Abstract
Non-Hodgkin's lymphoma (NHL) is seldom seen in the oral cavity, and has been reported with some frequency in HIV-positive patients. Oral HIV-related lymphomas exhibit an aggressive course and can mimic other oral tumors and infections that make early recognition and diagnosis difficult. This paper presents a case of NHL on the gingiva of a 28-year-old HIV-positive male patient.
Keywords: Gingiva, HIV, non-Hodgkin's lymphoma
INTRODUCTION
The occurrence of the non-Hodgkin's lymphoma (NHL) in the oral cavity in patients with Acquired Immune Deficiency Syndrome (AIDS) has been reported by various authors.[1,2] However, only three reports could be traced in which the oral manifestations of NHL were discussed in detail.[3,4] NHL is rarely seen in the oral cavity, and the gingiva is one of the uncommon intraoral sites to be involved. This paper reviews a case of primary NHL, which presented as a gingival mass in a HIV-infected patient.
CASE REPORT
A 28-year-old male patient reported to the dental clinic with a complaint of a swelling in the lower front teeth region since 2 months. The patient had been aware of a gradual increase in the size of the swelling, but had not experienced any local discomfort or bleeding. From his social history, the patient belonged to a high-risk group as he was found to be promiscuous. He smoked approximately 20 cigarettes a day since 10 years and he was also a known alcoholic for the same period. Extra-oral examination at the first visit revealed submandibular cervical lymphadenopathy, but no obvious facial asymmetry was noted. The lymphnodes were firm and non-tender. On intraoral examination, a reddish multilobular tissue mass of size 3 × 4 cm was noted involving both the labial and lingual aspect of the lower anterior teeth. Labially, it extended till the inner surface of lower lip. Lingually, it involved the sublingual region up to the lingual frenum [Figure 1]. The growth was not found to be ulcerated or any other secondary changes.
Figure 1.
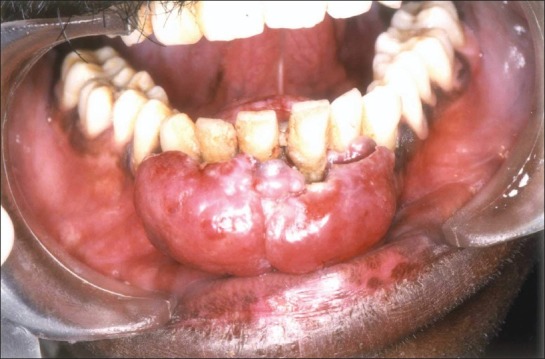
Intraoral picture showing the gingival mass
Intraoral periapical radiograph (IOPA) revealed severe bone loss up to the periapical region of mandibular incisors along with ill-defined borders [Figure 2].
Figure 2.
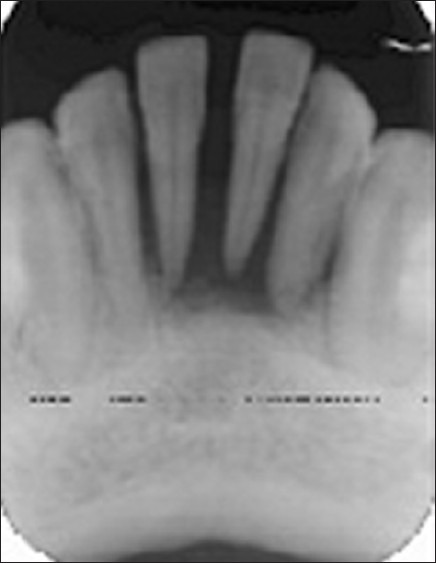
IOPA radiograph revealing severe bone loss in relation to mandibular incisors
The microscopic examination revealed a wedge of oral mucosa containing a dense, diffuse proliferation of malignant lymphoid cells in the connective tissue stroma. The neoplastic cells appear to be uniformly dark, round, and moderately differentiated and were also found to be infiltrating the adjacent tissues [Figure 3]. The neoplastic cells consisted of abundant pale cytoplasm, large vesicular nuclei with chromatin clumping. There is evidence of multiple, prominent nucleoli in proximity to the nuclear membrane. [Figure 4].
Figure 3.
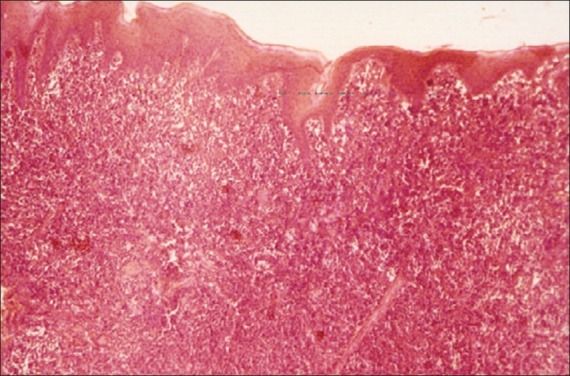
Photomicrograph showing dense, diffuse infiltration of malignant lymphoid cells in the connective tissue stroma (H and E, ×4)
Figure 4.
