Abstract
Background:
Pheochromocytoma/paragangliomas have been described to be associated with rare vascular abnormalities like renal artery stenosis. Coexistence of physiologically significant renal artery lesions is a compounding factor that alters management and prognosis of pheochromocytoma patients. Apart from individual case reports, data on such association in Indian population is not available. The aim of this study is to find the nature and prevalence of associated vascular abnormalities.
Materials and Methods:
From 1990 to 2010, a total of 50 patients were diagnosed with pheochromocytoma/paragangliomas. Hospital charts of these patients were reviewed retrospectively to identify those with unusual vascular abnormalities. Available literature was also reviewed.
Results:
Of the 50 patients with pheochromocytoma, 7 (14%) had coexisting vascular lesions including renal artery stenosis in 4, aortoarteritis in 1, aortic aneurysm in 1 and inferior vena cava thrombosis in 1. Pheochromocytoma was adrenal in 42 and extra adrenal in 8. Laparoscopic adrenalectomy was done in the patients. One patient with renal artery stenosis due to intimal fibrosis was subjected to percutaneous balloon angioplasty; the other three improved after adrenalectomy and lysis of fibrous adhesive bands. The patient with aortoarteritos was treated with oral steroids. Inferior vena cava thrombosis was reversed with anticoagulants. The patient with abdominal aortic aneurysm was advised for annual follow-up on account of its size of 4.5 cm and asymptomatic presentation.
Conclusion:
There are multiple mechanisms that can lead to renal artery stenosis and other vascular abnormalities in a case of pheochromocytoma. A high index of suspicion is necessary to enable both entities to be diagnosed preoperatively and allow proper planning of surgical therapy. Incomplete diagnosis may lead to persistent hypertension postoperatively in a case of associated renal artery stenosis.
Keywords: Aortic aneurysm, aortoarteritis, hypertension, pheochromocytoma, inferior vena cava thrombosis, renal artery stenosis
INTRODUCTION
Pheochromocytoma is an uncommon cause of hypertension. It is estimated to occur in 0.1-1% of hypertensive patients.[1] This chromaffin cell tumor may secrete catecholamines and other substances,[2] either continuously or intermittently, causing sustained or paroxysmal symptoms, respectively. As some patients may have recurrences after removal of the primary tumor,[3] follow-up is essential.
Renal artery aberrations and pheochromocytoma comprise correctable causes of surgical hypertension. The initial report citing the association between the two was published in 1958.[4] There may be a common pathophysiological mechanism mediated by catecholamine secretion,[5] or extrinsic compression.[6] The diagnostic dilemma of both lesions makes management more complex. If either lesion is missed hypertension may persist postoperatively. Proper diagnosis and management are imperative to achieve cure of secondary hypertension. Previous reports have been limited to one or two cases with the largest series describing 10 cases of pheochromocytoma with renal artery lesions from Cleveland Clinic Foundation.[7]
Aortoarteritis is a nonspecific inflammatory arteriopathy involving the aorta, its major branches and sometimes the pulmonary artery. However its association with pheochromocytoma has not yet been previously reported. One report has previously published coexistence of renal artery aneurysm with pheochromocytoma.[7] We hereby have reviewed the past medical records of pheochromocytoma patients presenting to our hospital and analyzed the patients with coexistent vascular lesions. To the best of our knowledge, ours is the first reported series emanating from India comprising of pheochromocytoma associated with aortoarteritis, aortic aneurysm, and inferior vena caval thrombosis without any tumoral invasion.
MATERIALS AND METHODS
From 1990 to 2010 a total of 50 patients were diagnosed to have pheochromocytoma. By thoroughly going through the patient data retrospectively, we could identify seven patients with associated unusual vascular abnormalities [Table 1]. Baseline parameters included patient age, gender, presenting symptoms and type of antihypertensive medications. Preoperative biochemical evaluation confirming the presence of a pheochromocytoma included plasma and urinary metanephrine levels, vanillylmandelic acid. Patients with pheochromocytoma, elevated serum creatinine, increased plasma renin activity, computerized tomography (CT) findings suggestive of a renal arterial lesion or impingement of renal artery, a small kidney, delayed nephrogram, and extra-adrenal pheochromocytoma at the renal hilum were evaluated for a coexisting renal arterial lesion. Presence of a physiologically significant renal artery stenosis was evaluated by greater than 70% arterial narrowing on arteriography and increased plasma renin activity. Radiological tests included abdominopelvic computed tomogram (CT), magnetic resonance imaging (MRI), aortography and venography as per the clinical scenario. The side and location of the pheochromocytoma and renal arterial lesion were recorded. Surgical/medical management and postoperative status were determined from a review of hospital records and/or telephonic calls to the patients.
Table 1.
Clinical profile of patients with additional vascular abnormality
One 40-year-old lady presented with cramping pain in both upper limbs. On examination, carotid and upper limb pulses were not palpable while lower limb pulses could be well palpated with normal systemic examination. There was no history of any visual symptoms, transient ischemic attack, limb claudication or abdominal pain. Diagnostic angiogram was done via right femoral artery under local anesthesia. Another 48-year-old male was evaluated for complaints of loin pain. Apart from other lab investigations, MRI abdomen and inferior vena cava (IVC) venogram via the right jugular vein was performed. A 38-year-old lady complained of vague abdominal pain and back pain with occasional nausea and vomiting. The patient was subjected to MRI scan.
RESULTS
A total of 50 patients (male: female =35:15) diagnosed as pheochromocytoma could be retrieved. Their mean age was 45.5 ± 23.3 years (range 25-72 years). Pheochromocytoma was adrenal in 42 patients and eight patients having extraadrenal paraganglioma. Out of the eight patients, the paraganglioma was periaortic in three cases, precaval in two cases, two retrocaval, and one intrarenal.
