Abstract
Nardostachys jatamansi is traditionally used in alternative medicine for treatment of neuropsychiatric disorders. We investigated the potential of N. jatamansi extract (NJE) in protecting against chronic stress-induced impairments in spatial learning and memory. The rats were exposed to 21 days of chronic restraint stress and simultaneously received 100 mg or 200 mg/kg body weight of NJE following which acquisition and retention of hippocampus-dependent spatial memory were tested in a partially-baited eight arm radial maze. Animals treated with 200 mg/kg body weight NJE had learning curves comparable to control unstressed animals, made significantly more correct choices (38%, P < 0.001), and fewer reference memory errors (53%, P < 0.01) on the eighth day of training compared to untreated stressed animals as well as stressed animals which received vehicle or a lower dose (100 mg/kg) of NJE. NJE-treated animals also made significantly higher correct choices (31%, P < 0.001) than untreated animals in a retention test 10 days after the training period. We propose that NJE has a protective effect of stress-induced impairments in hippocampus-dependent learning and memory behavior in rats.
Keywords: Chronic stress, hippocampus, learning, memory, Nardostachys jatamansi
INTRODUCTION
Nardostachys jatamansi (commonly called as Jatamansi) belonging to the family Valerianaceae is a medicinal herb found in parts of India, Tibet, and China. It is often used in Ayurvedic and Unani traditions of alternative medicine as a neuroprotective agent in the treatment of epilepsy, hysteria, insomnia, and convulsions.[1] The rhizomal extract of N. jatamansi (NJE) has been particularly studied for its effect on the central nervous system. It has been shown to possess anticonvulsant activity,[2] antidepressant activity,[3] and neuroprotective effects in a rat cerebral ischemia model.[4]
Although N. jatamansi is also recommended as a nootropic agent in Ayurveda, not many studies have investigated this aspect systematically. NJE has been reported to improve performance in elevated plus maze and passive avoidance tasks in young mice and also to ameliorate aging-related deficits in these tasks. Further, it has also been reported to reverse diazepam and scopolamine-induced amnesia.[5] The effect of NJE on hippocampus-dependent spatial memory has received scant attention. A reduction in the hippocampal volume and cell numbers has been reported in animal models of aging, depression, and alcoholism—conditions that have all been associated with memory loss in humans.[6,7] Chronic stress is associated with hippocampus-dependent learning and memory impairments have been reported in animals subjected to 6 h of physical restraint each day for 21 days.[8] Behavioral deficits in animal models of chronic stress have been associated with loss of hippocampal neurons, dendritic atrophy, and increase in dendritic spines and excrescences.[9–11] Such models therefore provide an useful approach to study the pharmacological potential of agents in preventing/reversing stress-related cognitive impairments.
Pretreatment with NJE has been reported to reduce markers of stress such as elevation of plasma corticosterone, gastric ulceration, and increase in adrenal and spleen weights in rats subjected to stress.[12,13] This antistress effect prompted us to explore the effects of NJE on stress-induced deficits in learning and memory. We investigated the potential of NJE in protecting against chronic restraint stress-induced impairments in spatial learning and memory in a partially baited eight arm radial arm maze (RAM) task. This model has been successfully used in the past to study changes in hippocampus-dependent cognitive tasks.[14,15] We hypothesized that NJE reduces the impact of chronic restraint stress on performance in this task.
MATERIALS AND METHODS
Subjects
Adult male Wistar rats aged 2–2.5 months (200–250 g body weight) obtained from the Central Animal House of K. S. Hegde Medical Academy, Mangalore, were used in the study. The Institutional Animal Ethics Committee for the use of animals in research approved the experimental protocols (Approval letter No. KSHEMA/AEC/044/2006, dated 18-09-2006). All efforts were made to minimize both the suffering and the number of animals used. The rats were reared in a light-controlled (12-h light dark) environment with ad libitum access to water and food except during RS and RAM training periods.
Experimental groups
The animals were divided into five groups each of ten animals. (1) Normal control (NC) did not undergo any stress procedure, (2) Restraint stress (RS) subjected to a 21 day chronic restraint stress protocol (as explained below), (3) RS + JM1: received 100 mg of N. jatamansi per kg body weight for 21 days along with chronic stress, (4) RS + JM2: rats received 200 mg of N. jatamansi per kg body weight for 21 days along with chronic stress, and (5) VC: received vehicle (Alcohol + Tween 80 + water—1:2:5) along with chronic stress for 21 days. In addition to these animals used in the behavioral experiments, an additional group of six animals was used for the evaluation of the effectiveness of the chronic restraint stress model by estimating serum corticosterone levels during the stress protocol (see below for details).
Chronic restraint stress
Chronic restraint stress involved placing the rats in a close-fitting wire mesh rodent restrainer for 6 h per day for 21 days. This form of stress has been reported to elevate corticosterone levels, increase adrenal and spleen weights, cause gastric ulcers, and to induce deficits in spatial learning and memory.[8,14]
Corticosterone analysis
Stress is associated with alterations in the corticosterone level. The serum corticosterone level can therefore be used to assess the effectiveness of experimental stress protocols. In order to establish the effectiveness of the chronic restraint stress model employed in this study, four blood samples were obtained from an additional group of six animals on Days 0, 3, 10, and 21 (Day 1 indicates the start of the stress procedure). Collection tubes containing sodium citrate as anticoagulant were used to sample from the retro-orbital venous plexus. Samples were centrifuged at 4° C for 15 min at 3000 rpm. Serum aliquots were aspirated and stored in sealable polypropylene microcentrifuge tubes at –70° C until assayed for serum corticosterone concentrations using enzymeimmunoassay (EIA) kit (Cayman chemicals Inc., Lewis, USA).
