Abstract
Context:
Effect of parenteral testosterone esters administration on bone-mineral density (BMD) and bone turnover in young age onset male hypogonadism is not studied in Indian subjects.
Aims:
To prospectively study the effect of short-term (6 months) replacement therapy with parenteral testosterone enanthate-propionate combination on BMD and bone turnover markers in hypogonadal adult patients.
Settings and Design:
Prospective, tertiary care academic center.
Materials and Methods:
Thirteen young, otherwise healthy hypogonadal males (age 25.5 ± 4.9 yrs, serum testosterone 2.56 ± 4.29 nmol/l) were subjected to BMD measurements (DXA) and estimation of urinary Crosslaps™ and serum osteocalcin at baseline. Twelve healthy age and BMI-matched males served as controls for BMD measurements. The hypogonadal patients were administered parenteral testosterone esters (as mixed enanthate and propionate) 250 mg i.m. every 2-3 weeks, and prospectively followed for 6 months. BMD and bone markers were studied at the end of 6 months.
Statistical Analysis Used:
Mann-Whitney nonparametric test, paired t-test and Pearson's test of two-tail significance.
Results:
At baseline, BMD was significantly lower in hypogonadal males as compared to that in controls. With testosterone replacement, there was significant improvement in BMD, both at trabecular and cortical sites, There was a decline in bone turnover with treatment (Ur Crosslaps™:creatinine ratio: pretreatment 72.8 ± 40.4, post-treatment 35.5 ± 23.8 μg/mmol, P = 0.098; serum osteocalcin: pre-treatment 41.0 ± 16.8, post-treatment 31.7 ± 2.1 ng/ml, P = 0.393).
Conclusions:
Short-term parenteral testosterone replacement significantly improves BMD at the hip, lumbar spine and forearm in hypogonadal young males.
Keywords: C-terminal telopeptide, collagen-Crosslaps™, osteocalcin
INTRODUCTION
Male hypogonadism in young adults is associated with low bone-mineral density (BMD) and fractures.[1–6] Testosterone (T) replacement in hypogonadal males improves the BMD significantly.[1–5,7–12] The bone turnover reduces on testosterone replacement with decrease in bone resorption markers and increase in osteoblastic activity.[1,5,10,11]
In India, combination of parenteral testosterone enanthate and propionate is a commonly used for testosterone replacement. We prospectively studied BMD and bone turnover in young untreated hypogonadal males, and monitored the effects of short-term parenteral testosterone replacement on bone density and bone turnover parameters.
MATERIALS AND METHODS
Twenty-four consecutive young adult men presenting with complaints of poor development of secondary sexual characters, impotence and/or infertility, and who were found to have serum T levels less than 10 nmol/L (adult reference range: 10-34 nmol/L), were screened for enrollment into our study. The exclusion criteria were chronic hepatic and renal disease, untreated hypothyroidism or hyperthyroidism, ingestion of glucocorticoids, residence in endemic fluorosis area, and malabsorption. Four subjects were found to have other diseases that might have affected bone turnover and hence were excluded. Seven subjects refused for inclusion in the study. Thirteen patients completed the initial work-up and were followed prospectively while on T-replacement therapy. A detailed clinical history was recorded, with particular emphasis on anosmia and factors that are known to cause testicular or pituitary failure. Informed consent was obtained from all the patients and Institutional Human Experiments Ethics Committee approved the study protocol.
A complete physical and anthropometric examination was undertaken at baseline, and serum T and gonadotropin levels were estimated. Pituitary imaging (contrast-enhanced computerized tomography or magnetic resonance imaging) was performed for all the cases with hypogonadotropic hypogonadism (n = 4).
Intramuscular testosterone was administered to all 13 subjects at 2-3 weeks intervals (Testoviron Depot™, German Remedies, Mumbai, India, containing testosterone propionate 250 mg and testosterone enanthate 250 mg). The testosterone supplementation was started initially in dose of 100 mg every 2 weeks for 1 month and subsequently increased to 250 mg every 2-3 weeks. Adequacy of dosage was assessed by estimating serum T-level at 10-14 days of the last injection.[10] A serum T level of >10 nmol/L was considered as adequate replacement therapy. Twelve age and body mass index (BMI)-matched healthy males from hospital staff served as control subjects.