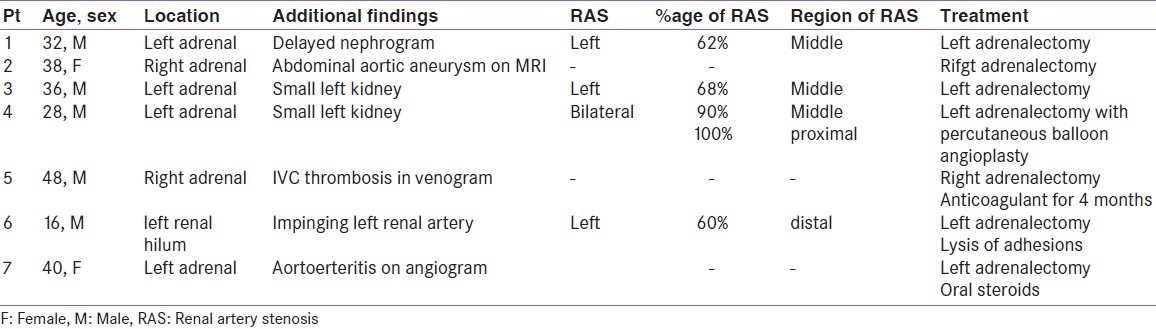
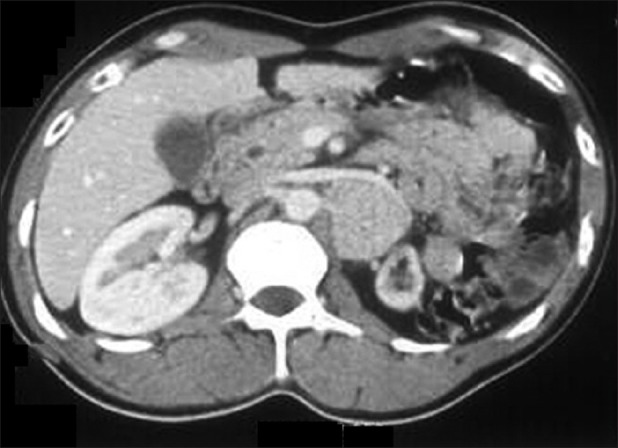
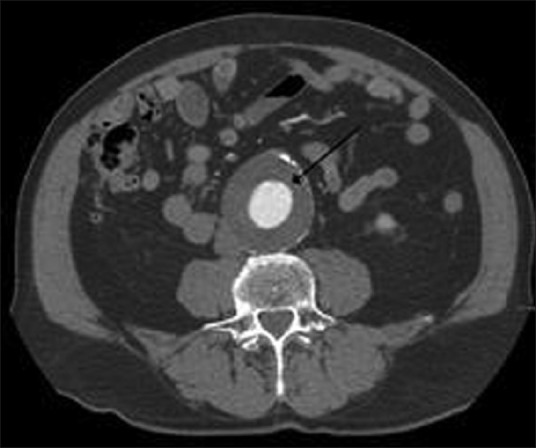
Seven patients were found to have associated vascular abnormalities. Of all the patients, seven had pheochromocytoma, with extraadrenal paraganglioma in none. Table 1 depicts the clinical parameters of the patients. There were five men and two women with mean age of 35.3 ± 21.4 years (range 16-44 years). The vascular lesions included renal artery stenosis in four patients, aortoarteritis, aortic aneurysm, and inferior vena cava thrombosis in one each patient. The renal artery stenosis was diagnosed by small ipsilateral kidneys in 2 [Figure 1], delayed nephrogram in 1 [Figure 2] and impingement of renal artery in one patient [Figure 3]. Of the seven patients pheochromocytoma was adrenal in six patients (four left sided and one right sided) and ectopic in one (at left renal hilum).
Figure 1.
CT scan of abdomen revealing small kidney on the right side
Figure 2.
Contrast-enhanced nephrogram showing delayed uptake by the left kidney
Figure 3.
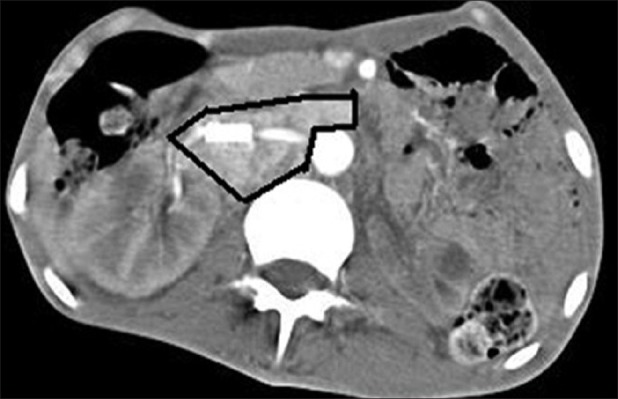
CT abdomen showing pheochromocytoma with left renal artery narrowing
Diagnostic angiogram of the 40-year-old female revealed irregular aortic arch, and total occlusion of both the common carotid and both the subclavian arteries [Figure 4]. He also had normochromic anemia with elevated ESR. MRI of the 48-year-old male presenting with loin pain revealed intraluminal thrombus extending proximally up to the confluence of hepatic veins immediately inferior to the right atrium without distal extension to femoral veins bilaterally [Figure 5]. IVC venogram of the same lady demonstrated multiple filling defects indicating occlusion of the IVC inferior to the right atrium [Figure 6]. MRI of the 45-year-old lady presenting with vague abdominal pain depicted 4.5 cm abdominal aortic aneurysm with 3 cm lumen [Figure 7].
Figure 4.
