Abstract
Objective:
Women with polycystic ovary syndrome (PCOS) may represent a large underappreciated segment of female population who is at increased cardiovascular risk because of the presence of cluster of metabolic abnormalities. The aim of our study was to assess atherosclerotic risk factors in women with PCOS.
Materials and Methods:
In a cross-sectional study, 50 women with PCOS and 50 age and weight-matched healthy controls were enrolled. Endothelial dysfunction by flow-mediated dilatation (FMD) of brachial artery, highly sensitive C-reactive protein (hs CRP), and carotid intima media thickness (CIMT) were measured in both cases and control groups.
Results:
The mean age of women with PCOS was 26.82 ± 3.26 years and Body-mass index (BMI) of 26.2 ± 4.8 kg/ m2. Thirty-six (72%) patients were overweight or obese,54% had central obesity and 12% had impaired glucose tolerance. Among the markers of atherosclerosis, hsCRP levels were nonsignificantly higher in patients with PCOS than in controls. The FMD was 12.18 ± 2.3% vs 8.3 ± 2.23% in patients with PCOS and controls respectively (P=0.01). CIMT was significantly different in two study groups (0.68 ± 0.11 in PCOS vs 0.52 ± 0.02 in normal subjects, (P=0.01). FMD had significant negative correlation with homeostasis model assessment (HOMA) index (r = −0.32, P=0.02) and hs CRP (r = −0.37, P=0.04) while hs CRP was correlated with BMI (r = 0.54, P=0.005), HOMA (r = 0.38, P=0.02) and FMD (r = -0.33, P=0.01). CIMT was significantly different in women with PCOS and control subjects, and it had significant correlation with age (r = 0.42, P=0.03), BMI (r = 0.36, P=0.01), waist circumference (r = 0.52, P=0.001) and HOMA (r = 0.31, P=0.04).
Conclusion:
Women with PCOS definitely have increased risk for future cardiovascular events. Clinicians should consider early cardiovascular screening and interventions to control all modifiable cardiovascular risk factors.
Keywords: Atherosclerosis, cardiovascular risk, metabolic syndrome, polycystic ovary syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy in women of child-bearing age with estimated prevalence of nearly 10%.[1,2] It is characterized by chronic anovulation, hyperandrogenism and/or polycystic ovaries. Its association with metabolic syndrome has placed it as ovarian manifestation of metabolic syndrome. Insulin resistance and its compensatory hyperinsulinemia is the central feature of PCOS, which it shares with metabolic syndrome. PCOS may represent a large underappreciated segment of female population who is at increased cardiovascular risk because of the presence of cluster of metabolic abnormalities such as glucose intolerance, hypertension, obesity and dyslipidemia. All of them have been associated with atherosclerosis. Glueck et al., have reported that 46% of women with PCOS have the metabolic syndrome.[3]
Although the only long-term study on women with PCOS has not shown an increase in mortality[4] due to coronary artery disease, there is growing evidence that risk is substantial.[5–7] However, the question remains whether PCOS increases the risk independently of the presence of obesity. The studies have demonstrated conflicting results on atherosclerotic markers in women with PCOS. In recent times, certain surrogate markers of atherosclerosis have been devised, which can help assess the cardiovascular risk noninvasively.
Endothelial dysfunction has been regarded as an early feature of atherosclerosis. Assessment of endothelial dysfunction by measuring flow-mediated dilatation (FMD) of the brachial artery is considered a potential tool for predicting coronary atherosclerosis.[8] Highly sensitive C-reactive protein (hsCRP)[9] and carotid intima media thickness (CIMT)[10] are other surrogate markers and good predictors of subclinical atherosclerosis and vascular events. We are facing the problem of metabolic syndrome and type 2 diabetes in epidemic proportions and not many studies have addressed this issue in our context. Therefore, this study was designed to evaluate early atherosclerotic markers in young women with PCOS and the age- and weight-matched control group.