Blood samples were collected after overnight fast at baseline and at the end of 6 months of T-replacement, and serum was separated and stored at −20°C till the time of assay. Serum alkaline phosphatase, albumin corrected total calcium, inorganic phosphorus and alkaline phosphatase were estimated on the same day by a TechniconR analyzer. A 24-hours urine collection was also done at the beginning and at the end of the study for estimation of urinary C-terminal telopeptide of type I collagen (CTx, Crosslaps™) levels and creatinine estimations. Serum osteocalcin (bone GLA protein) were measured at baseline and at the end of experiment.
Serum T was estimated by RIA (Diagnostic Products Corporation, LA, USA; sensitivity 0.14 nmol/L, interassay and intra-assay CV: 5.9-11%, 4.0-9.5%, respectively); osteocalcin by IRMA (Active Osteocalcin™; Diagnostic Systems Laboratories Inc., TX, USA; sensitivity 0.3 ng/ ml, interassay CV 3.3-5.3%, intra-assay CV 1.4-3.4%); and CTx by ELISA (Active Crosslaps™; Diagnostic Systems Laboratories Inc; sensitivity 50 μg/L, interassay CV 4.7-9.4%, intra-assay CV 2.9-5.7%). The values of CTx (Crosslaps™) were corrected for creatinine concentrations and the results expressed in μg Crosslaps™/mmol creatinine.
Areal BMD (aBMD) was measured at baseline in all cases and at the end of 6 months of adequate T-replacement in seven of them. The aBMD was measured at the nondominant forearm (distal 1/3, mid-1/3, ultradistal, total), hip (neck of femur, trochanter, intertrochanteric, total hip, ward's) and lumbar spine (AP, lateral) on a Hologic QDR 4500A dual-energy X-ray absorptiometer (Hologic, Waltham, MA, USA). The same operator, who was blind in respect to patient's treatment protocol, analyzed all scans. BMD was expressed as grams per square centimeter (g/cm2). The precision of BMD measurement in our laboratory was as follows: lumbar spine 1.0%; femoral neck 1.5%; Ward's triangle 2.8%; trochanter 1.6%.
Statistical analysis
Mann-Whitney nonparametric test was employed to compare between the BMD values at baseline of the cases and the controls. Paired t-test was performed for all biochemical and bone densitometry parameters of the patients before and following treatment. Correlation between rate of change in bone densitometry parameters and biochemical parameters was assessed by Pearson's test of two-tail significance.
RESULTS
Clinical
Thirteen young adult hypogonadal men presenting to us with complaints of absence of secondary sexual characters (n = 7), diminished libido or impotence (n = 6) were included in the study. The mean age and BMI of patients was 25.5 ± 4.9 years (M ± 1SD; range 20-35 years), and 22.2 ± 4.7 kg/m2 (range 14.9-34.4 kg/m2), respectively. Their pretreatment serum T-levels were 2.56 ± 4.29 nmol/L. Nine patients had eunuchoid body proportions,[13] and 6 had gynecomastia. Nine cases had hypergonadotropic hypogonadism (Klinefelter syndrome (47XXY)-1, mumps orchitis 1, idiopathic 7). Four subjects had hypogonadotropic hypogonadism. None had a history of anosmia.
Comparison of aBMD between Cases and Controls
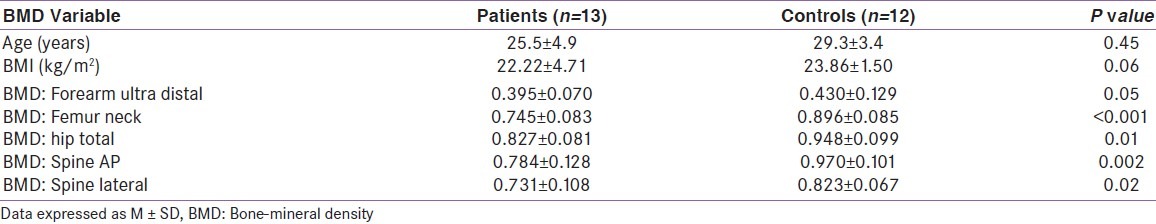
At baseline, hypogonadal males had significantly (P < 0.05) lower aBMD at the lumbar spine, hip and forearm [Table 1] as compared to controls subjects.
