Abstract
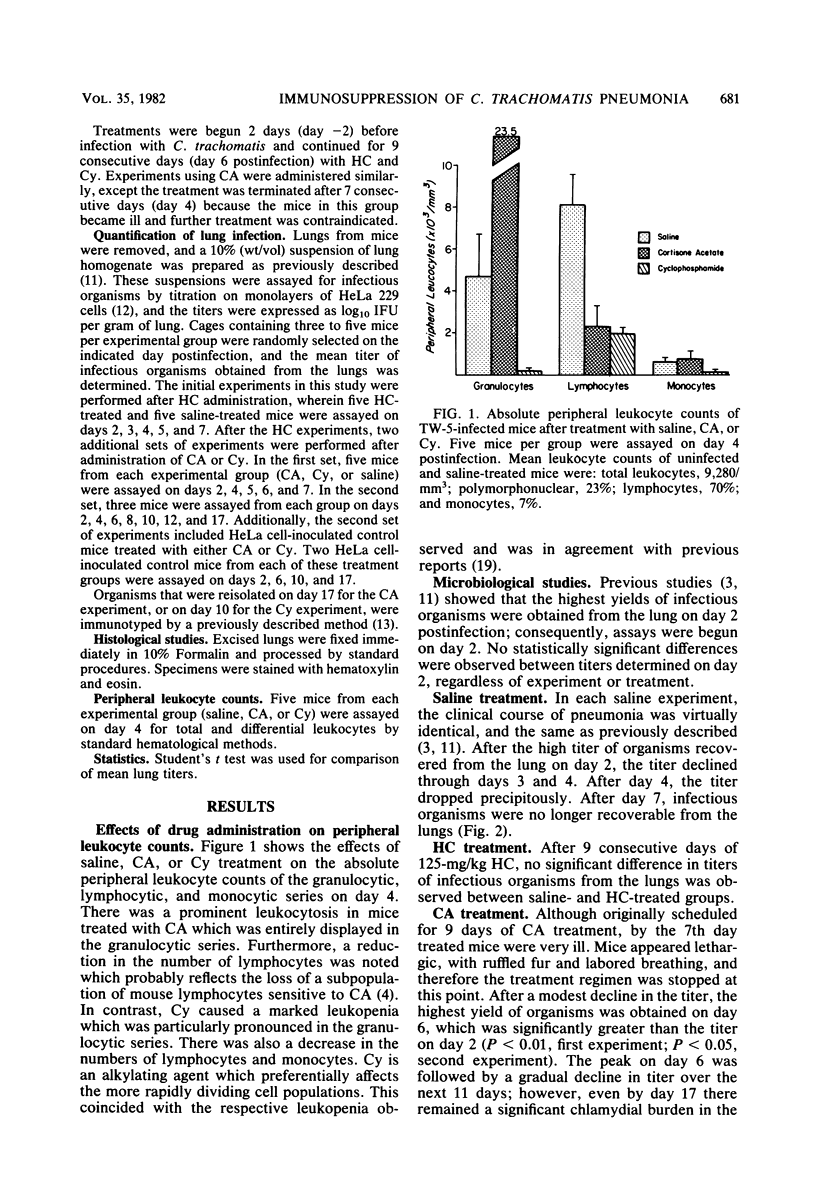
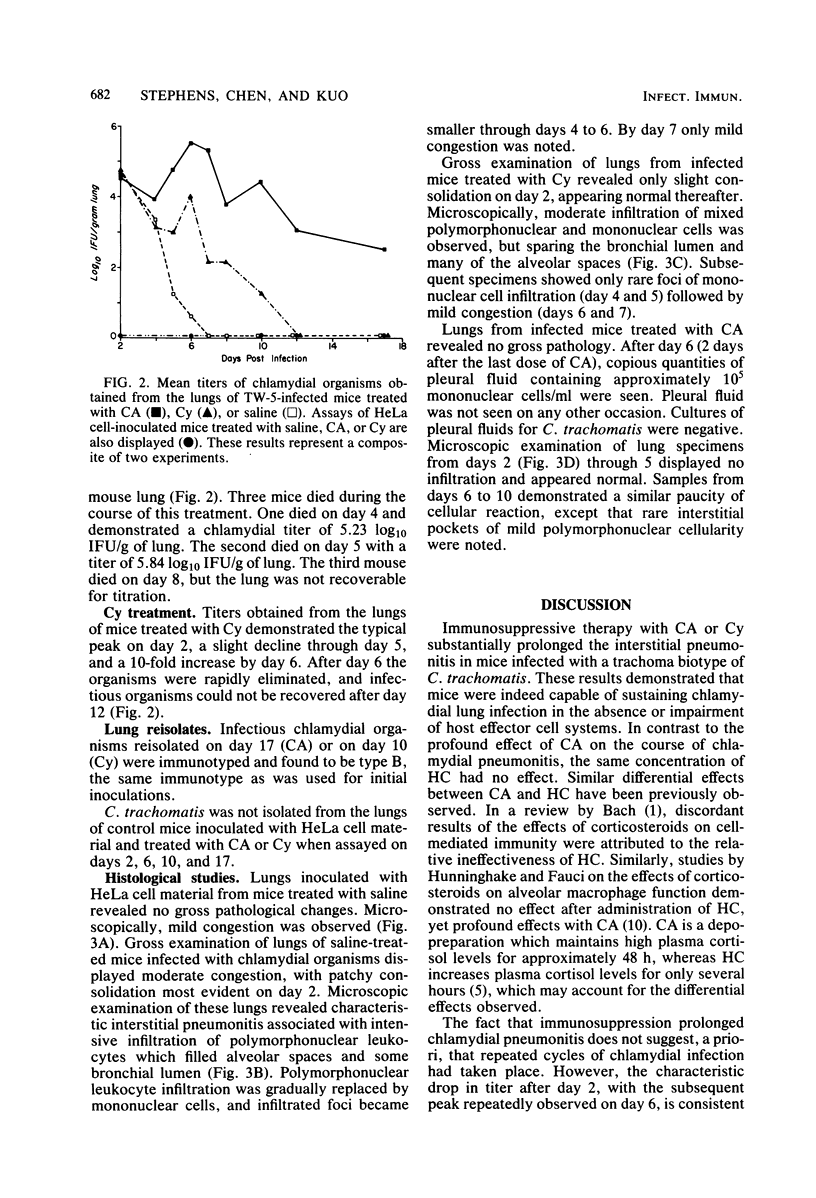
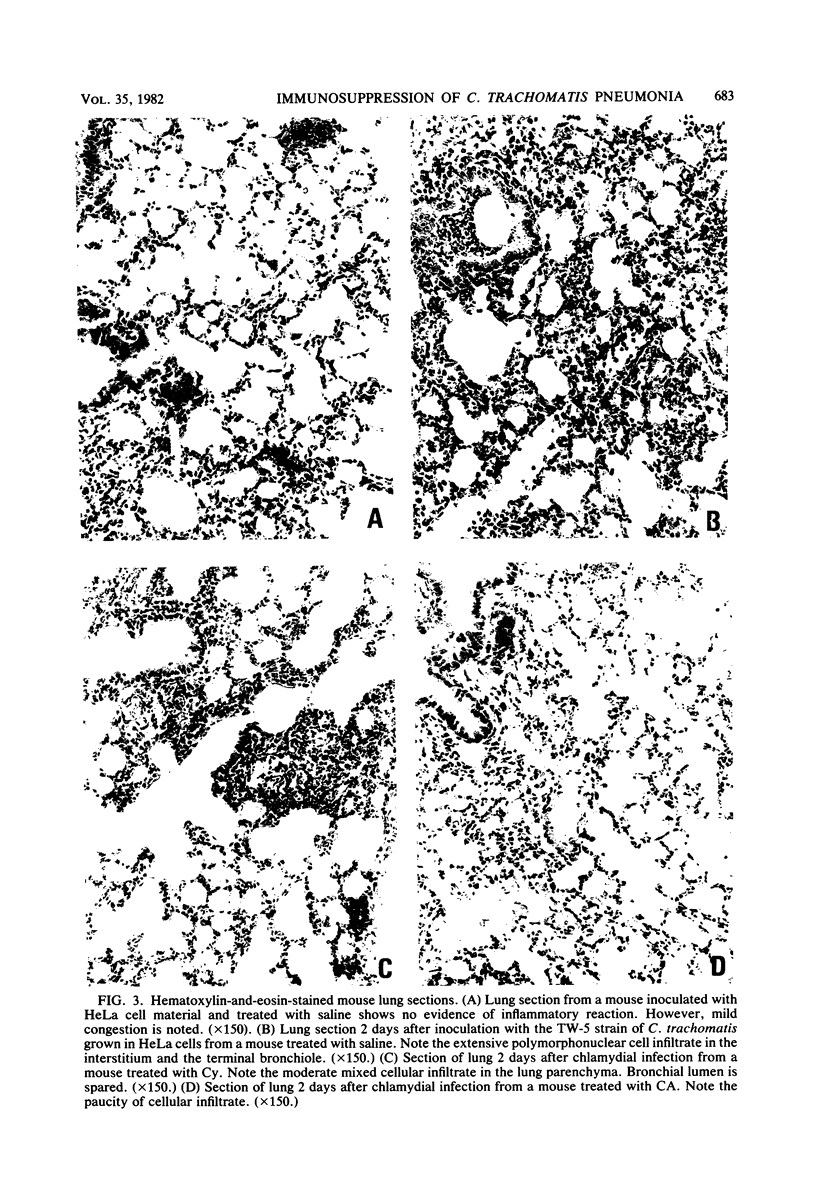
Suppression of the inflammatory reaction with daily doses of cortisone acetate or cyclophosphamide substantially prolonged the pulmonary infection in mice which had been intranasally inoculated with a trachoma biotype of Chlamydia trachomatis. Titration of organisms recovered from the lungs of treated mice revealed a drop in titer after day 2 (postinfection), followed by a prominent increase on day 6. In cyclophosphamide-treated mice the infection was resolved after day 12, whereas in cortisone acetate-treated mice a significant titer remained after day 17. In contrast, no organisms were recoverable after day 6 in control mice treated with saline or in mice treated with hydrocortisone succinate. Histologically, the ability of cortisone acetate and cyclophosphamide to inhibit the inflammatory reaction correlated with the respective course of chlamydial pneumonitis. This study demonstrated that mice were intrinsically capable of sustaining a lung infection induced by a human strain of S. trachomatis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beem M. O., Saxon E. M. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N Engl J Med. 1977 Feb 10;296(6):306–310. doi: 10.1056/NEJM197702102960604. [DOI] [PubMed] [Google Scholar]
- Chen W., Kuo C. A mouse model of pneumonitis induced by Chlamydia trachomatis: morphologic, microbiologic, and immunologic studies. Am J Pathol. 1980 Aug;100(2):365–382. [PMC free article] [PubMed] [Google Scholar]