Preparation and administration of the N. jatamansi extract
Botanically identified roots and rhizomes of N. jatamansi were purchased from a recognized and licensed supplier (Amsar Private Ltd, Indore, India). An ethanolic extract was prepared following standardized methods described earlier.[4] They were cut into small pieces, powdered and refluxed with 95% ethanol in a Soxhlet extractor for 6–8 h. The extract was evaporated to dryness under reduced pressure and temperature using a rotary vacuum evaporator, and the dried residue was stored at 4° C. The yield of the dry extract from the crude powder was about 10%. Previous studies investigating neurological actions of NJE have suggested 200 mg/kg body weight as an effective dose in mediating its antidepressant and memory-enhancing actions.[3,5] Accordingly, doses of 200 mg/kg body weight and 100 mg/kg were administered orally for 21 days.
Assessment of spatial memory using eight arm radial maze
The animals were assessed for spatial memory in a partially baited RAM. Learning and memory in the RAM were assessed in a separate set of animals as described earlier.[14] The eight arm radial maze consisted of eight equally spaced arms (42 × 12 × 12 cm3) radiating from an octagonal central platform. Prior to the training, the animals were kept on a restricted diet and body weight was maintained at 85% of their free feeding weight, with water available ad libitum. The animals were provided two sessions of acclimatization on two consecutive days prior to beginning of training. During these sessions, they were allowed us to explore the baited arms of the maze for 10 min.
During the training period, the rats were given two acquisition trials per day until they achieved learning criteria as specified below. At the beginning of each trial, the maze was thoroughly cleaned with 70% ethanol and four of the arms (2, 3, 5, and 7) were baited with food reinforcement (Kellogg's Planets and Stars™, Kellogg India Ltd., Mumbai, India). The rat was placed in the center of the octagon and was allowed a free choice. An arm choice was recorded when a rat ate a bait or reached the end of an arm. The maze arms were not rebaited, and only the first entry into the baited arm was recorded as a correct choice. An entry into an unbaited arm was considered a reference memory error (RME) and any re-entry was considered as a working memory error (WME). The trial continued until the rat entered all the four baited arms or 5 min had elapsed. At the end of the trial, the rat was returned to the home cage for a period of 1 h before running the second trial. Training was continued until the rats attained the criteria of 80% correct choice (at least four correct entries out of five). Data from the two trials for each day were averaged and entered into further analysis. Ten days after acquisition, rats were evaluated for retention of the task. Rats were given two trials, and the average of the scores percentage correct choice, reference, and WME was taken for analysis.
RESULTS
Effectiveness of the chronic stress procedure
Serum corticosterone levels were elevated by chronic restraint stress
A repeated easures ANOVA performed on the corticosterone levels measured on Days 0 (baseline), 3, 10, and 21 of chronic stress revealed a significant difference within-subjects [F(3, 12) = 22.547, P < 0.001)] [Figure 1]. Pairwise comparisons (adjusted for multiple comparisons by the Bonferroni method) revealed that Days 3 and 21 corticosterone levels were significantly higher than Day 0 (baseline) levels (P = 0.001 and P <0.05, respectively). Mean corticosterone levels on Day 10 was higher than Day 0, but this difference did not reach statistical significance in the post hoc pairwise tests. By Day 21 corticosterone levels were significantly lower than Day 3 levels (P = 0.001).
Figure 1.
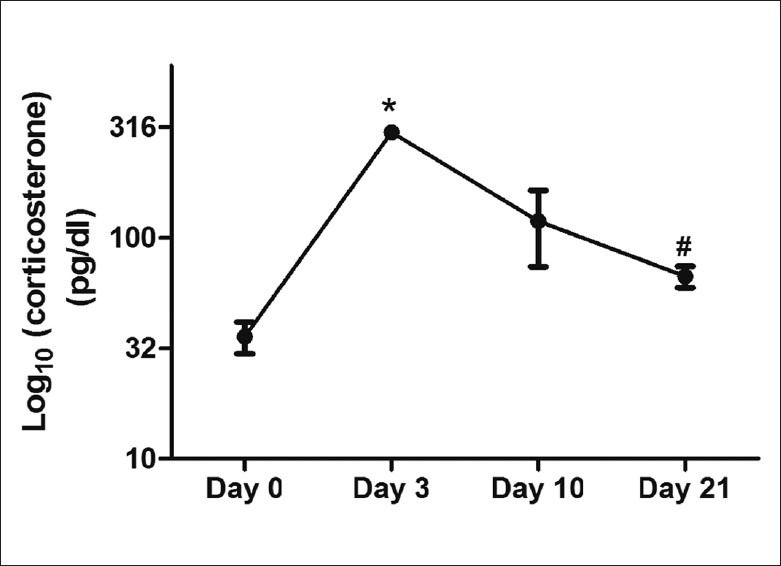
Serum corticosterone levels in the rats subjected to chronic RS for 21 days. Values represent mean ± standard error of mean (N = 6). Repeated measures ANOVA: F(3, 12) = 22.547, P < 0.001; pairwise comparisons (with Bonferroni correction for multiple comparisons): *P < 0.001, Day 3 > Day 0; #P < 0.01, Day 21 > Day 0; $P < 0.001, Day 21 < Day 3
Acquisition
% Correct choices during learning phase
A two -way repeated measures ANOVA (RM-ANOVA) was performed with training day (Day) as within-subjects factor and experimental group (Group) as between-subjects factor. A significant main effect was found for Day [P < 0.001, F (7, 315) = 24.37], group [P 0< 0.001, F = (4, 45) = 6.42] as well as the Day by Group interaction [P < 0.001, F = (28, 315) = 2.86] [Figure 2]. This suggests that the performance of the animals changed with days of training and that this change in performance differed between the groups.