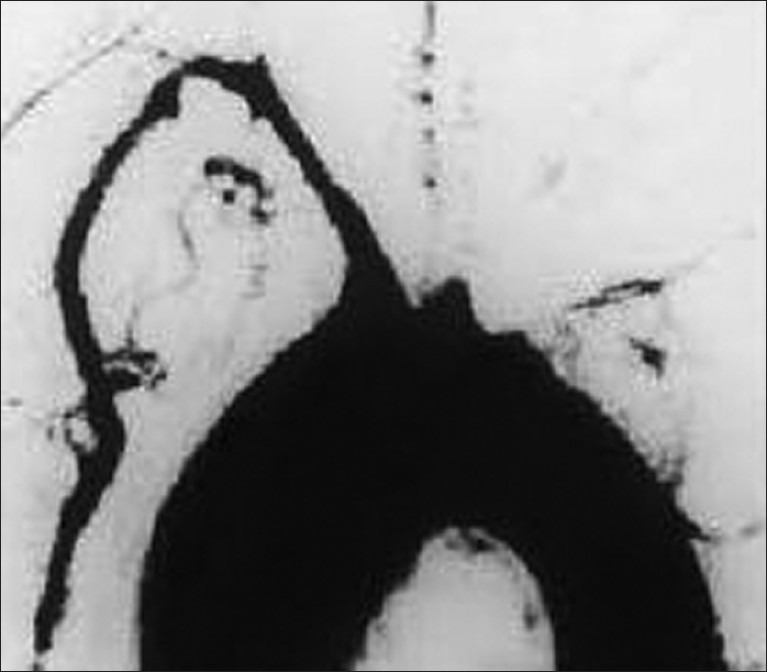
Intra-arterial digital subtraction angiogram of the arch of aorta showing total occlusion of both the common carotid and both the subclavian arteries
Figure 5.
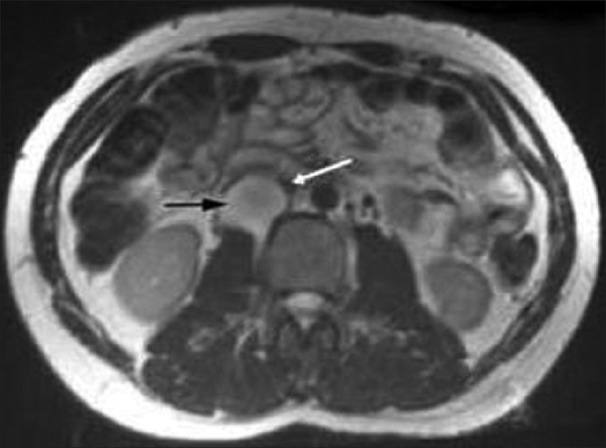
T2-weighted axial MRI demonstrating the mass (predominantly high signal) between the IVC and right kidney (black arrow) compressing the overlying IVC (white arrow)
Figure 6.
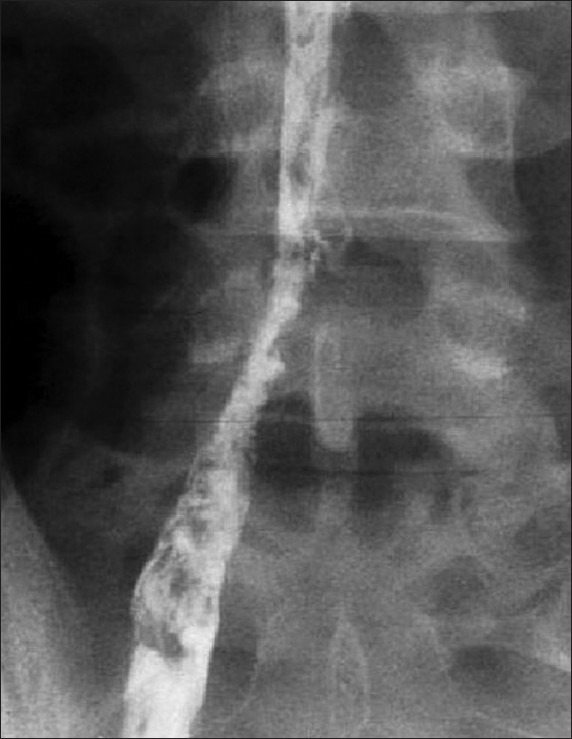
Inferior vena cava venogram showing multiple filling defects indicating occlusion of the IVC inferior to the right atrium
Figure 7.
MRI abdomen depicting 4.5 cm abdominal aortic aneurysm with 3 cm lumen
DISCUSSION
Two aspects of the current study involving pheochromocytoma patients are unusual: (1) presentation of four new cases of pheochromocytoma and renal artery lesion and (2) first reported association between pheochromocytoma with aortoarteritis, inferior vena cava thrombosis, and aortic aneurysm in Indian patients.
Concurrent pheochromocytoma and renal artery stenosis is rare. Harrison et al. first reported on a patient with a left adrenal and two extra-adrenal pheochromocytomas in the left renal hilum, which were excised.[4] A year later renovascular hypertension developed secondary to entrapment and narrowing of the entire extent of the ipsilateral renal artery in a dense postoperative cicatrix which was treated with secondary nephrectomy. Of the 87 cases in the literature pheochromocytoma was extra-adrenal in 40 (46%), adrenal in 47 (54%) and bilateral in 10.[8–12] Of the extra-adrenal pheochromocytomas 31 were in the renal hilum, 3 periaortic,[13,14] 3 precaval,[5,10] 2 para-adrenal,[8] 2 retrocava1,[15] and 1 intrarenal.[16] Of the 37 unilateral adrenal tumors 23 (62%) involved the left adrenal gland, and the majority were located in the inferomedial limb. Caudal growth of a tumor at this site could cause extrinsic compression of the renal artery. In our series, 42 (84%) patients had adrenal pheochromocytomas and 8 (16%) patients had extraadrenal paraganglioma. Out of the eight patients, the paraganglioma was periaortic in three cases, precaval in two cases, 2 retrocaval, and 1 intrarenal.
