commensal origin. Indian J Med Res. 2006;124:99–104. [PubMed] [Google Scholar]
- 9.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Hance LF, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: The Pneumococcal Global Serotype Project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanungo R, d’Lima D, Rajalakshmi B, Natarajan MK, Badrinath S. Throat carriage of pneumococci in healthy schoolchildren in Union Territory of Pondicherry. Indian J Med Res. 2000;112:100–3. [PubMed] [Google Scholar]
- 12.Henderson RH, Sundaresan T. Cluster sampling to asses immunization coverage: A review of experience with a simplified method. Bull World Health Organ. 1982;60:253–60. [PMC free article] [PubMed] [Google Scholar]
- 13.Murray PR, Baron EJ, Jorgensen JJ, Pfaller MA, Yelken RH. Manual of clinical microbiology. 8th ed. Washington, DC: ASM Press; 2003. [Google Scholar]
- 14.Corless CE, Guiver M, Borrow R, Edwards-Jones V, Fox AJ, Kaczmarski EB. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol. 2001;39:1553–8. doi: 10.1128/JCM.39.4.1553-1558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Performance standards for antimicrobial susceptibility testing. Twentieth informational supplement. CLSI document M100-S20. Wayne, PA: CLSI; 2010. Clinical and Laboratory Standards Institute. [Google Scholar]
- 16.Carvalho MGS, Steigerwalt AG, Thompson T, Jackson D, Facklam RR. Confirmation of nontypeable Streptococcus pneumoniae-like organisms isolated from outbreaks of epidemic conjunctivitis as Streptococcus pneumoniae. J Clin Microbiol. 2003;41:4415–7. doi: 10.1128/JCM.41.9.4415-4417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porat N, Greenberg D, Givon-Lavi N, Shuval DS, Trefler R, Segev O, et al. The important role of nontypable Streptococcus pneumoniae international clones in acute conjunctivitis. J Infect Dis. 2006;194:689–96. doi: 10.1086/506453. [DOI] [PubMed] [Google Scholar]
- 18.Sourav S, Patrica A, Sharma S, Kanungo R, Jayachandran S, Prashanth K. Detection of pneumolysin and autolysin genes among antibiotic resistant Streptococcus pneumoniae in invasive infections. Indian J Med Microbiol. 2010;28:34–9. doi: 10.4103/0255-0857.58726. [DOI] [PubMed] [Google Scholar]
- 19.Kanungo R, Bhaskar M, Kumar A, Badrinath S, Rajalakshmi B. Detection of pneumolysin in CSF for rapid diagnosis of pneumococcal meningitis. Indian J Med Res. 2004;119:75–8. [PubMed] [Google Scholar]