- Dracott B. N., Smith C. E. Hydrocortisone and the antibody response in mice. I. Correlations between serum cortisol levels and cell numbers in thymus, spleen, marrow and lymph nodes. Immunology. 1979 Oct;38(2):429–435. [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. Immunosuppressive and anti-inflammatory effects of glucocorticoids. Monogr Endocrinol. 1979;12:449–465. doi: 10.1007/978-3-642-81265-1_24. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. I. Redistribution of circulating T and b lymphocytes to the bone marrow. Immunology. 1975 Apr;28(4):669–680. [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hanna L., Merigan T. C., Jawetz E. Effect of interferon on TRIC agents and induction of interferon by TRIC agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1115–1119. doi: 10.1016/0002-9394(67)94092-5. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Divergent effects of cyclophosphamide administration on mononuclear killer cells: quantitative depletion of cell numbers versus qualitative suppression of functional capabilities. J Immunol. 1976 Jul;117(1):337–342. [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunologic reactivity of the lung. III. Effects of corticosteroids on alveolar macrophage cytotoxic effector cell function. J Immunol. 1977 Jan;118(1):146–150. [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Chen W. J. A mouse model of Chlamydia trachomatis pneumonitis. J Infect Dis. 1980 Feb;141(2):198–202. doi: 10.1093/infdis/141.2.198. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. Delayed hypersensitivity in lung diseases. Ann N Y Acad Sci. 1974;221:312–316. doi: 10.1111/j.1749-6632.1974.tb28231.x. [DOI] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Schachter J., Lum L., Gooding C. A., Ostler B. Pneumonitis following inclusion blennorrhea. J Pediatr. 1975 Nov;87(5):779–780. doi: 10.1016/s0022-3476(75)80309-x. [DOI] [PubMed] [Google Scholar]
- Tack K. J., Peterson P. K., Rasp F. L., O'Leary M., Hanto D., Simmons R. L., Sabath L. D. Isolation of Chlamydia trachomatis from the lower respiratory tract of adults. Lancet. 1980 Jan 19;1(8160):116–120. doi: 10.1016/s0140-6736(80)90604-2. [DOI] [PubMed] [Google Scholar]
- Truitt G. L., Mackaness G. B. Cell-mediated resistance to aerogenic infection of the lung. Am Rev Respir Dis. 1971 Dec;104(6):829–843. doi: 10.1164/arrd.1971.104.6.829. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Parker D. The effect of cyclophosphamide on the immune response. J Immunopharmacol. 1979;1(2):127–137. doi: 10.3109/08923977909026368. [DOI] [PubMed] [Google Scholar]
- Weston W. L., Claman H. N., Krueger G. G. Site of action of cortisol in cellular immunity. J Immunol. 1973 Mar;110(3):880–883. [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Drutz D. J., Sumaya C. V. Pneumonia due to Chlamydia trachomatis in the immunocompromised (nude) mouse. J Infect Dis. 1981 Feb;143(2):238–241. doi: 10.1093/infdis/143.2.238. [DOI] [PubMed] [Google Scholar]