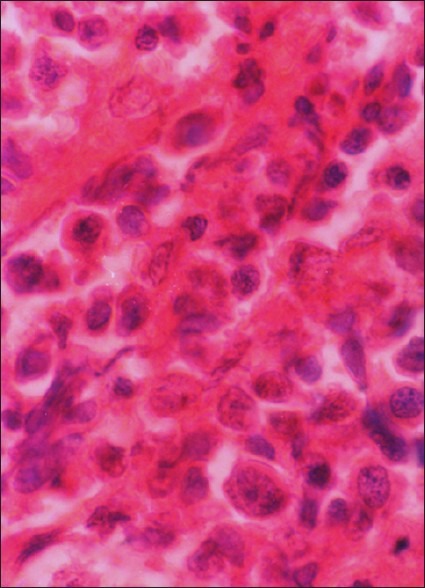
Photomicrograph showing neoplastic lymphoid cells (H and E, ×40)
The above features in correlation with clinical and radiological pictures were suggestive of a diffuse type primary extranodal large-cell NHL. The patient was referred to a regional cancer institute for further management.
DISCUSSION
NHL is the second most common HIV-associated tumor after Kaposi's sarcoma, the risk of getting NHL being 60 times greater in patients with HIV disease than in otherwise healthy persons. NHL occurs in 3% of individuals with HIV disease.[5] There is considerable variation in the frequency of oral NHL among reported patients’ groups. It is perhaps more frequent in homosexual males and injecting drug users than in other patients liable to HIV disease.[6]
The NHLs commonly involve the oropharyngeal lymphoid tissue comprising of Waldeyer's ring but only occasionally involve other oral tissues. The favored intraoral sites are the palatal mucosa and bone. The gingiva is one of the rarest intraoral sites and only isolated cases have been reported.[7,8] This is partially accounted for by the fact that lymphoid tissue is not normally found in the gingiva.[9]
In the largest series of intraoral lymphoma cases reported,[9] not a single case of gingival involvement has been reported. The diagnosis of malignant lymphoma can only be made by microscopic examination of the biopsy specimen.[10]
The etiology of NHL is unknown. However, the incidence of malignancy in patients who are immunodeficient has been reported as 10,000 times that of the general age matched population.[11,12] The incidence of diffuse large cell lymphomas is 0.3% in younger age and has a relative survival rate of 42.9% after 5 years.[13] Primary immunodeficiency syndromes such as Chediak-Higashi, Wiscott-Aldrich, and ataxia-telangiectasia are associated with an increased risk of developing lymphoma.[14] The treatment is dependent on both histological subtype and clinical staging of the disease. Generally, radiotherapy is used to treat local lesions, while chemotherapy is used for disseminated disease. Autologous hematopoietic stem-cell transplantation (HSCT) after high-dose chemotherapy has evolved as part of the standard of care for many patients with NHL. The success of this treatment modality hinges on collection of sufficient hematopoietic stem cells (HSC) for transplantation. Approximately 10–30% of patients are unable to collect the minimum number of HSC, defined as 2 × 106 CD34 cells/kg, to support high-dose chemotherapy and autologous HSCT. Plerixafor given either alone or with granulocyte colony-stimulating factor (G-CSF) has been reported in phases I and II clinical studies to significantly increase the number of peripheral blood CD34 cells and CD34 cell collection.[15]
Adolescents (age 15–21 years) compared with younger children with mature B-cell NHL have been historically considered to have an inferior prognosis. According to French–American–British Mature B-Cell Lymphoma 96 (FAB LMB 96) trial, the LDH level at diagnosis, mediastinal disease, and combined Bone marrow-positive/CNS-positive involvement are independent risk factors in children with mature B-cell NHL.[16]
The incidence of NHL in patients infected with HIV is relatively high. It was reported that between 4% and 10% of patients affected by AIDS will develop NHL. NHL in patients with AIDS confers a poor prognosis, not only because of the sequelae of progressive lymphoma, but also because of an increased incidence of opportunistic infection.[11] The overall prognosis was significantly worse among HIV-positive patients. Median survival was 34 months in immunocompetent vs. 9 months in HIV-positive patients.[17] Early clinical stage and a complete response to chemotherapy were associated with longer survival. There was no apparent difference in survival with regimens more intensive than cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).[18]
The intraoral NHL commonly shows a strong resemblance to acute necrotizing and inflammatory lesions of the gingiva. Thus, clinical diagnosis may be difficult to make, especially when it exhibits primary with solitary manifestations. It is, therefore, imperative that clinicians include NHL in the differential diagnosis of oral lesions in persons belonging to the high-risk groups.
