Abstract
Introduction:
Non-alcoholic fatty liver disease (NAFLD) is associated with Type 2 diabetes (T2DM) and the metabolic syndrome, and can progress to chronic liver disease. We examined the incidence of elevated (>35 iu/l) alanine transaminase (ALT), as a surrogate marker for NAFLD, in patients with newly diagnosed T2DM.
Materials and Methods:
Retrospective analysis of ALT with metabolic parameters, in 606 consecutive patients presenting to district wide education sessions for newly diagnosed T2DM.
Results:
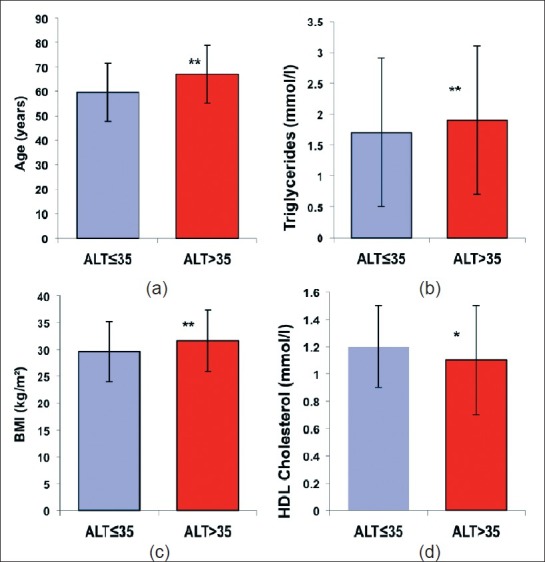
ALT was elevated in 155 patients (25.6% (95% CI 22.1, 29.2)), who tended to be older (mean difference 7.3 years (5.2, 9.5), P < 0.001), heavier (body mass index (BMI) mean difference 2.0 kg/m2 (1.0, 3.0), P < 0.001), and more likely to be male (M:F raised ALT 104:51, normal ALT 219:232, P < 0.001), with higher triglycerides (median difference 0.2 mmol/l, P = 0.001) and lower HDL cholesterol (mean difference 0.09 mmol/l (0.02, 0.15), P = 0.001). There were no statistically significant differences in HBA1C or total cholesterol.
Conclusions:
In a well-defined population of newly diagnosed people with T2DM, there is a high incidence of abnormal ALT levels, which is associated with features of the metabolic syndrome (obesity and lipid abnormalities), but not glycemic control.
Keywords: Alanine transaminase, non-alcoholic fatty liver disease, Type 2 diabetes
INTRODUCTION
Type 2 diabetes (T2DM) is associated with a clinical spectrum of liver abnormalities collectively known as non-alcoholic fatty liver disease (NAFLD). NAFLD is a clinico-histopathological diagnosis characterized by hepatocellular steatosis which is usually macrovesicular, in the absence of other risk factors for chronic liver disease particularly alcohol drugs or chronic viral hepatitis,[1] which can progress to steatohepatitis, fibrosis, and ultimately cirrhosis. Serum alanine aminotransferase (ALT), which is a widely available serum marker of liver damage, is elevated in about 20% of children and adolescents with T2DM, and in most cases this is attributable to NAFLD.[2] Westerbacka J et al.[3] had demonstrated that ALT was closely associated with liver fat unlike Aspartate transaminase (AST) and gamma glutamyl transferase (GGT) and hence, ALT is used as a surrogate marker for many epidemiological studies.[4]
The incidence of NAFLD in patients with newly diagnosed T2DM has not been studied. We examined the incidence of elevated ALT, as a surrogate marker for NAFLD, in a well-defined population of patients with newly diagnosed T2DM, and attempted to characterize those with higher ALT measurements.