Figure 2.
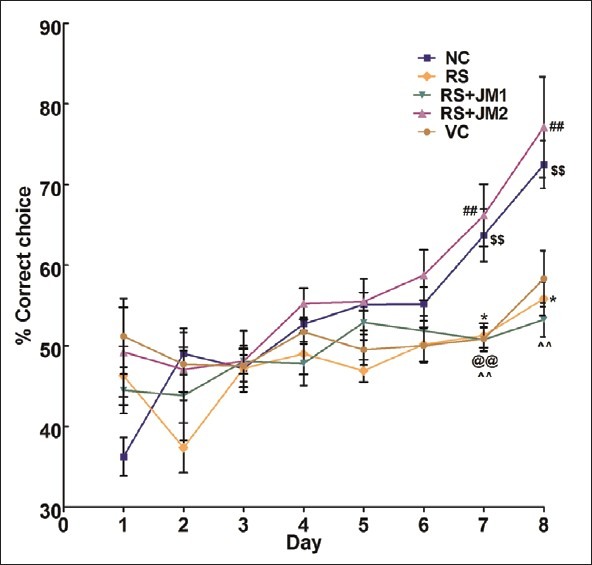
Mean (SEM) percent correct choices during acquisition of partially baited radial arm maze. NC, normal control; RS, restraint stress; VC, vehicle control; RS+JM1, stress + Jatamansi extract 100 mg, RS+JM2: Stress + Jatamansi extract 200 mg. Two-way repeated measures ANOVA: P < 0.001, F = (28, 315) = 2.86 for Group × Day interaction; Simple main effect of Groups on Day 7: P < 0.001, F(4, 45) = 9.18; simple main effect of Groups on Day 8: P < 0.001, F(4, 45) = 8.29. Bonferroni adjusted pair wise comparisons: *P < 0.05, RS < NC; ##P < 0.01, RS + JM2 > VC; $$P < 0.01, RS + JM2 > RS; @@P < 0.01 VC > NC; ∧∧P < 0.01 RS + JM1 < NC
Performance on Day 8 was further analyzed within the RM-ANOVA framework to assess the group differences in learning. A significant simple main effect was found for groups at this time point [P < 0.001, F(4, 45) = 8.29]. Pairwise comparisons, adjusted for multiple comparisons using the Bonferroni method revealed a significantly higher %CC for NC animals on Day 8 compared to RS animals (P < 0.001). The RS + JM2 group showed significantly higher %CC compared to the RS and the VC groups (P < 0.001). The VC and the RS + JM1 groups did not differ significantly from the RS group [Figure 3].
Figure 3.
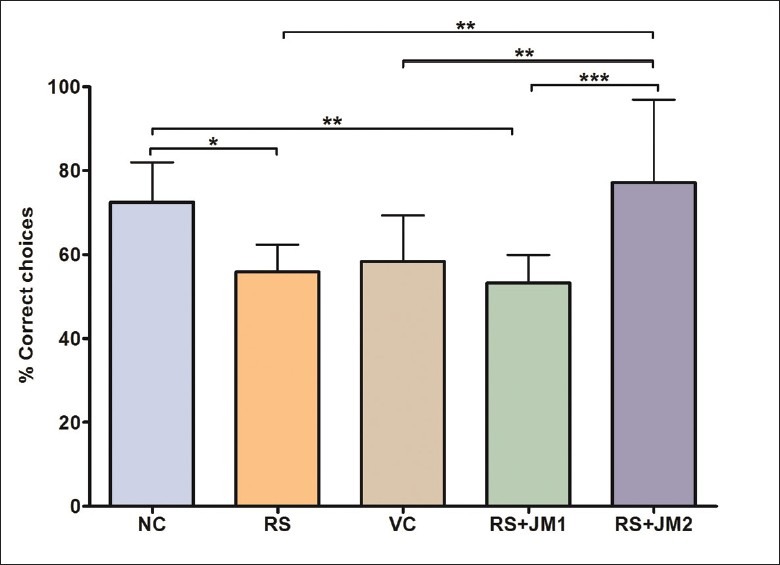
Comparison of mean (SD) percent correct choice on Day 8. One-way ANOVA P < 0.001, F(4, 45) = 8.29; Post hoc Tukey's test: *P < 0.05, RS < NC; **P < 0.01: RS + JM1 < NC, RS + JM2 > RS, RS + JM2 > VC; ***P < 0.001: RS + JM2 > RS + JM1
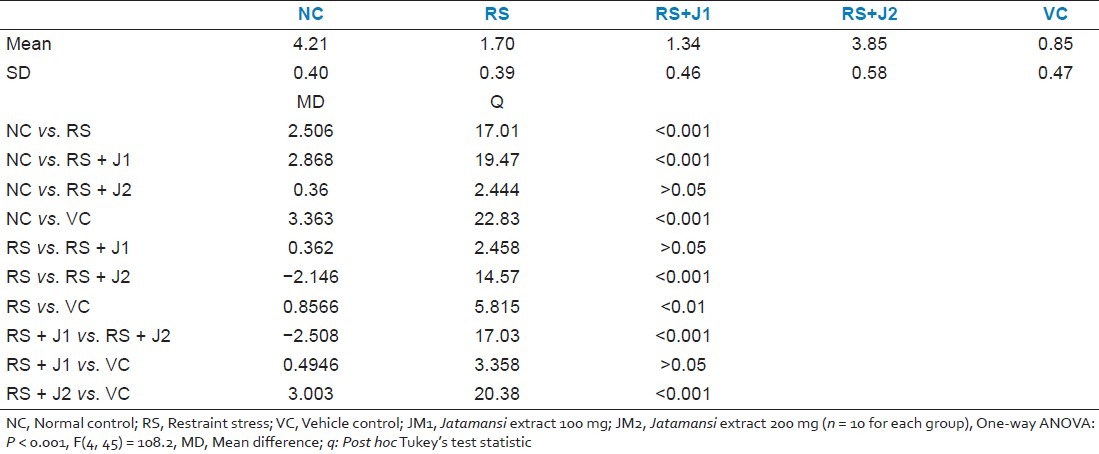
A trend analysis performed to more precisely characterize the interaction between Day and Group revealed that the linear effect of Days by Group interaction was significant [P < 0.001, F(4, 45) = 6.31] with no such significant effects at higher-order nonlinear trends. A one-way ANOVA test was used to compare the slopes of the linear regression of learning curves. This showed significant between groups difference in the slopes of the learning curves [P < 0.001, F(4, 45) = 108.2]. Post hoc Tukey's tests revealed that the slope of the learning curve for the RS group was significantly lower than that of the NC group and that the slope for the RS+JM2 group was significantly higher than both the RS and VC groups but did not differ from the slope of the NC group [Table 1].