The simultaneous occurrence of pheochromocytoma and renal artery stenosis is very rare. Some pathophysiological mechanisms have been proposed,[10] including (a) an ipsilateral tumor compressing the renal artery, (b) prolonged catecholamine inducing an arterial vasospasm, that can bring about changes in the renal artery wall. Norepinephrine secreted preponderantly by extra-adrenal pheochromocytomas is an even more potent vasoconstrictor. Cases of transient renal artery stenosis have been reported in the literature.[11,15] (c) A periarterial adhesion following the resection of the adrenal tumor; (d) generalized neuroectodermal dysplasia with pheochromocytoma and neurofibromatosis, and (e) an independent occurrence of stenotic lesions of the renal artery (e.g., atherosclerosis, fibromuscular dysplasia). Spastic phase of fibromuscular dysplasia can result from markedly elevated local catecholamine levels due to associated pheochromocytoma, initiating a sustained arterial spasm ultimately resulting in dysplasia-like morphological changes in the muscular elements of the arterial wall (organic phase of fibromuscular dysplasia).[17] Extrinsic compression of the renal artery by a pheochromocytoma seems to be the most common cause of the association,[7] which can lead to myointimal proliferation over the long term further reducing the arterial luminal diameter. However, even pheochromocytoma without renal artery stenosis can be accompanied by elevated plasma renin activity[17] that may be induced by direct stimulatory effect of catecholamines on renin release and can lead to secondary hyperaldosteronism. This suggests that measuring plasma renin activity in a peripheral vein does not reveal an association between renal arterial stenosis and pheochromocytoma.
Also called “pseudostenosis,” transient renal artery narrowing possesses special features with therapeutic implications. Such stenosis is more evident during a hypertensive pheochromocytoma crisis, which may on occasion be triggered by aortography.[18] Alternatively, stenosis may be mild and hemodynamically insignificant. These stenoses have been noted to regress completely following administration of α-adrenergic blockers for treatment of the concurrent pheochromocytoma.
Preoperative biochemical evaluation is directed towards establishing evidence of catecholamine hypersecretion and documentation of differential renal ischemia. Elevation of urinary and plasma metanephrines establishes the diagnosis of pheochromocytoma.[19] The biochemical diagnosis of pheochromocytoma is made by measuring 24 hour urine fractionated metanephrines (sensitivity 98% and specificity 98%) and fractionated catecholamines.[20] The specificity is high for 24 hours urinary VMA (95%) and 24 hours urinary total metanephrines (99%). Plasma metanephrines are the screening test of choice for patients considered at high risk for pheochromocytoma and for those suspected of having a familial form. In our series of patients, increased levels of urinary metanephrine were detected in 42 of 50 (84%) plasma catecholamines in 28/32 patients tested (90%), and vanillylmandelic acid was positive in 23 of 30 (77%). Thus, renal artery stenosis and other associated vascular abnormalities do not interfere with the biochemical diagnosis of a pheochromocytoma. However, patients with pheochromocytoma have catecholamine-induced stimulation of renin-angiotensin-aldosterone system leading to elevated plasma renin activity which normalizes following either α blockade or pheochromocytoma. Accompanying renal vasoconstriction induced by elevated circulating norepinephrine can also lead to renal ischemia and hyperreninemia.[21] Other factors implicated in causation of hyperreninemia include the decreased plasma volume in patients with a pheochromocytoma, salt restriction, and diuretics. Thus, hyperreninemia cannot be interpreted as conclusive evidence of renal artery stenosis in patients with a pheochromocytoma. Additional studies like Doppler ultrasonography, gadolinium-enhanced 3D magnetic resonance angiography, and contrast-enhanced arteriography must be used to lateralize the renal ischemia. Renal vein renin ratios can establish the functional significance of the angiographically demonstrated stenosis. The current study group had elevated plasma renin activity in 18 of 20 patients (90%), aldosterone was increased in 8 of 12 (66%), and renal vein renin ratio lateralized to the ischemic kidney in 10 of 13 (77%).
The importance of preoperative diagnosis of the pheochromocytoma and renal artery stenosis is obvious. Hypertension would persist postoperatively if either etiology was not surgically corrected. Major cardiac complications occurred in five of nine patients[14] and perioperative mortality occurred in 2 of 16 in whom the pheochromocytoma was not suspected preoperatively and renal artery stenosis was the only diagnosis. Preoperative undiagnosed renal artery stenosis does not subject the patient to additional risk. There was no perioperative mortality in four patients in whom the diagnosis of a coexisting renal artery stenosis was missed before surgery.[1] In a previous study,[7] Renal artery lesions included renal artery stenosis in eight patients, renal artery aneurysm in 1, and postangiographic dissection occlusion in 1. Renal artery stenosis was due to extrinsic compression in three patients, intimal fibroplbsia in 2, possible catecholamine-induced vasoconstriction in 2, iatrogenic in 1, and atherosclerosis in 1.[7] In our study group renal artery stenosis was due to extrinsic compression in two patients, intimal fibroplbsia in 1, and possible catecholamine-induced vasoconstriction in 1.
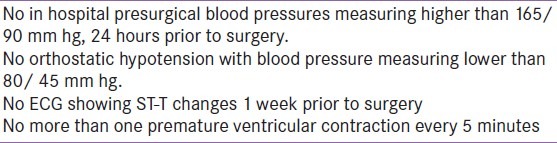
Preoperative optimization of blood pressure requires adequate α blockade in increasing doses followed by b blockade. Roizen criteria for significant a blockade are used [Table 2].[22] Surgical resection is the appropriate treatment, cures 90% patients. Laparoscopic removal is done commonly. Laparotomy is reserved for tumors >8 cm and local invasion.[23] Both the approaches are equally good in terms of overall survival. The greatest intraoperative concern is the release of catecholamines leading to life-threatening hypertension. Hypertensive crises can cause myocardial infarction, heart failure, dysrhythmias, and cerebral hemorrhage. Severe hypertension can occur at any time throughout the surgery but induction, intubation, and tumor palpation tend to be the times of greatest catecholamine release. In all of our patients presenting with coexistent vascular lesions, laparoscopic resection was carried out in view of their size below 8 cm. In the overall study population, total of 42 underwent laparoscopic resection while eight patients underwent laparotomy. Induction agents should be titrated slowly to maintain normotension. A short acting narcotic such as fentanyl, with its minimal myocardial depression, in combination with a sedative/hypnotic is preferable. Adequate depth of anesthesia is required so that the patient does not respond to the stimulus of intubation.[24] Vecuronium or rocuronium with few cardiovascular effects may be used for muscle relaxation. Pancuronium should be avoided because of its sympathomimetic effects. Inhalational agents (isoflurane, sevoflurane, desflurane) may be used with or without intravenous agents. Other medications known to trigger release of catecholamines like metoclopramide, pentazocine, droperidol, atracurium, succinylcholine, SSRIs, MAO inhibitors, imipramine, opioids, and curare should be avoided.[25] Intraoperatively alpha blockade is continued with phentolamine. Its most common side effect is reflex tachycardia due to the baroreceptor reflex following alpha 2 blockade. Labetalol should be used to control tachycardia. Calcium channel blockers and nitroprusside can be used a second line therapies.[25] In most of our cases, IV phentolamine was used.