MATERIALS AND METHODS
Approval for this study was granted by the Local Audit Committee. All newly diagnosed T2DM patients from the Poole area attend three education sessions at Poole Hospital Diabetes Centre. At the first session, body mass index (BMI) is measured and venous blood taken for glycated hemoglobin (HbA1C), ALT, and lipid profile. Results were entered onto a computer database (Proton, CCL Computing, UK). Data were obtained retrospectively. Patients were divided into two groups: those who had ALT within the normal range (ALT ≤35 iu/l), and those who had elevated ALT (ALT > 35 iu/l). Power calculations were carried out a priori, with 600 subjects required to allow adequate numbers in each group. Variables with normal distribution (Age, BMI, HbA1c, total cholesterol, and HDL cholesterol) were compared using student t-test, whereas triglycerides were compared using Mann-Whitney U-test, and sex was compared using Chi-squared test.
RESULTS
Data were retrieved from 650 consecutive patients who attended the education sessions between June 2004 and April 2005 of whom 44 had incomplete data sets. Data from 606 patients were therefore analyzed. ALT was >35 iu/l in 155 patients (25.6% (95% CI 22.1, 29.2). Differences between the ALT ≤ and the ALT > 35 groups are shown in Table 1. There were significant differences between the groups in age (older if ALT > 35) as shown in Figure 1a, sex (more likely to be male if ALT > 35), BMI as shown in Figure 1c, triglycerides (both higher if ALT >35) as shown in Figure 1b and HDL cholesterol (lower if ALT >35) as shown in Figure 1d. There were no significant differences between the groups in glycemic control or total cholesterol measurement.
Table 1.
Patient characteristics and metabolic parameters
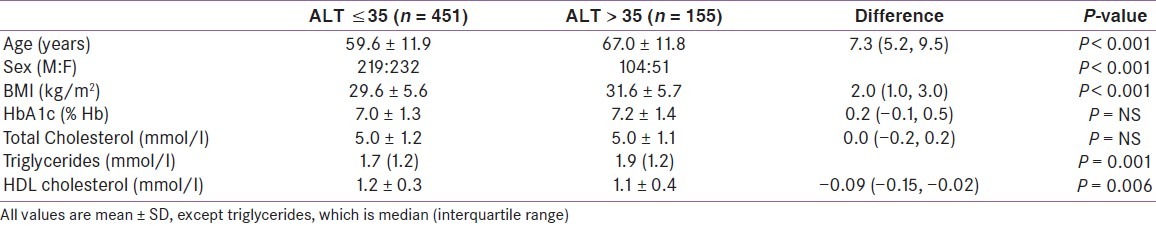
Figure 1.
Patient Characteristics in ALT = 35 & ALT > 35 groups All values are mean SD, except Triglycerides, which is median (interquartile range), (a) Age in ALT = 35 & ALT > 35 groups, (b) Triglycerides in ALT = 35 & ALT > 35 groups, (c) BMI in ALT = 35 & ALT > 35 groups, (d) Total & HDL-cholesterol in ALT = 35 & ALT > 35 groups, (*P value=0.006, **P value <0.001)
DISCUSSION
Our study shows a high incidence of elevated ALT in a well-defined population of newly diagnosed people with T2DM. Elevated ALT was used as a surrogate marker of NAFLD, which whilst both insensitive and non-specific, does suggest that this condition may be common within people with T2DM. Elevated ALT was found to have a statistically significant association with increasing age, obesity, elevated triglyceride levels, and low HDL cholesterol levels, but was not significantly associated with glycemic control. Manifestations of the metabolic syndrome, which often precedes T2DM, such as obesity, hyperinsulinemia, peripheral insulin resistance, hypertriglyceridemia, and hypertension, have been previously suggested as a cause of NAFLD.[5] This study is in keeping with the current understanding of the pathogenesis of NAFLD as a hepatic manifestation of the metabolic syndrome itself, as elevated ALT was commonly elevated in people with diagnosis of T2DM, suggesting that development of NAFLD may precede the diagnosis of T2DM.
Strong epidemiological, biochemical, and therapeutic evidence support the premise that the primary pathophysiogical derangement, in most patients with NAFLD, is insulin resistance.[6] Insulin resistance leads to increased lipolysis, triglyceride synthesis, increased hepatic uptake of free fatty acids, and accumulation of hepatic triglyceride.[5,7–12] Our data, demonstrating higher serum triglycerides and lower HDL cholesterol in the raised ALT group, support this hypothesis.