Table 1.
Baseline bone-mineral density (g/cm2) in hypogonadal and healthy males
Clinical response to testosterone replacement therapy
T-replacement was well tolerated by all the patients. One patient experienced transient increase in gynecomastia and mastalgia after starting T. None of patients had deranged liver function tests at beginning and at the end of treatment. All cases achieved circulating T-levels of >10 nmol/L (mean 16.22 ± 3.57 nmol/L, range 11.09-20.8 nmol/L) at the dose of 250 mg every 3 weeks. All the cases were followed up for minimum of six (range 6-11 months), and their aBMD and bone turnover markers were assessed.
Bone-mineral density response to testosterone replacement therapy
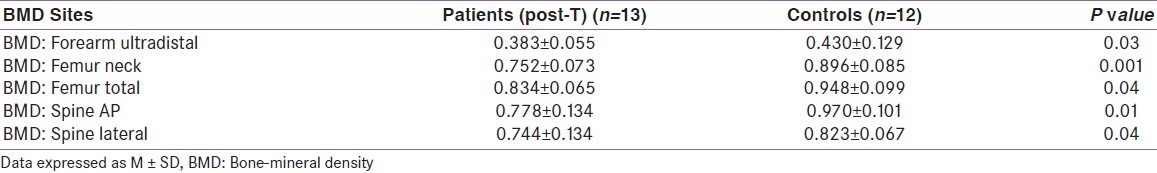
There was an increase in aBMD at all 11 sites following T-replacement. The gain was statistically significant at six sites (distal 1/3 forearm, total forearm, neck femur, trochanter, total hip, AP spine) [Table 2]. However, the aBMD values at the end of 6 months were significantly lower than that of the control subjects at all sites except one (femur-intertrochanteric region) [Table 3]. The degree of gain in aBMD did not correlate statistically to baseline BMD.
Table 2.
Biochemical parameters and bone-mineral density (g/m2) at baseline and 6 months of testosterone replacement in study patients
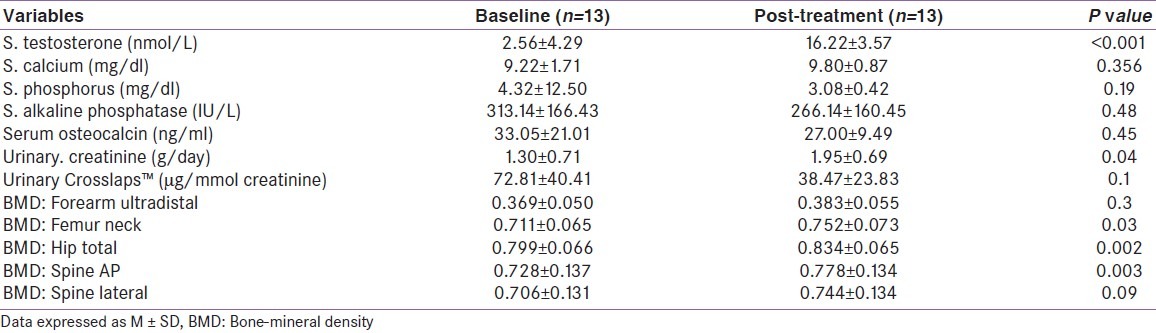
Table 3.