Table 1.
Comparison of the slopes of the % correct choices over days during partially baited radial arm maze acquisition
Reference memory errors during the learning phase
A two-way repeated measures ANOVA (RM-ANOVA) was performed with training day (Day) as within-subjects factor and the experimental group (Group) as between-subjects factor. A significant main effect was found for Day [P < 0.001, F (7, 315) = 10.23], Group [P < 0.001, F = (4, 45) = 4.33] as well as the Day by Group interaction [P < 0.001, F = (28, 315) = 3.10] [Figure 4]. This suggests that the RME rates changed with days of training and that these changes differed between the groups.
Figure 4.
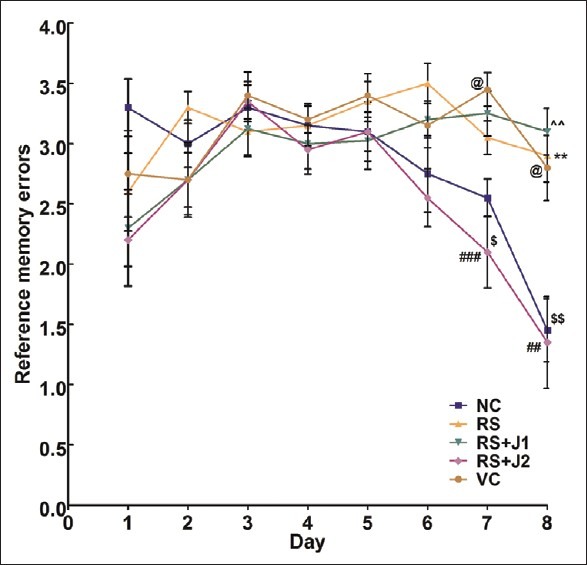
Mean (SEM) reference memory errors during acquisition of partially baited radial arm maze. NC, normal control; RS, restraint stress; VC, vehicle control; RS + JM1, Stress + Jatamansi extract 100 mg; RS + JM2: Jatamansi extract 200 mg. Two-way repeated measures ANOVA: P < 0.001, F = (28, 315) = 3.10 for Group × Day interaction. Simple main effect of Groups on Day 7: P < 0.001, F(4, 45) = 8.14. Simple main effect of Groups on Day 8: P < 0.001, F(4, 45) = 9.61. Bonferroni adjusted pairwise comparisons: **P < 0.01, RS < NC; ##P < 0.01, RS + JM2 > VC; ###P < 0.01, RS + JM2 > VC; $P < 0.05, RS + JM2 > RS; $$P < 0.01, RS + JM2 > RS; @P < 0.05 VC > NC; ∧∧P < 0.01 RS + JM1 < NC
Reference memory errors on Day 8 were further analyzed within the RM-ANOVA framework to assess the group differences in learning. A significant simple main effect was found for groups at this time point [P < 0.001, F(4, 45) = 9.61]. Pairwise comparisons, adjusted for multiple comparisons using the Bonferroni method revealed a significantly fewer RME for NC animals on Day 8 compared to RS animals (P < 0.01). The RS+JM2 group made significantly less RME compared to the RS and VC groups (P < 0.01). The VC and the RS+JM1 groups did not differ significantly from the RS group [Figure 5].
Figure 5.