There are weaknesses of our study particularly the lack of comprehensive alcohol and drug history, although our cohort comes from a center where all newly diagnosed patients from a defined geographical area attended a well-recognized education program. We can therefore use our data as a true incidence of increased ALT in a population of newly diagnosed T2DM. Our study demonstrates statistically significant associations between the components of metabolic syndrome and raised ALT, which suggests that this can be used as a marker for NAFLD, albeit a non-specific one. Waist circumference to assess central obesity, which is a better parameter for metabolic syndrome was not measured. This can be considered as another limitation. However, we used BMI which is an accepted alternative to be a marker of metabolic syndrome.[13,14] Also, lack of follow up data in this retrospective study was an additional limitation.
Individuals with NAFLD are at a higher risk of developing fibrosis and chronic liver disease and an increased risk of all-cause death.[15] Furthermore, there is increasing evidence that NAFLD may also independently increase an individual's risk of cardiovascular disease. Our study suggests that it may be possible to identify people with T2DM who are at higher risk of developing NAFLD.[16] In these individuals attention could be focussed on modification of metabolic risk factors, such as weight loss, treatment of hypertension, and control of dyslipidemia rather than just tighter glycemic control,[17] thereby potentially preventing significant mortality and morbidity.
In summary, this study demonstrates a high incidence of elevated ALT in patients with newly diagnosed T2DM, suggesting that the onset of the liver abnormalities associated with dysglycemia may precede the diagnosis of T2DM itself. These abnormal ALT levels are associated with features of the metabolic syndrome, but not glycemic control.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Brunt EM. Non-alcoholic steatohepatitis: Definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 2.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–31. doi: 10.1016/S0140-6736(07)60821-6. [DOI] [PubMed] [Google Scholar]
- 3.Westerbacka J, Corner A, Tiikkainen M, Tamminen M, Vehkavaara S, Häkkinen AM, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: Implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–9. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 4.Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22:437–43. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- 5.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: Further evidence for an etiologic association. Hepatology. 2002;35:367–72. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 6.Polyzos SA, Kountouras J, Zavos C. Nonalcoholic Fatty liver disease: The pathogenetic roles of insulin resistance and adipocytokines. Curr Mol Med. 2009;9:299–314. doi: 10.2174/156652409787847191. [DOI] [PubMed] [Google Scholar]
- 7.Sheth SB, Gordon FD, Chopra S. Non alcoholic steatohepatitis. Ann Intern Med. 1997;126:137–45. doi: 10.7326/0003-4819-126-2-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 9.Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–9. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 10.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with non-alcoholic steatohepatitis: Insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–61. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- 11.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of non-alcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 12.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of non-alcoholic fatty liver disease. Ann Intern Med. 2005;143:722–8. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 13.Wei M, Gaskill SP, Haffner SM, Stern MP. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican Americans: A 7-year prospective study. Obes Res. 1997;5:16–23. doi: 10.1002/j.1550-8528.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 14.Stevens J, Couper D, Pankow J, Folsom AR, Duncan BB, Nieto FJ, et al. Sensitivity and specificity of anthropometrics for the prediction of diabetes in a biracial cohort. Obes Res. 2001;9:696–705. doi: 10.1038/oby.2001.94. [DOI] [PubMed] [Google Scholar]
- 15.Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13:4539–50. doi: 10.3748/wjg.v13.i34.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Targher G, Marra F, Marchesini G. Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: Causal effect or epiphenomenon? Diabetologia. 2008;51:1947–53. doi: 10.1007/s00125-008-1135-4. [DOI] [PubMed] [Google Scholar]
- 17.Day CP. Non-alcoholic fatty liver disease: Current concepts and management strategies. Clin Med. 2006;6:19–25. doi: 10.7861/clinmedicine.6-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]