Comparison of bone-mineral density (g/cm2) between hypogonadal and controls healthy subjects following testosterone replacement
Biochemical response to testosterone replacement therapy
There was an expected significant increase in serum T-levels replacement as compared to baseline (P < 0.001). There was no significant difference in serum calcium and inorganic phosphorus levels before and during T replacement (P > 0.05). There was a wide scatter of the serum osteocalcin levels, both at pretreatment and post-treatment period (pretreatment: 33.05 ± 21.01; post-treatment: 27.00 ± 9.49 ng/ml), and the difference between the means was not significant (P = 0.448). Serum levels of alkaline phosphatase decreased following T therapy, but they failed to normalize (pretreatment - 313.14 ± 166.43 IU/L, post-treatment: 266.14 ± 160.45 IU/L; P = 0.482). Urinary CTx levels were suppressed following T replacement, though this, too, did not achieve significance (P = 0.098). Gain in a BMD at distal 1/3rd and mid-forearm correlated positively with change in serum levels of alkaline phosphatase. aBMD change did not have any correlation with the change in serum osteocalcin, urinary CTx levels or circulating T levels.
DISCUSSION
Our study showed significantly lower aBMD at all the sites in young hypogonadal males, as compared to age- and BMI-matched controls. Adequate T-replacement therapy for 6 months increased the aBMD significantly at all trabecular sites. During treatment, mean serum osteocalcin, serum alkaline phosphatase and urinary CTX:creatinine ratio declined in our study subjects, though these were not statistically significant.
Various authors have reported significantly lower BMD at lumbar spine,[1,3–5,8,10–13] femoral neck,[3,5,12] total hip,[8,13] and distal radius[1,2,8,9] in hypogonadal subjects as compared to eugonadal males. However, BMD at cortical sites (proximal 1/3 radius) has been reported lower.[2] We also observed significantly lower aBMD in our hypogonadal men at the hip, lumbar spine and distal 1/3 forearm.
The effects of testosterone replacement on BMD and bone turnover have been studied. Various testosterone replacement therapy increases the BMD at all trabecular sites.[1,5,10,11] However, transdermal testosterone patch (5 mg/day) for 12 months did not result in beneficial effect on BMD despite attaining normal serum testosterone levels.[7] We found significant improvement in aBMD at all skeletal sites studied following 6 months of parenteral T-therapy with a mixture of testosterone-propionate and -enanthate. Despite significant rise in bone density in our study population, aBMD at all but one of the sites still remained significantly lower than that of the controls at the end of the study period [Table 3].
Long-term T replacement has resulted in BMD comparable to age-dependent normal range in one study[4] while in another study, BMD remained subnormal in another study.[11] Serial study of volumetric BMD by QCT of lumbar spine in 72 hypogonadal patients for 16 years showed greatest increase in BMD during the first year of treatment in previously untreated patients.[8] Long-term testosterone treatment maintained BMD in the age-dependent reference range in all 72 hypogonadal men, independent of the type of hypogonadism. Transdermal testosterone patches applied to the scrotum were as effective in normalizing BMD as intramuscular testosterone enanthate injections.[4] In another long-term (mean 70-76 months) study,[11] spinal BMD in idiopathic hypogonadotropic hypogonadism remained significantly lower than in controls, while there was no difference in femoral neck BMD. There was no difference in spinal or femoral neck BMD between Klinefelter's syndrome and control population. Long-term studies are required in Indian hypogonadal subjects to assess the BMD at various skeletal sites.
Most studies agree that T suppresses bone-resorption parameters while bone formation markers have been variably shown to be increased,[10,14,15] decreased[1,5] or no change[7] with therapy. In our patients, serum levels of total alkaline phosphatase and osteocalcin, and urinary CTx levels declined with T therapy, though these changes were not statistically significant. The lack of significance in these trends may be due to the small study population and wide scatter in values.
We did not find any correlation between the rise in serum T levels and the gain in aBMD in our subjects. However, some studies have found positive correlation of serum testosterone with spine BMD.[3] The lowering of serum osteocalcin and urinary CTx levels also did not correlate with the rise in aBMD. Surprisingly, the change in BMD at distal 1/3rd and middle 1/3rd of the forearm correlated positively with the change in serum alkaline phosphatase levels. This finding is difficult to explain, as the alkaline phosphatase in our cohort actually declined during T therapy along with a concomitant rise in aBMD. This needs to be studied in a larger group of subjects.