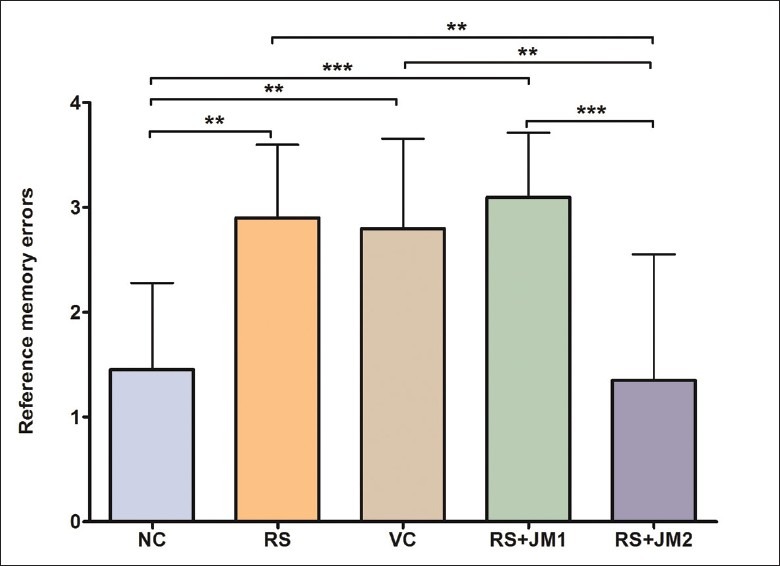
Comparison of mean (SD) reference memory errors on Day 8. One-way ANOVA P < 0.001, F(4, 45) = 9.61; Post hoc Tukey's test: **P < 0.01: RS > NC, RS > RS + JM2, VC > RS + JM2, VC >NC; ***P < 0.01: RS + JM1 > NC, RS + JM1 >RS + JM2
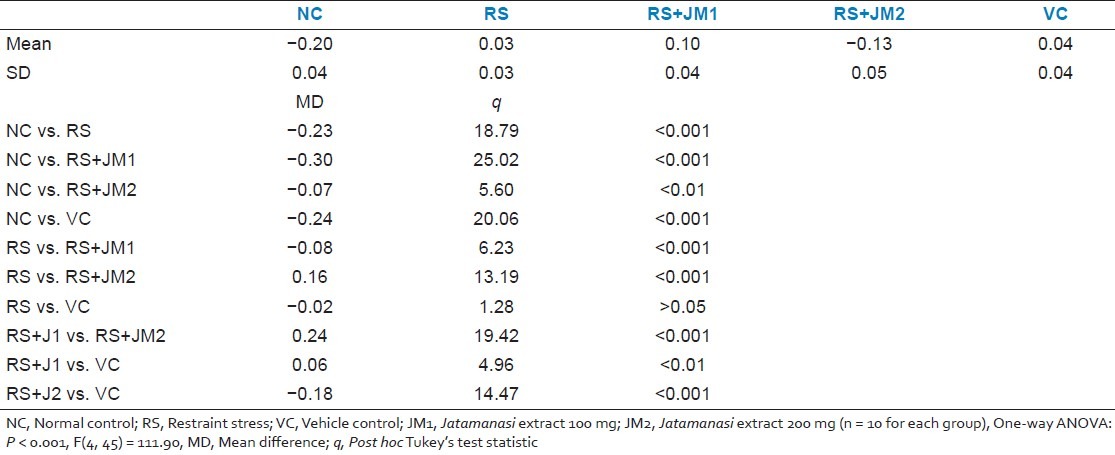
Trend analysis performed to characterize the interaction between Day and Group revealed that the linear effect of Days × Group interaction was significant [P < 0.001, F(4, 45) = 6.79] with no such significant effects at higher-order nonlinear trends. The RS and VC groups had slopes which did not deviate significantly from zero, i.e., showed no change in RME rates over time. However, the NC and the JM treated, stressed groups had negative slopes which were significantly non-zero suggesting that the RME decreased linearly with number of training days. A one-way ANOVA test was used to compare the slopes of the linear regression of RME over days curves. This showed significant between groups difference in the slopes of the RME curves [P < 0.001, F(4, 45) = 111.90]. Post hoc Tukey's tests revealed that the slope of the RME curve for the NC group was significantly lower than that of the RS group and that the slope for the RS+JM2 group was significantly lower than both the RS and VC [Table 2].
Table 2.
Comparison of the slopes of the reference memory errors over days during partially baited radial arm maze acquisition
Working memory errors during the learning phase
A two-way repeated measures ANOVA (RM-ANOVA) was performed with the training day (Day) as within-subjects factor and the experimental group (Group) as between-subjects factor. A significant main effect was found for Day [P < 0.001, F(7, 315) = 18.72], Group [P < 0.001, F = (4, 45) = 1.86] as well as the Day by Group interaction [P < 0.001, F = (28, 315) = 2.71] [Figure 3]. Exploration of the simple main effects of Group within the different time points (Days) suggested significant effects on Day 1 [P < 0.001, F(4, 45) = 6.22], Day 6 [P < 0.049, F(4, 45) = 2.60], and Day 7 [P < 0.045, F(4, 45) = 2.65]. Pairwise comparison of WME between the groups at these time points with Bonferroni adjustment for multiple comparisons was performed. On Day 1, the NC group had significantly higher WME compared to each of the other groups (P < 0.01). On Day 6, the only significant difference was between NC and VC (P < 0.05). No other significant between-group differences were found with post hoc pairwise comparisons [Figure 6].