Table 2.
Roizen's criteria for appropriate preoperative alpha blockade and surgical optimization
Following early ligation of the vein draining the pheochromocytoma, intravenous fluid administration is essential for volume expansion. The sudden drop in catecholamines can lead to significant hypotension, requiring aggressive fluid replacement with a combination of crystalloids and colloids. Pressors may be necessary to maintain blood pressure in severe hypotension but they are best avoided and contraindicated if the patient is hypovolemic. Often the hypotension of pheochromocytoma is refractory to agents such as norepinephrine, epinephrine, and dopamine because of the desensitization of sympathetic receptors to the previously persistently high levels of catecholamines.[24]
The goals of operation include (1) removal of the tumor, (2) preservation of functioning renal tissue, and (3) correction of physiologically significant renal artery stenosis. The markedly high blood pressure due to the pheochromocytoma might provide a sufficient pressure head to maintain a near normal blood flow to the kidney despite ipsilateral renal artery stenosis. Excision of the pheochromocytoma would lower the blood pressure, thus significantly decreasing the blood flow across the stenotic lesion. A previously borderline renal artery stenosis could now become significant in producing renal ischemia. Therefore, failure to repair the stenosis at pheochromocytoma excision may lead to postoperative renal artery thrombosis, resulting in renal demise. A second surgery to correct the renal artery stenosis is hazardous and has resulted in secondary nephrectomy in 9 of 12 such cases (75%).
In the presence of equivocal findings, manometric or doppler flow pressure studies of the involved vessel need to performed once the pheochromocytoma has been resected.[8,14] Confirmation of a significant gradient across the stenotic site would mandate an attempt at renal revascularization.[10,13] In cases of pheochromocytoma, being inextricably adherent to the renal pedicle or there is evidence of ischemic nephropathy nephrectomy should be considered after confirmation of a normal contralateral renal unit. In patients with a solitary kidney autotransplantation with or without bench repair is the preferred option.[10] In the event of persistent or missed renal artery stenosis balloon angioplasty may be a superior alternative to secondary renal revascularization.[12] Minimally invasive techniques are being increasingly used for resection of adrenal tumors and to treat renal artery lesions. Laparoscopic adrenalectomy is performed by either the transperitoneal or retroperitoneal approach.[26] Similarly, percutaneous balloon angioplasty has come to be the first line of treatment for the majority of cases of renal artery stenosis.[27] The use of endovascular stents has further extended the applicability of percutaneous revascularization techniques. Open surgical revascularization is now reserved for angioplasty failures or some ostial lesions. The seven patients with vascular lesions in our series underwent laparoscopic adrenalectomy. Out of the four patients with RAS, three recovered after adrenalectomy with lysis of adhesive fibrous strands, where as one patient with intimal fibrosis required percutaneous balloon angioplasty. Figure 8 describes the diagnostic algorithm for treating patients with pheochromocytoma and suspected coexisting renal arterial lesion.[7]
Figure 8.
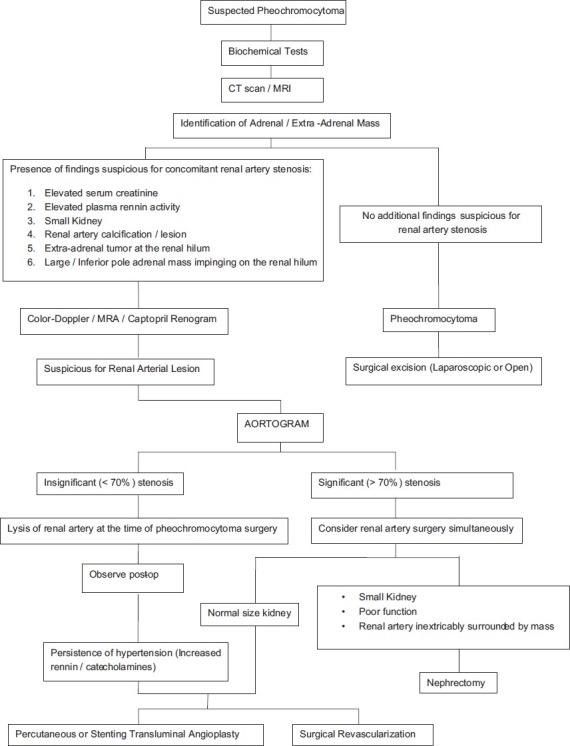
Suggested diagnostic and therapeutic algorithm for treating patients with pheochromocytorna and suspected coexisting renal arterial lesion. J Urol 2000; 164: 296-301
The simultaneous occurrence of pheochromocytoma and aortoarteritis is not yet described in the literature. Aortoarteritis in this case could be because of (1) persistently high circulating catecholamines may damage vascular endothelium resulting in intimal fibrosis in large and medium vessels leading to aortoarteritis, (2) association of pheochromocytoma with systemic lupus erythematous and Behcet's disease might explain the triggering of an autoimmune phenomenon leading to aortoarteritis. But an immunological study from India of 50 patients with aortoarteritis did not find any role of an autoimmune mechanism in aortoarteritis,[28] and (3) a chance association between these 2 conditions.
