Abstract
Objective:
We evaluated the thyroid function tests in individuals with metabolic syndrome to explore the possibility of thyroid receptor resistance.
Materials and Methods:
The study was a cross-sectional study. It included 40 patients (group I) and 20 healthy individuals served as controls (group II). Patients in group I fulfilled the three or more of the NCEP ATP III (National Cholesterol Education Programme – Adult Treatment Panel III) criterion to define the metabolic syndrome. Blood sugar and serum insulin levels were measured in both the groups. All the patients (group I) had insulin resistance as per the HOMA IR (the homeostasis model for insulin resistance) model. The HOMA IR value obtained in group II individuals served as a reference mark to define insulin resistance. T3, T4, TSH levels were measured as indicators of thyroid functions. There was an increase in TSH levels with normal T3 and T4 in group I indicating that increased TSH probably due to thyroid receptor resistance may be a part of metabolic syndrome rather than a state of hypothyroidism.
Results:
T3 and T4 levels were comparable in patients and controls. There was a significant increase in TSH levels in patients as compared to the controls.
Conclusion:
Raised TSH in patients with metabolic syndrome independent of lowered T3 and T4 suggest it to be a part and parcel of this syndrome.
Keywords: Homeostasis model assessment for insulin resistance, insulin resistance, metabolic syndrome, thyroid function tests, thyroid receptor resistance
INTRODUCTION
Metabolic syndrome (syndrome X, insulin resistance syndrome) consists of constellation of metabolic abnormalities which include central obesity, hyperglycemia plus insulin resistance, high triglycerides plus low HDL cholesterol, and hypertension (the deadly quartet).[1] Third National health and Nutrition Examination Surveys (NHANES III) revealed the age adjusted prevalence of metabolic syndrome to be 34% in men and 35% in women in the Unites States.[2] In a multiethnic study in Singapore, 28.8% of Indians, 24.2% of Malaysians, and 14.8% of Chinese had metabolic syndrome.[3] With rapid industrialization and urbanization, the prevalence of metabolic syndrome has increased dramatically. By NCEP (National Cholesterol Education Programme) criterion, 41.1% of Asian Indians were suffering from metabolic syndrome[4] whereas the prevalence of metabolic syndrome was 11.2% in Chennai urban population.[5]
Obesity, a key component of metabolic syndrome, occurs due to increased energy intake, decreased energy expenditure, or a combination of both, thus leading to positive energy balance. Thyroid hormones up-regulate metabolic pathways relevant to resting energy expenditure, hence, obesity and thyroid functions are often correlated. On one hand, obesity per se causes alterations in thyroid hormones, i.e. increased thyroid hormone levels,[6] increased TSH with no effect on T3 and T4,[7–9] or increase in TSH and T3 with no effect on T4[10–12]; on the other hand, subclinical hypothyroidism as a result of slow metabolism can lead to the obesity.[13] It is still not clear whether these alterations in thyroid hormones are a cause or an effect of obesity (metabolic syndrome).
One fact is clear that clinicians often interpret increased TSH levels with normal thyroid hormone levels in obese persons as an evidence of subclinical hypothyroidism and prescribe thyroxine replacement therapy to reinforce the euthyroid status which already exists. It has also been noted that the unnecessary use of thyroxine replacement can lead to its toxicity. The mechanism of normal levels of T3, T4 with increased TSH in metabolic syndrome is not defined, but it has been hypothesized that metabolic syndrome is associated with insulin resistance due to the defect in postreceptor signal transduction in target tissue, a similar mechanism of thyroid receptor resistance might be operating in these obese persons.[7] Thus, in order to explore the possibility of thyroid receptor resistance to TSH, this study was designed to find alterations in metabolic syndrome (obese insulin resistant individuals, defined by the HOMA model).[14]
MATERIALS AND METHODS
Study design
The study was carried out in 40 patients with metabolic syndrome fulfilling three or more NCEP ATP III criteria (namely waist circumference >102 cm in men and >88 cm in women, blood pressure ≥130/≥85 mmHg, triglycerides ≥150 mg/dl, HDL cholesterol <40 mg/dl in males and <50 mg/dl in females, and fasting glucose ≥110 mg/ dl). Twenty nonobese persons were taken as the control (group II). We excluded the subjects with a history of thyroid diseases or radioiodine treatment, antithyroid treatment, thyroid autoantibody positive subjects, and pregnant women. All patients gave a written consent, and the institutional review board at the University of Health Sciences approved the study protocol.