MATERIALS AND METHODS
We conducted a cross-sectional hospital-based study in a medical college hospital that enrolled 50 patients aged 20-35 years from outpatient departments of medicine/endocrinology and gynecology who were diagnosed as PCOS according to Rotterdam criteria.[11] Two of three criteria were sufficient for confirmation of the diagnosis. We included 50 age- and weight-matched normal healthy women as controls with normal menstrual cycles, with no evidence of hyperandrogenism, and with normal ovarian morphology on pelvic ultrasonography. All study participants gave written informed consent, and the study protocol was approved by Institutional Ethics committee. All patients who had secondary causes of hyperandrogenism such as hyperprolactinemia, late-onset congenital adrenal hyperplasia, androgen-secreting tumor, Cushing's disease, hypothyroidism, end-stage liver or kidney disease, pregnancy, hypertension and diabetes mellitus were excluded.
A detailed history and clinical examination for features of virilization and hirsutism according to Ferriman-Gallwey score was done. A thorough physical examination was performed, including measurement of weight, height and waist and hip circumferences. Weight was measured with the subject wearing light clothing without shoes, and height was measured using a stadiometer. Body-mass index (BMI) was calculated by using the formula: weight (in kg)/height (in meters)2. Waist circumference (WC) was measured with the patient standing, at a point midway between the lower costal margin and the iliac crest in the mid-axillary line. Blood pressure was measured manually with a sphygmomanometer. Overweight (BMI >23 kg/m2) and central obesity (WC >80 cm) were defined by Asian criteria.[12] The biochemical profile included lipids, glucose, insulin and liver and renal function tests. The serum levels of prolactin, thyroxin, total testosterone, Dehydroepiandrosterone sulfate, follicle-stimulating hormone, luteinizing hormone (LH) and 17-hydroxy progesterone were measured in all cases and controls. Homeostasis model assessment (HOMA) method for insulin resistance was calculated by the formula: Fasting serum insulin (micro units/mL) × fasting serum glucose (mill moles/L)/22.5.
Flow-mediated dilatation measurement
Endothelial function was measured noninvasively by ultrasonographic assessment of right brachial artery dimensions.[13] The diameter of the right brachial was measured twice, first at rest, then after inducing reactive hyperemia with the help of pneumatic cuff. It was carried out by a blinded sonologist, after an overnight fast in a cool, quiet room, with B mode ultrasound scanner (Seimens, Munich, Germany) using 10 MHz linear transducer. The diameter of right brachial artery was measured 2-8 cm above the antecubital space in the end-diastolic phase from one media–adventitia interface to the other at the clearest part three times and an average was taken. After the detection of the right transducer position, skin was marked and arm kept in same position. The blood pressure cuff was tied on the upper arm and inflated to supra-systolic levels and kept inflated for 4 minutes. Sixty seconds after the cuff was released, brachial artery dimensions were again measured. The maximum diameter measurement was defined as the average of three consecutive diameters measurements. The hsCRP concentration was determined using an immunoturbidimetric method (Randox, Mauguio, France) in mg/dL. CIMT was measured by B mode ultrasound using linear probe at frequency of 10 MHz. The common carotid arteries were scanned at the level of bifurcation on either side and mean value was used for analysis. The intima media thickness was measured in the far wall of the arteries at sites identified as diffuse and continuous projections with the greatest distance between the luminal–intimal interface and media–adventitial interface but without atherosclerotic plaques. Localized lesions >2 mm thickness were considered to be atherosclerotic plaques. CIMT was assessed by single observer who was blinded for the diagnosis.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 15.0 was used for statistical analysis. Results were expressed as mean ± SD. The characteristics of distribution were tested with Kolmogorov-Smirnof test. Highly skewed variables were analyzed after logarithmic transformation. Spearman rank correlations were used for these variables. When variables showed persistent skewed deviation, Mann-Whitney ‘U’ test was used. Differences between means were analyzed by Student's unpaired ‘t’ test. P value <0.05 was considered statistically significant. Analysis of correlations between parameters was performed by using Pearson's correlation coefficient and regression analysis was done to predict FMD and CIMT.