Signs and symptoms in patients with Takayasu's arteritis (aortoarteritis) can show considerable clinical variation with regard to severity, duration, and quality.[29] The onset of the disease is often insidious and it progresses at a variable rate from the active inflammatory phase to the chronic, sclerotic phase with intimal hyperplasia, medial degeneration, and adventitial fibrosis.[30] Stenotic lesions are more common than aneurysms.[31] The narrowing starts near the orifice of the artery and extends for a variable distance; progressive narrowing leads to occlusion with ischemic symptoms. In such an extreme form of brachiocephalic arteritis, all or most of the arch vessels are occluded and the entire circulation to the brain is provided through collateral vessels. Our patient did not have these symptoms.
Glucocorticoids effectively suppress the systemic symptoms and usually arrest progression of Takayasu arteritis. The normochromic anemia and elevated acute phase reactants also return to normal.[32] In our patient we noticed improvement in anemia and ESR following treatment with steroids. Arterial stenosis may reverse and ischemic symptoms may improve in early cases. However, the vascular response is diminished once fibrous tissue has formed in the involved vessels or thrombosis has occurred. Angiographic improvement on adequate, long-term steroids, as reported by some workers[33] was not seen in this case. CT or MRI can also be used to follow the response to therapy.[34] No improvement of arterial obstruction has been reported in other studies.[35] Kerr et al.[21] reported a relapse in half the patients who had initially achieved remission on steroids. Rapidly increasing aneurysmal size (more than 1 cm/year) has been reported despite glucocorticoid therapy.[34] Percutaneous transluminal angioplasty or bypass grafts may be considered in late cases when irreversible arterial stenosis has occurred and significant ischemic symptoms are present.[36] Angioplasty is preferable when the lesions are amenable to catheter-based therapy. However, percutaneous intervention is less likely to be successful when stenoses or occlusions affect lengthy portions of an artery or the artery is heavily scarred. Continued inflammation in a treated segment may result in restenosis following angioplasty, with or without stenting; restenosis is less likely following bypass grafting than angioplasty, when performed after initiation of treatment, or if revascularization is followed by antiinflammatory therapy.[37]
There are case reports citing the coexistence of pheochromocytoma and IVC thrombosis.[38,39] The simultaneous occurrence of pheochromocytoma and IVC thrombosis can be explained by (1) local compression leading to alteration in blood flow and stasis, (2) sustained hypertension leading to vascular endothelial injury and hypercoagulbility, (3) association of pheochromocytoma with systemic lupus erythematous and Behcet's disease might explain the triggering of an autoimmune phenomenon leading to aortoarteritis, and (4) An underlying anatomic abnormality or coagulation disorder. It also could be a chance association between these two conditions. In our case local compression due to coexisting pheochromocytoma might have been causative.
Recent advances in the utilization of ultrasound, CT and MRI imaging as well as endovascular procedures have resulted in an increase in detection rates of IVC anomalies.[40] Contrast venography remains the standard for diagnosis of IVC thrombosis with the advantage of access for immediate treatment if required. However, it is an invasive procedure associated with a 2-10% incidence of postprocedural DVT.[41] Duplex ultrasound scanning (USS) has become an accurate non-invasive method of diagnosing IVC thrombosis and is often the first-line investigative modality.[41] However, duplex USS is operator dependant and can be limited by body habitus or the presence of bowel gas and may occasionally fail to identify any IVC anomaly.[42] CT imaging is a rapid noninvasive method which can accurately diagnose and assess the extent of thrombus as well as delineate any associated abdominal or pelvic abnormality.[41] MRI imaging is now replacing CT as the optimal investigative tool avoiding radiation and giving more accurate delineation of thrombus as well as any IVC anomaly. MRI is also used to follow-up patients to determine morphological changes in the thrombus following therapy.[43]
Treatment options in the case of IVC thrombus without anatomical variance include anticoagulation, mechanical thrombectomy, systemic thrombolytic therapy, transcatheter regional thrombolysis, pulse-spray pharmacomechanical thrombolysis, and angioplasty.[44] There is no specific literature describing the ideal duration of anticoagulation in these instances; however, case evidence identifies a trend toward treatment for a minimum of 1 year with the interplay of hypercoagulability disorders needing to be factored into any decision. Surgical reconstruction of the IVC and bypass of an aberrant section are both recognized modalities reserved for the most severe cases and are associated with morbidity and mortality risk.[45] Endovascular stent placement in combination with angioplasty is recommended in the cases of residual stenosis and chronic IVC occlusion.[45] A caval anomaly is a permanent risk factor for venous stasis and thrombosis and that anticoagulant treatment should be lifelong.[46] Since our patient had no anatomic abnormality or any other predisoping factors, we decided to give the treatment for 4 months only and stopped it then after documenting radiologic luminal restoration [Figure 9].
Figure 9.
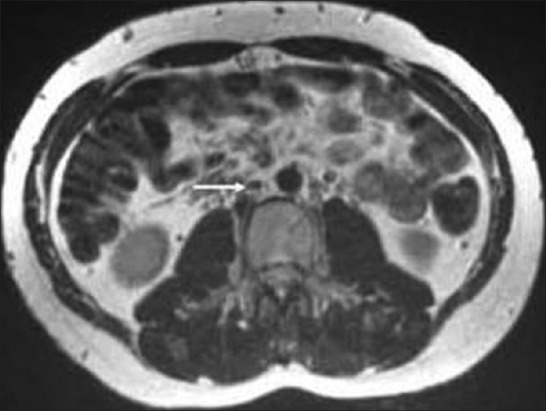
T2-weighted axial MRI comparable in position and image acquisition to Figure 4 demonstrating complete resolution of IVC thrombosis (white arrow) after 4 months of oral anticoagulation therapy
Though cases of renal artery stenosis, renal artery aneurysm have been described,[7] we found an uncommon association with abdominal aortic aneurysm. Isolated case reports citing the coexistence of pheochromocytoma and abdominal aortic aneurysm exist in literature.[47–49] Possible etiological factors associating aortic aneurysm with pheochromocytoma are (1) persistent exposure to high catecholamines leads to damage and subsequent weakening of vascular wall, (2) associated cigarettes smoking, increasing age, hypertension, and atherosclerosis, (3) coexistence of vasculitis like takayasu's disease, giant cell arteritis and cystic medial necrosis due to marfan syndrome and Ehlers Danlos syndrome, (4) chance association.