Anthropometric parameters, thyroid function tests, blood glucose, and serum insulin levels
Body weight (kg) and height (m) were measured using standardized techniques and equipment. The BMI was calculated as weight divided by squared height (kg/m2). The waist circumference was measured with a paper tape horizontally at the level of umbilicus in the standing position. The hip circumference was measured at the level of greater trochanter. A waist-to-hip ratio was calculated as waist circumference divided by hip circumference. Blood pressure was measured from the left arm in the sitting position with apparatus at the level of heart. Venous blood samples were taken after 12 h of fasting, separated and frozen at -8°C until analysis. The serum levels of TSH (reference range, 0.17–4.05 μIU/ml), T3 (reference range, 70–200 ng/dl), and T4 (reference range, 5.5–13.5 μg/dl) were measured as indicators of thyroid function using I-125 gamma counter (IC 4702A, Electronics Corporation of India, India) with radioimmunoassay kits (IRMAK 9, RIAK 4/4A, and RIAK 5/5A, respectively; Board of Radiation and Isotope Technology, Navi Mumbai, India). The serum insulin levels (reference range, 1–30 mU/l) were also measured using a radioimmunoassay kit (RIAK 1, Board of Radiation and Isotope Technology, Navi Mumbai, India). Blood sugar levels (reference range, 60–100 mg/dl) were estimated by the glucose oxidase-peroxidase (GOD-POD) method using the glucose analyzer (Techno 168, Logotech India Pvt. Ltd., India). Fasting serum concentrations of triglycerides and HDL were measured using commercially available kits.
Homeostasis model assessment for insulin resistance
To define the insulin resistance, homeostasis model assessment for insulin resistance (HOMA IR) was calculated by the following formula[14]:

Statistical analysis
The data were analyzed using SPSS software (version 12.0 for windows, SPSS, Inc., Chicago, IL). Statistical significance was calculated by the Student ‘t’-test. An unpaired ‘t’-test was used for intergroup comparison. The P value was calculated for statistical significance. The P value <0.05 was taken as significant. Data were expressed as mean±SD.
RESULTS
General characteristics of the subjects in both groups
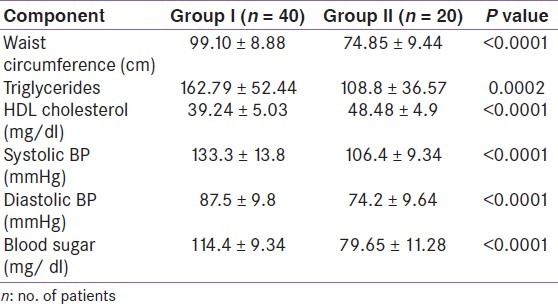
The mean age of the patients (group I) and the controls (group II) was 38.24±9.17 years and 34.16±11.61 years, respectively. A female-to-male ratio (sex ratio) was 3:2 in both the groups. The mean waist circumference was 99.10±8.88 cm in patients and 74.85±9.44 cm in controls (P <0.0001). The mean systolic and diastolic blood pressure were 133.3±13.8 mmHg and 87.5±9.8 mmHg, respectively, in group I patients whereas the mean systolic and diastolic blood pressure were 111.6±11.6 mmHg and 74.2±9.64 mmHg, respectively, in group II individuals and the difference was statistically significant. The mean blood sugar levels were 114.4±9.34 mg/dl in group I individuals as compared to 79.65±11.28 mg/dl in controls (P <0.0001). Similarly, there was a significant difference in triglyceride levels (162.79±52.44 vs. 108.8±36.57 mg/dl; P = 0.0002) and HDL cholesterol levels (39.24±5.03 vs. 48.48±4.9 mg/ dl; P <0.0001) in both groups [Table 1].
Table 1.
Individuals with metabolic syndrome (group I) and controls (group II)
Homeostasis model assessment for insulin resistance
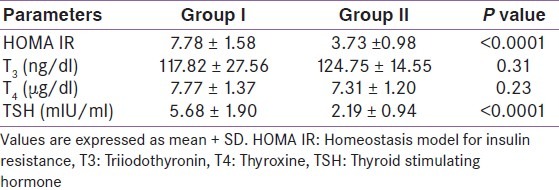
HOMA IR (homeostasis model assessment for insulin resistance) was calculated in both groups. The standardized value of HOMA IR (mean+2SD = 5.7) obtained in group II was taken as a reference value to define the insulin resistance. The mean HOMA IR was 7.78±1.58 in group I and 3.73±0.98 in group II individuals, and the difference was significant (P <0.0001).