We have treated our patients with a combination of testosterone-enanthate and T–propionate as this is a commonly used parenteral depot T available in our country. Transdermal or oral testosterone preparations are sparingly used in our country due to cost factor and nonavailability. We did not find any significant changes in bone turnover in our patients, which may be due to the small size of our study cohort. This needs further study in a larger group of hypogonadal subjects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 2.Devogelaer JP, De Cooman S, Nagant de Deuxchaisnes C. Low bone mass in hypogonadal males. Effect of testosterone substitution therapy, a densitometric study. Maturitas. 1992;15:17–23. doi: 10.1016/0378-5122(92)90057-b. [DOI] [PubMed] [Google Scholar]
- 3.Choi HR, Lim SK, Lee MS. Site-specific effect of testosterone on bone mineral density in male hypogonadism. J Korean Med Sci. 1995;10:431–5. doi: 10.3346/jkms.1995.10.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2386–90. doi: 10.1210/jcem.82.8.4163. [DOI] [PubMed] [Google Scholar]
- 5.Guo CY, Jones TH, Eastell R. Treatment of isolated hypogonadotropic hypogonadism effect on bone mineral density and bone turnover. J Clin Endocrinol Metab. 1997;82:658–65. doi: 10.1210/jcem.82.2.3758. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JA, Riggs MW, Spiekerman AM. Testosterone deficiency as a risk factor for hip fractures in men: A case-control study. Am J Med Sci. 1992;304:4–8. doi: 10.1097/00000441-199207000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Merza Z, Blumsohn A, Mah PM, Meads DM, McKenna SP, Wylie K, et al. Double-blind placebo-controlled study of testosterone patch therapy on bone turnover in men with borderline hypogonadism. Int J Androl. 2006;29:381–91. doi: 10.1111/j.1365-2605.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 8.Vieira da Costa J, Pereira-Lima JF, da Costa Oliveira M. Bone mineral density in early-onset hypogonadism and the effect of hormonal replacement. J Clin Densitom. 2004;7:334–40. doi: 10.1385/jcd:7:3:334. [DOI] [PubMed] [Google Scholar]
- 9.Schubert M, Bullmann C, Minnemann T, Reiners C, Krone W, Jockenhovel F. Osteoporosis in male hypogonadism: responses to androgen substitution differ among men with primary and secondary hypogonadism. Horm Res. 2003;60:21–8. doi: 10.1159/000070823. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Swerdloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, et al. Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin Endocrinol (Oxf) 2001;54:739–50. doi: 10.1046/j.1365-2265.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 11.De Rosa M, Paesano L, Nuzzo V, Zarrilli S, Del Puente A, Oriente P, et al. Bone mineral density and bone markers in hypogonadotropic and hypergonadotropic hypogonadal men after prolonged testosterone treatment. J Endocrinol Invest. 2001;24:246–52. doi: 10.1007/BF03343854. [DOI] [PubMed] [Google Scholar]
- 12.Canale D, Vignali E, Golia F, Martino E, Pinchera A, Marcocci C. Effects of hormonal replacement treatment on bone mineral density and metabolism in hypogonadal patients. Mol Cell Endocrinol. 2000;161:47–51. doi: 10.1016/s0303-7207(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 13.Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res. 2005;20:1785–91. doi: 10.1359/JBMR.050606. [DOI] [PubMed] [Google Scholar]
- 14.Fintini D, Grossi A, Brufani C, Fiori R, Ubertini G, Pecorelli L, et al. Bone mineral density and body composition in male children with hypogonadism. J Endocrinol Invest. 2009;32:585–9. doi: 10.1007/BF03346513. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Eyre DR, Clark R, Kleinberg D, Newman C, Iranmanesh A, et al. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men--A clinical research center study. J Clin Endocrinol Metab. 1996;81:3654–62. doi: 10.1210/jcem.81.10.8855818. [DOI] [PubMed] [Google Scholar]