Figure 6.
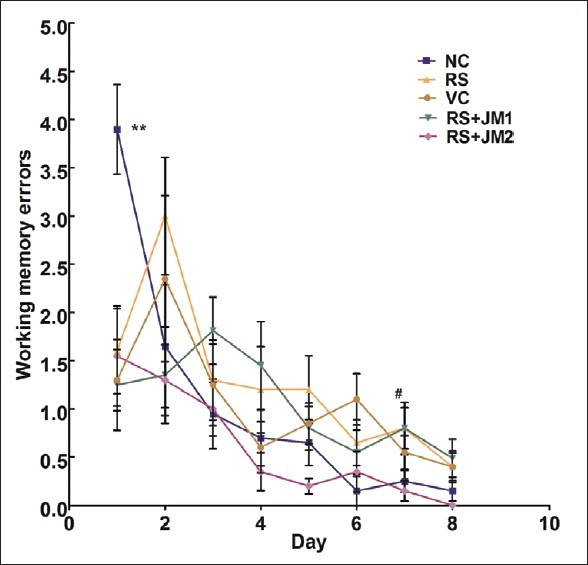
Mean (SEM) working memory errors during acquisition of partially baited radial arm maze. NC, normal control; RS, restraint stress; VC, vehicle control; RS + JM1, stress + Jatamansi extract 100 mg; RS+ JM2, stress + Jatamansi extract 200 mg. Two-way repeated measures ANOVA: P < 0.001, F= (28, 315) = 2.71 for Group × Day interaction. Simple main effect of Groups on Day 1: P < 0.001, F(4, 45) = 6.22; simple main effect of Groups on Day 6: P < 0.049, F(4, 45) = 2.60; simple main effect of Groups on Day 7: P < 0.045, F(4, 45) = 2.65. Bonferroni adjusted pairwise comparisons: **P < 0.01 for NC compared to each of the other groups; #P < 0.05 for VC > NC
The differences on Day 1 are possibly due to high variability in WME and the lack of significant differences in later days of training suggests that the groups did not differ in the WME during the acquisition of the partially baited RAM task.
Retention
% Correct choice (%CC) during retention testing
A one-way ANOVA was used to analyze the % correct choices during the retention testing trial performed on the tenth day following completion of the partially baited RAM task. A significant group difference was found [P < 0.01, F(4, 45) = 5.90)] which was further explored with post hoc Tukey's pairwise comparisons. This revealed significantly lower % CC by the RS group compared to the NC group. The RS + JM2 group showed significantly higher %CC compared to the RS and VC groups (P < 0.001). The VC and the RS+JM1 groups did not differ significantly from the RS group [Figure 7].
Figure 7.
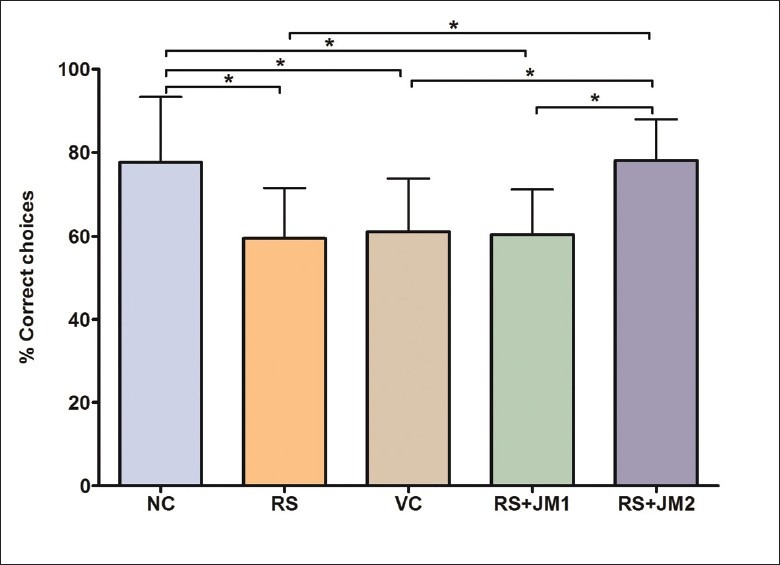
Comparison of mean (SD) % correct choices during retention testing. One-way ANOVA P < 0.01, F(4, 45) = 5.90; Post hoc Tukey's test: *P < 0.05: NC > RS, NC > VC, NC > RS + JM1, RS + JM2 > RS, RS + JM2 > VC, RS + JM2 < RS + JM1
Reference memory errors during retention testing
A one-way ANOVA was used to analyze the RME during the retention testing trial performed on the tenth day following completion of the partially baited RAM task. A significant group difference was found [P < 0.01, F(4, 45) = 4.37)] which was further explored with post hoc Tukey's pairwise comparisons. This revealed significantly higher RME by the VC and RS+JM1 groups compared to the NC group. The RS+JM2 group showed a trend for lower RME compared to the RS and VC groups, but this was not statistically significant [Figure 8].
Figure 8.