Abdominal aortic aneurysms occur more frequently in males than in females, and the incidence increases with age. Abdominal aortic aneurysms ≥ 4.0 cm may affect 1-2% of men older than 50 years. At least 90% of all abdominal aortic aneurysms >4.0 cm are related to atherosclerotic disease, and most of these aneurysms are below the level of the renal arteries. Prognosis is related to both the size of the aneurysm and the severity of coexisting coronary artery and cerebrovascular disease. The risk of rupture increases with the size of the aneurysm: the 5-year risk for aneurysms <5 cm is 1-2%, whereas it is 20-40% for aneurysms >5 cm in diameter. The formation of mural thrombi within aneurysms may predispose to peripheral embolization.[50] For abdominal aneurysms, the current treatment guidelines for abdominal aortic aneurysms suggest elective surgical repair when the diameter of the aneurysm is greater than 5 cm. However, recent data suggest medical management for abdominal aneurysms with a diameter of less than 5.5 cm.[51] Because of the asymptomatic 4.5 cm aneurysm, our patient was advised for periodic follow-up.
CONCLUSION
Coexistence of a pheochromocytoma and renal artery stenosis has been reported more often recently than noted by previous literature reviews, and the actual incidence may be even higher. To our knowledge we report the largest single institution experience from India to date. Even associations with aortoarteritis, inferior vena cava thrombosis, and abdominal aortic aneurysm are not yet reported on Indian patients. There are multiple mechanisms by which renal artery stenosis may present with either an adrenal or ectopic pheochromocytoma. A high index of suspicion is necessary for both entities to be diagnosed preoperatively, thereby permitting proper planning of surgical therapy. Discovery of an ectopic pheochromocytoma in the renal hilum, delayed nephrogram, and small kidney should alert the physician to a possible coexisting renal arterial lesion. The clinician should be aware of the entity of transient renal artery stenosis and avoid intervention for the same before a trial of adrenergic blockers. Pheochromocytoma resection and renal revascularization when indicated can be performed safely in a single operative session, although the pheochromocytoma should be excised first. When the degree of renal artery stenosis is unclear, intraoperative Doppler flow determinations may be helpful. Missing a functionally significant lesion may lead to persistent hypertension postoperatively.
ACKNOWLEDGMENTS
All the authors would extend their heartfelt thanks to Dr. Jagadeesh Tangudu, M Tech, MS, PhD and Mrs Sowmya Jammula, M Tech for their immense and selfless contribution toward manuscript preparation, language editing, and final approval of the text.
Footnotes
Source of Support: Nil
Conflict of Interest: No
REFERENCES
- 1.Mannelli M, Ianni L, Cilotti A, Conti A the National Study Group on Adrenal Tumors of the Italian Society of Endocrinology. Pheochromocytoma in Italy: A multicentric retrospective study. Eur J Endocrinol. 1999;141:619–24. doi: 10.1530/eje.0.1410619. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki A, Yumita S, Kimura S, Miura Y, Yoshinaga K. Immunoreactive corticotropin-releasing hormone, growth hormone-releasing hormone, somatostatin and peptide histidine methionine are present in adrenal pheochromocytomas but not in extra-adrenal pheochromocytoma. J Clin Endocrinol Metab. 1990;70:996–9. doi: 10.1210/jcem-70-4-996. [DOI] [PubMed] [Google Scholar]
- 3.Plouin PF, Chatellier G, Fofol I, Corvol P. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension. 1997;29:1133–9. doi: 10.1161/01.hyp.29.5.1133. [DOI] [PubMed] [Google Scholar]
- 4.Harrison JH, Gardner FH, Dammin GJ. A note on pheochromocytoma and renal hypertension. J Urol. 1958;79:173–8. doi: 10.1016/S0022-5347(17)66254-2. [DOI] [PubMed] [Google Scholar]
- 5.Del Gaudio A. Pheochromocytoma and renal artery stenosis. Int Surg. 1985;70:153–8. [PubMed] [Google Scholar]
- 6.Sato T, Sakamoto S, Maebashi M, Suzuki C, Sudo K. Hypertension due to combination of pheochromocytoma and unilateral renal ischemia by tumor compression. Jpn Heart J. 1967;8:202–8. doi: 10.1536/ihj.8.202. [DOI] [PubMed] [Google Scholar]
- 7.Gill IS, Meraney AM, Bravo EL, Novick AC. Pheochromocytoma coexisting with renal artery lesions. J Urol. 2000;164:296–301. [PubMed] [Google Scholar]
- 8.Naidich TP, Sprayregen S, Goldman AG, Siegelman SS. Renal artery alterations associated with pheochromocytoma. Angiology. 1972;23:488–99. doi: 10.1177/000331977202300804. [DOI] [PubMed] [Google Scholar]
- 9.Clinicopathologic conference. Persistent hypertension after resection of a pheochromocytoma. Am J Med. 1982;73:97–104. doi: 10.1016/0002-9343(82)90936-6. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman JJ. Pheochromocytoma and stenosis of the renal artery. Surg Gynecol Obstet. 1983;156:11–5. [PubMed] [Google Scholar]