RESULTS
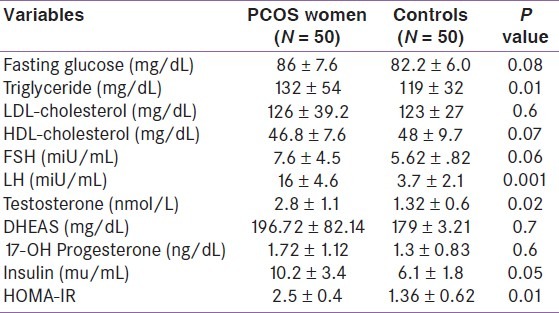
The anthropometric data of women with PCOS and controls are listed in Table 1. The mean age of women with PCOS was 26.82 ± 3.26 years and BMI of 26.2 ± 4.8 kg/m2. Thirty-six (72%) patients were overweight or obese, 54% had central obesity and 12% had impaired glucose tolerance (plasma glucose 2 h post 75 g glucose 140–199 mg/dL). There were no statistically significant differences in BMI, WC, hip circumference, waist/hip ratio, and mean systolic and diastolic blood pressure between cases and controls subjects. Table 2 summarizes the metabolic and hormonal profile of PCOS patients and control subjects that showed significant differences in levels of total testosterone, LH, insulin and HOMA index, while no difference could be demonstrated in other hormone levels between PCOS patients and control subjects.
Table 1.
Clinical characteristics of women with polycystic ovary syndrome and control subjects
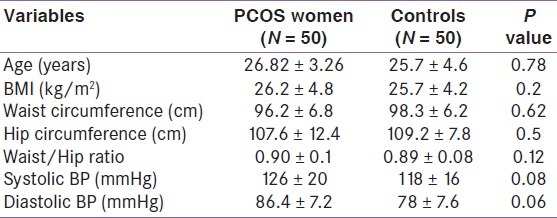
Table 2.
Hormonal data of women with polycystic ovary syndrome and control subjects
Among the markers of atherosclerosis [Table 3], hsCRP levels were higher in patients with PCOS than in controls but the difference was not statistically significant.
Table 3.
Atherosclerosis markers in women with polycystic ovary syndrome and control subjects

The baseline brachial artery diameter was 3.78 ± 0.23 mm in women with PCOS and 3.5 ± 0.42 mm in controls. The difference was not statistically significant. On the contrary, after inducing reactive hyperemia, the mean brachial artery dimensions were 4.23 ± 0.12 and 4.08 ± 0.17(P = 0.02).The FMD was 12.18 ± 2.3% vs 8.3 ± 2.23% in patients with PCOS and controls, respectively(P = 0.01).
CIMT was significantly different in two study groups (0.68 ± 0.11 in PCOS vs 0.52 ± 0.02 in normal subjects, P = 0.01). FMD had significant negative correlation with HOMA index (r = −0.32, P = 0.02) and hsCRP (r = −0.37, P = 0.04) while hsCRP was correlated with BMI (r = 0.54, P = 0.005), HOMA (r = 0.38, P = 0.02) and FMD (r = −0.33, P = 0.01). CIMT was significantly different in women with PCOS and control subjects, and it had significant correlation with age (r = 0.42, P = 0.03), BMI (r = 0.36, P = 0.01), WC (r = 0.52, P = 0.001) and HOMA (r = 0.31, P = 0.04).
Multiple regression analysis was carried out with CIMT as dependent variable. In the studied subjects (n = 100), PCOS status, age and BMI were the independent predictors of CIMT. Similarly for FMD, HOMA and hsCRP were the independent predictors.
DISCUSSION
Women with PCOS have a cluster of metabolic abnormalities. The proposed definition for this is female metabolic syndrome or syndrome XX[14] that starts early in adolescence and can lead to the development of premature atherosclerosis. The studies done on various atherosclerotic risk markers in women with PCOS have reported conflicting results. The variability of methodology that includes definition of PCOS, definition of metabolic syndrome, method of recruitment of PCOS group, selection of controls and the age, race or weight of the participants makes the interpretation of the results difficult in terms of prediction of the cardiovascular risk in the patients of PCOS.