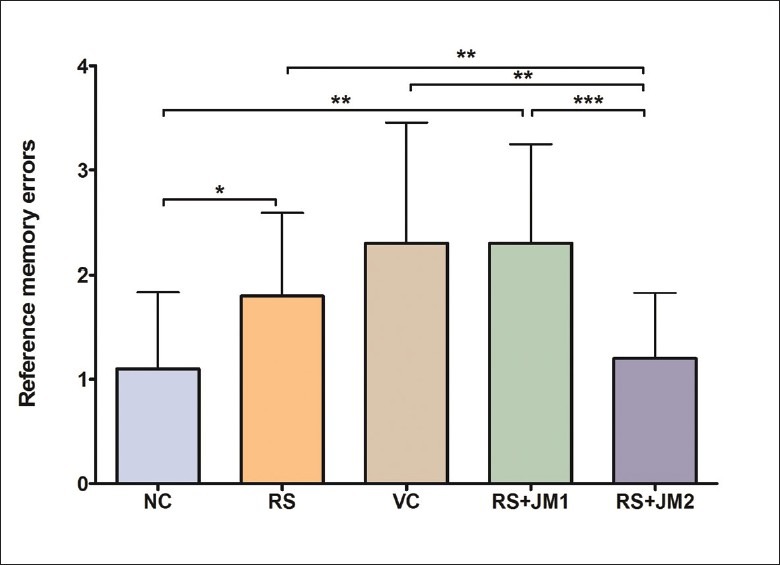
Comparison of mean (SD) RME during retention testing. One-way ANOVA P < 0.01, F(4, 45) = 4.37. Post hoc Tukey's test: *P < 0.05: NC > RS; **P < 0.01: NC < RS + JM1, RS + JM2 < RS, RS + JM2 < VC; ***P < 0.001: RS + JM2 < RS + JM1
DISCUSSION
The current study assessed the effects of concurrent treatment with NJE on hippocampus-dependent spatial learning and memory in rats subjected to 21 days of chronic RS. The salient findings in the current study are that chronic restraint stress impairs learning of the eight-arm radial maze task and that this impairment is ameliorated by concurrent treatment with NJE. Animals treated with 200 mg/kg body weight NJE had learning curves comparable to control unstressed animals, made significantly more correct choices and fewer reference memory errors on the eighth day of training compared to untreated stressed animals as well as stressed animals which received vehicle or a lower dose (100 mg/kg) of NJE. Compared to untreated animals, NJE-treated animals also made significantly higher correct choices and fewer RME in a retention test 10 days after the training period.
To the best of our knowledge, this is the first report of the protective effects of NJE on cognitive functions in chronically stressed animals. The stress model used in our study was effective as evidenced by the changes in plasma corticosterone during the stress period [Figure 1]. The chronic RS procedure resulted in impairments in the acquisition and retention of the partially baited radial maze task has been reported in earlier studies using this model.[15–17]
NJE has been demonstrated to protect against acute restraint and cold stress induced elevation in plasma corticosterone, increase in adrenal and spleen weights and gastric ulceration.[18] These findings were associated with a reversal of the stress-induced elevation of lipid peroxidase and NO levels and decrease in catalase activity in the brain and therefore the anti-stress effect of NJE was attributed to its anti-oxidant properties. This study provides preliminary evidence for the protective effect of NJE in chronic stress.
NJE has been reported in an earlier study to improve performance in passive avoidance tasks and to reverse drug-induced impairments in this task.[5] Findings from this study indicate that NJE has a protective effect on hippocampus-dependent spatial memory and learning as well. Chronic restraint stress has been shown to result in impaired learning and retention in spatial memory tasks like the RAM task[15,17] and the water maze task.[19] Such impairment has been associated with increase in circulating corticosteroids,[20] alterations in the hippocampal morphology-like retraction of the apical dendrites in the CA3 region of the hippocampus,[21] changes in the hippocampal dendritic spine number and shape,[22] loss of cells,[10] reduced neurogenesis in the dentate gyrus,[23] reduced BDNF expression,[24] impaired long-term potentiation at hippocampal synapses,[25] and alterations in neurotransmitter levels.[26] The protective effect of NJE on stress-induced deficits in the RAM task may be mediated through modulatory influences on one or more of these multiple factors. In this context, we have recently demonstrated that NJE treatment was associated with an elevation in AChE activity levels in the frontal cortex of both chronically stressed animals as well as unstressed controls.[27] Acute administration of NJE has been reported to enhance GABA levels in the rat brain while subchronic administration has been associated with increase in monoamines like NE, DA, 5-HT, 5-HIAA as well.[28] The modulatory effects of NJE on neurotransmitter systems may therefore be important in mediating its protective effects on hippopcampal function. Further studies are required to investigate these possibilities.
A few other studies have investigated the possible mechanisms of the action of NJE on the central nervous system in general. Studies in a mouse model of depression have suggested altered monoamine oxidase (MAO-A and MAO-B) activities[3] and changes in the levels of neurotransmitters such as norepinephrine, dopamine, serotonin, 5-hydroxyindoleacetic acid, and γ-aminobutyric acid.[28] Enhanced anti-oxidant defenses, restoration of dopamine and its metabolites, enhanced striatal dopaminergic D2 receptor binding, and tyrosine hydroxylase expression have been correlated with the antiparkinsonism activity of NJE.[29] It has also been proposed that GABAergic neuronal stimulation by NJE may antagonize the effect of glutamate and protect the neurons from cerebral ischemia. Some of these mechanisms may also play a role in mediating the effects of NJE on stress-induced cognitive impairment demonstrated in this study.