Comparison of thyroid functions in obese (group I) and control (group II) subjects
Thyroid function tests were done in both groups. The mean T3 levels were comparable in both the groups (117.82±27.56 vs. 124.75+14.55 ng/dl; P = 0.31). Similarly the difference in mean T4 levels was also nonsignificant in both the groups (7.77±1.37 vs. 7.31±1.20 μg/dl; P = 0.23). Mean TSH levels in group I individuals were higher as compared to group II (5.68±1.90 μIU/ml vs. 2.19±0.94 μIU/ml) and the rise was statistically significant (P <0.0001) as shown in Table 2.
Table 2.
HOMA IR and thyroid function tests in both groups
DISCUSSION
Metabolic syndrome can be associated with endocrinal and nonendocrinal disorders and has widespread consequences. Alterations in thyroid functions, though well known yet, are not recognized clinically and there is inconsistency in the thyroid functions in metabolic syndrome. The rise in TSH with normal T3 and T4 is mostly reported,[7–9] but the rise in TSH with alteration in T3 without any effect on T4[10–12] or increased thyroid hormone levels[6] also have been described in obesity. These disturbances in the thyroid function do not have any physiological basis, because it cannot be explained by the feedback mechanism. By this study, we tried to explore the possibility of thyroid receptor résistance akin to insulin resistance in metabolic syndrome and made interesting observations.
Forty obese individuals fulfilling the NCEP ATP III criteria were taken as cases and 20 healthy individuals served as controls. Patients with prior history of thyroid disease, history of antithyroid treatment, with positive antithyroid antibody and pregnancy were excluded. All the subjects were subjected to the thyroid hormone assay. The persons in both groups were made comparable with regards to age and sex but had significant difference with regards to components of metabolic syndrome as displayed in Table 1.
Keeping in mind that metabolic syndrome is associated with insulin resistance with hyperinsulinemia as a metabolic consequence[15]; it is possible that these patients might have thyroid receptor resistance resulting in persistent elevated TSH levels. The stimulus for increased TSH could be accounted by the elaboration of certain hormones namely leptin, adiponectin from the fatty tissue which cross the blood–brain barrier and stimulate the hypothalamic pituitary axis.[16,17] To explore the thyroid receptor resistance, we took the help of HOMA IR to define insulin resistance. The cut off limit of HOMA IR was derived from the individuals of group II as 5.7 (mean+2SD). All patients in group I were insulin resistant as per the HOMA IR model.
The mean levels of T3 (117.82±27.56ng/dl) and T4 (7.77±1.37μg/dl) in group I and mean T3 (124.75±14.55 ng/dl) and T4 levels (7.31±1.20 μg/dl) in group II were comparable; however, the mean levels of TSH in group I (5.68 ± 1.90 μIU/ml) were significantly higher than in group II (2.19 ± 0.94 μIU/ml). The raised TSH levels with normal T3 and T4 could not be explained on the basis of the feedback system. These findings also do not explain the state of subclinical hypothyroidism as all these patients were antithyroid peroxidase antibody negative.
Our finding suggest that insulin-resistant obese persons had higher TSH levels as compared to normal individuals but T3 and T4 levels were comparable in both groups. Raised TSH thus could be a metabolic consequence of metabolic syndrome rather than a state of subclinical hypothyroidism.
From our findings, it can be hypothesized that raised TSH levels in obese persons with insulin resistance (metabolic syndrome) could be due to association of thyroid receptor resistance to TSH similar to insulin resistance. The normal levels of T3 and T4 support this hypothesis. The stimulus for rise in TSH could also be due to hormones secreted by adipose tissue. Since we did not estimate these hormones (leptin, resistin, adiponectin, etc.) in our study, thus are not able to comment on the effect of these hormones on thyroid function tests.
To conclude, it is suggested that these findings are preliminary and require evaluation on a large scale with inclusion of various hormones elaborated by adipose tissue.