CONCLUSION
Therefore, it has been suggested that inclusion of NHL in differential diagnosis of intraoral soft tissue masses in suspected HIV cases. Importance of early diagnosis to ensure appropriate treatment and to develop awareness among health care workers, especially dentists, of the increased risk of oral NHL among HIV population appears to be imperative.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Brahim JS, Katz RW. Non-Hodgkin's lymphoma of the hard palate mucosa and buccal gingiva associated with AIDS. J Oral Maxillofac Surg. 1988;46:328–30. doi: 10.1016/0278-2391(88)90021-3. [DOI] [PubMed] [Google Scholar]
- 2.Groot RH, Van Merkesteyn JP. Oral manifestations of Non-Hodgkin's lymphoma in HIV infected patients. Int J Oral Maxillofac Surg. 1990;19:194–6. doi: 10.1016/s0901-5027(05)80387-x. [DOI] [PubMed] [Google Scholar]
- 3.Augar GE, Burns JC. Non-Hodgkin's lymphoma of the oral cavity associated with AIDS. Oral Surg. 1989;67:433–6. doi: 10.1016/0030-4220(89)90387-3. [DOI] [PubMed] [Google Scholar]
- 4.Wilson TG, Wright JM. Non- Hodgkin's lymphoma of the gingiva: Review of the literature-Report of a case. J Periodont. 1986;57:155–8. doi: 10.1902/jop.1986.57.3.155. [DOI] [PubMed] [Google Scholar]
- 5.Porter SR, Stock C. Oral plasmablastic lymphoma in previously undiagnosed HIV disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endol. 1999;87:730–40. doi: 10.1016/s1079-2104(99)70170-8. [DOI] [PubMed] [Google Scholar]
- 6.Bigger RJ, Rabkin CS. The epidemiology of acquired immuno deficiency syndrome related lymphomas. Curr Opin Oncol. 1992;1:833–93.7. doi: 10.1097/00001622-199210000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Bulut E, Bekçioğlu B, Günhan O, Sener I. Diffuse large B-cell lymphoma with oral manifestations. J Craniofac Surg. 2011;22:1144–7. doi: 10.1097/SCS.0b013e318210b940. [DOI] [PubMed] [Google Scholar]
- 8.Raghavendra K, Ankit S, Vathsala N. Diffuse Large B-cell lymphoma of mandible: A case report. Med Oral Patol Oral Cir Bucal. 2009;14:e421–4. [PubMed] [Google Scholar]
- 9.Eisenbud L, Seiabba J. Oral presentations in Non-Hodgkin's Lymphoma: Review of thirty-one cases. Part 1 Data analysis. Oral Surg. 1983;56:151. doi: 10.1016/0030-4220(83)90281-5. [DOI] [PubMed] [Google Scholar]
- 10.Martinelli-Kläy CP, Martinelli CR, Martinelli C, Dias JB, Cheade TC, Lombardi T. Primary extranodal non-Hodgkin lymphoma of the gingiva initially misdiagnosed as dental abscess. Quintessence Int. 2009;40:805–8. [PubMed] [Google Scholar]
- 11.Nittayanata W, Chung Panich S. AIDS-related Non-Hodgkin's lymphoma presenting as delayed healing of an extraction wound. Br Dent J. 1996;181:102–4. doi: 10.1038/sj.bdj.4809172. [DOI] [PubMed] [Google Scholar]
- 12.Ogden RA, Good R. A Occurrence of malignancy in immunodeficiency diseases. Cancer. 1971;28:89–98. doi: 10.1002/1097-0142(197107)28:1<89::aid-cncr2820280117>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Greiner TC, Medeiros LJ, Jaffe ES. Non-Hodgkin's lymphoma. Cancer. 1995;75:370–80. doi: 10.1002/1097-0142(19950101)75:1+<370::aid-cncr2820751319>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 14.Oden GR. Lymphoma of oral soft tissues. Br Dent J. 1986;161:9–11. doi: 10.1038/sj.bdj.4805887. [DOI] [PubMed] [Google Scholar]
- 15.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with Non-Hodgkin's Lymphoma. J Clin Oncol. 2009;27:4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 16.Cairo MS, Sposto R, Gerrard M, Auperin A, Goldman SC, Harrison L, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell Non-Hodgkin's Lymphoma: Results of the FAB LMB 96 Study. J Clin Oncol. 2012;30:387–93. doi: 10.1200/JCO.2010.33.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattaneo C, Facchetti F, Re A, Borlenghi E, Majorana A, Bardellini E, et al. Oral cavity lymphomas in immunocompetent and human immunodeficiency virus infected patients. Leuk Lymphoma. 2005;46:77–81. doi: 10.1080/10428190400007789. [DOI] [PubMed] [Google Scholar]
- 18.Castillo JJ, Winer ES, Stachurski D, Perez K, Jabbour M, Milani C, et al. Prognostic factors in chemotherapy-treated patients with HIV-associated Plasmablastic lymphoma. Oncologist. 2010;15:293–9. doi: 10.1634/theoncologist.2009-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]