To summarize, we demonstrate the ameliorative effects of NJE administration on RAM learning and memory in a chronic restraint stress model. This protective effect was seen at an oral dose of 200 mg/kg, but not at 100 mg/kg. We propose that these effects may be mediated through modulatory influences of NJE on hippocampal function.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Uniyal MR, Issar RK. Commercially and traditionally important medicinal plants of Mandakini Valley of UttaraKhand, Himalayas. J Res Indian Med. 1969;4:83. [Google Scholar]
- 2.Rao VS, Rao A, Karanth KS. Anticonvulsant and neurotoxicity profile of Nardostachys jatamansi in rats. J Ethnopharmacol. 2005;102:351–6. doi: 10.1016/j.jep.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 3.Dhingra D, Goyal PK. Inhibition of MAO and GABA: Probable mechanisms for antidepressant-like activity of Nardostachys jatamansi DC in mice. Indian J Exp Biol. 2008;46:212–8. [PubMed] [Google Scholar]
- 4.Salim S, Ahmad M, Khan SZ, Ahmad SA, Islam F. Protective effect of Nardostachys jatamansi in rat cerebral ischemia. Pharmacol Biochem Behav. 2003;74:481–6. doi: 10.1016/s0091-3057(02)01030-4. [DOI] [PubMed] [Google Scholar]
- 5.Joshi H, Parle M. Nardostachys jatamansi improves learning and memory in mice. J Med Food. 2006;9:113–8. doi: 10.1089/jmf.2006.9.113. [DOI] [PubMed] [Google Scholar]
- 6.Heine VM, Maslam S, Joel M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–75. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 7.Herrera DG, Yague AG, Johnsen–Soriano S. Selective impairment of hippocampal neurogenesis by chronic alcoholism: Protective effects of an antioxidant. Proc Natl Acad Sci U S A. 2003;100:7919–24. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunanda, Rao BS, Raju TR. Chronic restraint stress impairs acquisition and retention of spatial memory task in rats. Curr Sci. 2000;17:1581–4. [Google Scholar]
- 9.Sunanda, Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons--a quantitative study. Brain Res. 1995;694:312–7. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- 10.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–11. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–5. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 12.Glavin GB, Murison R, Overmier JB, Pare WP, Bakke HK, Henke PG, et al. The neurobiology of stress ulcers. Brain Res Rev. 1991;16:301–43. doi: 10.1016/0165-0173(91)90012-w. [DOI] [PubMed] [Google Scholar]
- 13.Welch BL, Welch A. Sustained effects of brief daily stress (fighting)upon brain and adrenal catecholamines and adrenal, spleen, and heart weights of mice. Physiology. 1969;64:100–7. doi: 10.1073/pnas.64.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srikumar BN, Raju TR, Rao BS. The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience. 2006;143:679–88. doi: 10.1016/j.neuroscience.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Titus AD, Rao BS, Harsha HN, Ramkumar K, Srikumar BN, Singh SB, et al. Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience. 2007;145:265–78. doi: 10.1016/j.neuroscience.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 16.Bindu B, Rekha J, Kutty BM. Postinsult enriched housing improves the 8-arm radial maze performance but not the Morris water maze task in ventral subicular lesioned rats. Brain Res. 2005;1063:121–31. doi: 10.1016/j.brainres.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 17.Srikumar BN, Raju TR, Shankaranarayana Rao BS. Contrasting effects of bromocriptine on learning of a partially baited radial arm maze task in the presence and absence of restraint stress. Psychopharmacology. 2007;193:363–74. doi: 10.1007/s00213-007-0801-4. [DOI] [PubMed] [Google Scholar]
- 18.Lyle N, Bhattacharyya D, Sur TK, Munshi S, Paul S, Chatterjee S, et al. Stress modulating antioxidant effect of Nardostachys jatamansi. Ind J Biochem Biophy. 2009;46:93–8. [PubMed] [Google Scholar]
- 19.Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav Brain Res. 2008;187:41–7. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 21.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 22.Donohue HS, Gabbott PL, Davies HA, Rodriguez JJ, Cordero MI, Sandi C, et al. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: A stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 23.Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- 24.Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–39. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Spedding M. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: Importance for psychiatric diseases. Neurotox Res. 2004;6:233–44. doi: 10.1007/BF03033225. [DOI] [PubMed] [Google Scholar]
- 26.Sunanda, Rao BS, Raju TR. Restraint stress-induced alterations in the levels of biogenic amines, amino acids, and AChE activity in the hippocampus. Neurochem Res. 2000;25:1547–52. doi: 10.1023/a:1026606201069. [DOI] [PubMed] [Google Scholar]
- 27.Karkada G, Shenoy KB, Halahalli H, Karanth KS. Differential effect of Nardostachys jatamansi rhizome extract on acetylcholinesterase in different regions of brain in rats under chronic stress. Biomedicine. 2011;31:13–21. [Google Scholar]
- 28.Prabhu V, Karanth KS, Rao A. Effects of Nardostachys jatamansi on biogenic amines and inhibitory amino acids in the rat brain. Planta Med. 1994;60:114–7. doi: 10.1055/s-2006-959429. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad M, Yousuf S, Khan MB, Hoda MN, Ahmad AS, Ansari MS, et al. Attenuation by Nardostachys jatamansi of 6-hydroxydopamine-induced parkinsonism in rats: behavioral, neurochemical, and immunohistochemical studies. Pharmacol Biochem Behav. 2006;83:150–60. doi: 10.1016/j.pbb.2006.01.005. [DOI] [PubMed] [Google Scholar]