CONCLUSION
To conclude, it is suggested that these findings are preliminary and require evaluation on a large scale with inclusion of various hormones elaborated by adipose tissue.
Footnotes
Source of Support: Nil
Conflict of Interest: No
REFERENCES
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Tan CE, Ma S, Wai D, Chew SK, Tai ES. Can we apply the National Cholesterol Education Program Adult Treatment Panel definition of the metabolic syndrome to Asians? Diabetes Care. 2004;27:1182–6. doi: 10.2337/diacare.27.5.1182. [DOI] [PubMed] [Google Scholar]
- 4.Ramchandran A, Snehlata C, Satyavani K, Sivasankari S, Vijay V. Metabolic syndrome in urban Asian Indian adults: A population study using modified ATP III criteria. Diabetes Res Clin Pract. 2003;60:199–204. doi: 10.1016/s0168-8227(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 5.Mohan V, Shanthirani S, Deepa R, Premalatha G, Sastry NG, Saroja R. Intraurban differences in the prevalence of the metabolic syndrome in Southern India-The Chennai Urban Population Study (CUPS No.4) Diabet Med. 2001;18:280–7. doi: 10.1046/j.1464-5491.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- 6.Michalaki MA, Vagenakis AG, Leonardou AS, Argentou MN, Habeos IG, Makri MG, et al. Thyroid Function in Humans with Morbid Obesity. Thyroid. 2006;16:73–8. doi: 10.1089/thy.2006.16.73. [DOI] [PubMed] [Google Scholar]
- 7.Bastemir M, Akin F, Alkis E, Kaptanoglu B. Obesity is associated with increased serum TSH level, independent of thyroid function. Swiss Med wkly. 2007;137:431–4. doi: 10.4414/smw.2007.11774. [DOI] [PubMed] [Google Scholar]
- 8.Tagliaferri M, Berselli ME, Calò G, Minocci A, Savia G, Petroni ML, et al. Subclinical hypothyroidism in obese patients: Relation to resting energy expenditure, serum leptin, body composition, and lipid profile. Obes Res. 2001;9:196–201. doi: 10.1038/oby.2001.21. [DOI] [PubMed] [Google Scholar]
- 9.Kundsen N, Lamberg P, Rasmussen LB, Bulow I, Perrild H, Ovessen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–24. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 10.Stichel H, I’Allemand D, Gruter A. Thyroid function and obesity in children and adolescents. Horm Res. 2000;54:14–9. doi: 10.1159/000063431. [DOI] [PubMed] [Google Scholar]
- 11.Reinehr T, deSousa G, Andler W. Hyperthyrotropinemia in obese children is reversible after weight loss and is not related to lipids. J Clin Endocrinol Metab. 2006;91:3088–91. doi: 10.1210/jc.2006-0095. [DOI] [PubMed] [Google Scholar]
- 12.Kiortsis DN, Durack I, Turpin G. Effects of a low-calorie diet on resting metabolic rate and serum triiodothyronine levels in obese children. Eur J Pediatr. 1999;158:446–50. doi: 10.1007/s004310051117. [DOI] [PubMed] [Google Scholar]
- 13.Bhowmick SK, Dasari G, Levens KL, Rettig KR. The prevalence of elevated serum thyroid-stimulating hormone in childhood/adolescent obesity and of autoimmune thyroid diseases in a subgroup. J Natl Med Assoc. 2007;99:773–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Lele RD, Marita AR. Insulin resistance and obesity. In: Das AK, Rao PV, Madhu SV, Mohan V, editors. Textbook of diabetes mellitus. 2nd ed. Hyderabad: Research Society for the Study of Diabetes in India; 2008. pp. 307–13. [Google Scholar]
- 16.Ghizzoni L, Mastorakos G, Ziveri M, Furlini M, Solazzi A, Vottero A, et al. Interactions of leptin and thyrotropin 24-hour secretory profiles in short normal children. J Clin Endocrinol Metab. 2001;86:2065–72. doi: 10.1210/jcem.86.5.7452. [DOI] [PubMed] [Google Scholar]
- 17.Ortiga-Carvalho TM, Oliveira KJ, Soares BA, Pazos-Moura CC. The role of leptin in the regulation of TSH secretion in the fed state: in vivo and in vitro studies. J Endocrinol. 2002;174:121–5. doi: 10.1677/joe.0.1740121. [DOI] [PubMed] [Google Scholar]