There is increasing evidence that suggests atherosclerosis is a chronic inflammatory process. Several large-scale prospective studies have shown that inflammatory markers like hsCRP provide an adjunctive method for assessment of cardiovascular risk. Many studies have also shown high hsCRP levels in women with PCOS[15–17] in contrast to others that say it is related only to obesity.[18] In our study, hsCRP level was not significantly higher in women with PCOS than the controls and it had correlation with BMI, FMD and decreased insulin sensitivity. Kim et al.[19] have reported higher hsCRP levels even in lean PCOS women. A meta-analysis of cardiovascular risk markers that included hsCRP by Toulis et al.,[20] concluded that women with PCOS have increased incidence of inflammatory markers compared with controls.
One of the earliest processes in the pathogenesis of atherosclerosis is impaired endothelial dysfunction that can be quantified by noninvasive methods. In FMD of brachial artery, dilatation is measured ultrasonographically after inducing brachial artery ischemia, which leads to release of endothelial nitric oxide and relaxation of vascular smooth muscle. FMD is a marker of sub-clinical atherosclerosis since it is well correlated with impaired endothelial function in the coronary arteries.[21] A link has been established between insulin resistance and endothelial dysfunction. Paradisi et al.,[22] first reported that PCOS is characterized by endothelial dysfunction. Although exact mechanism how insulin resistance leads to endothelial dysfunction is not clear, it has been proposed that overproduction of free fatty acids and inflammatory cytokines such as tumor necrosis factor alpha and leptin cause endothelial dysfunction, which is contributed by oxidative stress.[23,24] In our study, FMD was significantly different in cases and control subjects. It also had correlation with HOMA index.
CIMT is an easy and reliable marker of early atherosclerotic changes and is widely used to predict cardiovascular events. Multiple investigators have found that patients with PCOS have a greater prevalence of abnormal CIMT than the general population. The significant difference was demonstrated in CIMT between women with PCOS and controls in our study, similar to reports by Lakhani and Talbott et al.[25,26] In multiple regression analysis, PCOS status, age and BMI were the independent predictors of CIMT, while HOMA and hsCRP were the independent predictors of FMD.
Orio et al.,[27] also found an early impairment of endothelial structure and function and greater CIMT in normal-weight women with PCOS. In addition, Talbott and colleagues[17] also found a relationship between the degree of CIMT thickening in PCOS patients and levels of CRP. The insulin resistance is the key component of PCOS, which is present in both obese and lean women with PCOS[28] and is linked with the increased cardiovascular risk markers. The diagnosis of PCOS implies cardiovascular risk. Although a recent study published by Schmidt et al.,[29] after 21 years follow-up in PCOS women of postmenopausal age does not entail an evident increase in cardiovascular events, Meyer et al.,[30] in a systematic review and meta-analysis concluded that women with PCOS are at greater risk of premature atherosclerosis.
Indian women are reported to have a high prevalence of PCOS[31] and Indian patients have higher fasting insulin levels and greater insulin resistance compared with white women with PCOS.[32,33] A high prevalence of impaired glucose tolerance and diabetes mellitus has been reported.[34,35] Sundararaman et al.,[36] reported south Indian women with reproductive abnormalities had greater insulin resistance and CIMT compared with controls. The difference persisted when non-obese women with PCOS were compared with controls. Not many studies in India have addressed this issue and none of them have used the same surrogate markers of early atherosclerosis[37,38] as have been studied by us. The limitations of our study were small sample size and that we did not analyze obese and lean PCOS with obese and lean controls separately.
CONCLUSION
Women with PCOS definitely have increased markers of atherosclerosis independent of obesity, so they are at risk for future cardiovascular events because of the presence of metabolic derangements, although larger studies with well-defined PCOS population may be required to draw a more robust conclusion.
Clinicians should consider early cardiovascular screening and interventions to control all modifiable cardiovascular risk factors in women who are diagnosed to have PCOS.
ACKNOWLEDGEMENT
We are thankful to Prof. Ashok Chandra for his intellectual inputs and support in preparation of the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: No
REFERENCES
- 1.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–61. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 3.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–15. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 4.Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long term follow-up: A retrospective cohort study. Clin Endocrinol. 2000;52:595–600. doi: 10.1046/j.1365-2265.2000.01000.x. [DOI] [PubMed] [Google Scholar]
- 5.Talbott EO, Zborowski JV, Rager JR, Boudreaux MY, Edmundowicz DA, Guzick DS. Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5454–61. doi: 10.1210/jc.2003-032237. [DOI] [PubMed] [Google Scholar]
- 6.Guzick DS. Cardiovascular risk in women with polycystic ovarian syndrome. Semin Reprod Endocrinol. 1996;14:45–9. doi: 10.1055/s-2007-1016308. [DOI] [PubMed] [Google Scholar]
- 7.Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:1357–63. doi: 10.1210/jc.2005-2430. [DOI] [PubMed] [Google Scholar]
- 8.Verma S, Buchnan MR, Anderson TJ. Endothelial dysfunction testing a biomarker of vacular disease. Circulation. 2003;108:2054–9. doi: 10.1161/01.CIR.0000089191.72957.ED. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease: Detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 10.O’Leary DH, Polak JF. Intima -media thickness: A tool for atherosclerosis imaging and event prediction. Am J Cardiol. 2002;90:18L–21. doi: 10.1016/s0002-9149(02)02957-0. [DOI] [PubMed] [Google Scholar]
- 11.The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, authors. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Snehlatha C, Vishwanathan V, Ramachandran A. Cut off values for normal anthropometric variables in Asian Indians. Diabetes Care. 2003;26:1380–4. doi: 10.2337/diacare.26.5.1380. [DOI] [PubMed] [Google Scholar]
- 13.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelium-dependent flow-mediated vasodilation of brachial artery. A report of the International Brachial Artery Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 14.Sam S, Dunaif A. Polycystic Ovary Syndrome: Syndrome XX? Trends Endocrinol Metab. 2003;14:365–70. doi: 10.1016/j.tem.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Boulman N, Levy Y, Leiba R, Shachar S, Linn R, Zinder O, et al. Increased C-reactive protein levels in the polycystic ovary syndrome: A marker of cardiovascular disease. J Clin Endocrinol Metab. 2004;89:2160–5. doi: 10.1210/jc.2003-031096. [DOI] [PubMed] [Google Scholar]
- 16.Tarkun I, Arsan Z, Turemen E, Sahin T, Duman C. Enthothelial dysfuction in young women with polycystic ovary syndrome: Relationship with insulin resistance and low grade inflammation. J Clin Endocrinol Metab. 2004;89:5592–6. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 17.Talbott EO, Zborowski JV, Boudreaux MY, McHugh-Pemu KP, Sutton-Tyrrell K, Guzick DS. The relationship between C-reactive protein and carotid intima-media wall thickness in middleaged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:6061–7. doi: 10.1210/jc.2003-032110. [DOI] [PubMed] [Google Scholar]
- 18.Ketel IJ, Stehouwer CD, Henry RM, Serne EH, Hompes P, Homburg R, et al. Greater arterial stiffness in polycystic ovary syndrome (PCOS) is an obesity-But not a PCOS-associated phenomenon. J Clin Endocrinol Metab. 2010;95:4566–75. doi: 10.1210/jc.2010-0868. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Han JE, Kim YS, Won HJ, Yoon TK, Lee WS. High sensitivity C-reactive protein and its relationship with impaired glucose regulation in lean patients with polycystic ovary syndrome. Gynecol Endocrinol. 2012;28:259–63. doi: 10.3109/09513590.2011.613967. [DOI] [PubMed] [Google Scholar]
- 20.Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou SA, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Hum Reprod Update. 2011;17:741–60. doi: 10.1093/humupd/dmr025. [DOI] [PubMed] [Google Scholar]
- 21.Kazmierski M, Michalewska-Wludarczyk A, Krzych LJ, Tendera M. Diagnostic value of flow mediated dilatation measurement for coronary artery lesions in men under 45 years of age. Cardiol J. 2010;17:288–92. [PubMed] [Google Scholar]
- 22.Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103:1410–5. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 23.Arcaro G, Cretti A, Balzano S, Lechi A, Muggeo M, Bonora E. Insulin causes endothelial dysfunction in humans. Circulation. 2002;105:576–85. doi: 10.1161/hc0502.103333. [DOI] [PubMed] [Google Scholar]
- 24.Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. 2005;90:5711–6. doi: 10.1210/jc.2005-0011. [DOI] [PubMed] [Google Scholar]
- 25.Lakhani K, Hardiman P, Seifalian A. Intima-media thickness of elastic and muscular arteries in young women with polycystic ovaries. Atherosclerosis. 2004;175:353–9. doi: 10.1016/j.atherosclerosis.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20:2414–21. doi: 10.1161/01.atv.20.11.2414. [DOI] [PubMed] [Google Scholar]
- 27.Orio F, Palomba S, Cascella T, De Simone B, Di Biase S, Russo T, et al. Early impairement of endothelial structure and function in young normal weght women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:4588–93. doi: 10.1210/jc.2003-031867. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt J, Landin-Wilhelmsen K, Brännström M, Dahlgren E. Cardiovascular disease and risk factors in PCOS women of postmenopausal age: A 21-year controlled follow-up study. Clin Endocrinol Metab. 2011;96:3794–803. doi: 10.1210/jc.2011-1677. [DOI] [PubMed] [Google Scholar]
- 29.Meyer ML, Malek AM, Wild RA, Korytkowski MT, Talbott EO. Carotid artery intima-media thickness in polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update. 2011;18:112–26. doi: 10.1093/humupd/dmr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodin DA, Bano G, Bland JM, Taylor K, Nussey SS. Polycystic ovaries and associated metabolic abnormalities in Indian subcontinent Asian women. Clin Endocrinol. 1998;49:91–9. doi: 10.1046/j.1365-2265.1998.00492.x. [DOI] [PubMed] [Google Scholar]
- 31.Norman RJ, Mahabeer S, Master S. Ethnic differences in insulin and glucose response to glucose between white and Indian women with polycystic syndrome. Fertil Steril. 1995;63:58–62. doi: 10.1016/s0015-0282(16)57297-5. [DOI] [PubMed] [Google Scholar]
- 32.Wijeyaratne CN, Balen AH, Barth JH, Belchetz PE. Clinical manifestations and insulin resistance (IR) in polycystic ovary syndrome(PCOS) among South Asians and Caucasians: Is there a difference? Clin Endocrinol. 2002;57:343–50. doi: 10.1046/j.1365-2265.2002.01603.x. [DOI] [PubMed] [Google Scholar]
- 33.Ganie MA, Khurana ML, Eunice M, Gupta N, Dwivedi SN, Gulati M, et al. Prevalence of glucose intolerance among adolescent and young women with polycystic ovary syndrome in India. Indian J Endocrinol Metab. 2004;6:9–14. [Google Scholar]
- 34.Kulshreshtha B, Ganie MA, Praveen EP, Gupta N, Lal Khurana M, Seith A. Insulin response to oral glucose in healthy, lean young women and patients with polycystic ovary syndrome. Gynecol Endocrinol. 2008;24:637–43. doi: 10.1080/09513590802342858. [DOI] [PubMed] [Google Scholar]
- 35.Sundararaman PG, Manomani R, Sridhar GR, Sridhar V, Sundaravalli A, Umachander M. Risk of atherosclerosis in women with polycystic ovary syndrome: A study from South India. Metab Syndr Relat Disord. 2003;1:271–5. doi: 10.1089/1540419031361435. [DOI] [PubMed] [Google Scholar]
- 36.Allahbadia GN, Merchant R. Polycystic ovary syndrome in the Indian Subcontinent. Semin Reprod Med. 2008;26:22–34. doi: 10.1055/s-2007-992921. [DOI] [PubMed] [Google Scholar]
- 37.Kalra A, Nair S, Rai L. Association of obesity and insulin resistance with dyslipidemia in Indian women with polycystic ovarian syndrome. Indian J Med Sci. 2006;60:447–53. [PubMed] [Google Scholar]
- 38.Maitra A, Pingle RR, Menon PS, Naik V, Gokral JS, Meherji PK. Dyslipidemia with particular regard to apolipoprotein profile in association with polycystic ovary syndrome: A study among Indian women. Int J Fertil Womens Med. 2001;46:271–7. [PubMed] [Google Scholar]