- 11.Kishikawa H, Tsuji H, Takagi I, Yamakawa Y, Shimozato T, Honda K, et al. Hemorrhagic necrosis of pheochromocytoma associated with reversible renal artery stenosis. Jpn J Surg. 1986;16:46–51. doi: 10.1007/BF02471069. [DOI] [PubMed] [Google Scholar]
- 12.Burns AP, O’Connell PR, Murnaghan DJ, Brady MP. Bilateral adrenal pheochromocytomas associated with unilateral renal artery stenosis. Postgrad Med J. 1989;65:943–7. doi: 10.1136/pgmj.65.770.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Way CW, III, Michelakis AM, Alper BJ, Hutcheson JK, Rhamy RK, Scott HW., Jr Renal vein renin studies in a patient with renal hilar pheochromocytoma and renal artery stenosis. Ann Surg. 1970;172:212–7. doi: 10.1097/00000658-197008000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alvestrand A, Bergström J, Wehle B. Pheochromocytoma and renovascular hypertension. A case report on a review of the literature. Acta Med Scand. 1977;202:231–6. [PubMed] [Google Scholar]
- 15.Kohara K, Mikami H, Ogihara T, Hashizume K, Kumahara Y, Kojima Y, et al. Extra-adrenal pheochromocytoma manifesting renovascular hypertension. J Clin Hypertens. 1987;3:303–9. [PubMed] [Google Scholar]
- 16.Bezirdjian DR, Tegtmeyer CJ, Leef JL. Intrarenal pheochromocytoma and renal artery stenosis. Urol Radiol. 1981;3:121–2. doi: 10.1007/BF02927822. [DOI] [PubMed] [Google Scholar]
- 17.Stanley JC, Gewertz BL, Bove EL, Sottiurai V, Fry WJ. Arterial fibrodysplasia. Histopathologic character and current etiologic concepts. Arch Surg. 1975;110:561–6. doi: 10.1001/archsurg.1975.01360110107018. [DOI] [PubMed] [Google Scholar]
- 18.Brewster DC, Jensen SR, Novelline RA. Reversible renal artery stenosis associated with pheochromocytoma. JAMA. 1982;248:1094–6. [PubMed] [Google Scholar]
- 19.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of Pheochromocytoma which test is best? JAMA. 2002;287:1427–34. doi: 10.1001/jama.287.11.1427. [DOI] [PubMed] [Google Scholar]
- 20.Kudva YC, Sawka AM, Young WF., Jr Clinical review 164: The laboratory diagnosis of adrenal pheochromocytoma: The Mayo clinic experience. J Clin Endocrinol Metab. 2003;88:4533–9. doi: 10.1210/jc.2003-030720. [DOI] [PubMed] [Google Scholar]
- 21.Freiha FS, Kavaney PB, Cunningham JJ, Fajardo LF, Castellino RA. Extraadrenal pheochromocytoma causing renal artery stenosis.Relationship to hypertension and renin levels. Urology. 1973;2:303–7. doi: 10.1016/0090-4295(73)90471-8. [DOI] [PubMed] [Google Scholar]
- 22.Roizen MF, Horrigan RW, Koike M, Eger IE, 2nd, Mulroy MF, Frazer B, et al. A prospective randomized trial of four anesthetic techniques for resection of pheochromocytoma. Anesthesiology. 1982;57:A43. [Google Scholar]
- 23.Assalia A, Gagner M. Laparoscopic adrenalectomy. Br J Surg. 2004;91:1259–74. doi: 10.1002/bjs.4738. [DOI] [PubMed] [Google Scholar]
- 24.Grant F. Anesthetic considerations in the multiple endocrine neoplasia syndromes. Curr Opin Anaesthesiol. 2005;18:345–52. doi: 10.1097/01.aco.0000169245.88506.13. [DOI] [PubMed] [Google Scholar]
- 25.Dortzbach K, Gainsburg DM, Frost EA. Variants of pheochromocytoma and their anaesthetic implications. Middle East J Anesthesiol. 2010;20:897–905. [PubMed] [Google Scholar]
- 26.Gill IS, Schweizer D, Nelson D. Laparoscopic versus open adrenalectomy in 210 patients: Cleveland Clinic experience with 210 cases. J Urol. 1997;161:21. Abstract 70. [Google Scholar]
- 27.Paulsen D, Kløw NE, Rogstad B, Leivestad T, Lien B, Vatne K, et al. Preservation of renal function by percutaneous transluminal angioplasty in ischaemic renal disease. Nephrol Dial Transplant. 1999;14:1454–61. doi: 10.1093/ndt/14.6.1454. [DOI] [PubMed] [Google Scholar]
- 28.Chopra P, Datta RK, Dasgupta A, Bhargava S. Immunological studies in aortoarteritis. Indian J Med Res. 1982;76:436–43. [PubMed] [Google Scholar]
- 29.Ishikawa K. Patterns of symptoms and prognosis in occlusive thromboaortopathy (Takayasu's disease) J Am Coll Cardiol. 1986;8:1041–6. doi: 10.1016/s0735-1097(86)80380-1. [DOI] [PubMed] [Google Scholar]
- 30.Sekiguchi M, Suzuki J. An overview on Takayasu's arteritis. Heart Vessels Suppl. 1992;7:6–10. doi: 10.1007/BF01744537. [DOI] [PubMed] [Google Scholar]
- 31.Kerr GS, Hallanhan CW, Giordano J, Leawitt RV, Fauci AS, Rottem M, et al. Takayasu's arteritis. Ann Intern Med. 1994;120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kerr GS. Takayasu's arteritis. Rheum Dis Clin North Am. 1995;21:1041–58. [PubMed] [Google Scholar]
- 33.Ishikawa K. Effects of prednisolone therapy on arterial angiographic features in Takayasu's disease. Am J Cardiol. 1991;68:410–3. doi: 10.1016/0002-9149(91)90845-c. [DOI] [PubMed] [Google Scholar]
- 34.Sueyoshi E, Sakamoto I, Hayashi K. Aortic aneurysms in patients with Takayasu's arteritis: CT evaluation. Am J Roentgenol. 2000;175:1727–33. doi: 10.2214/ajr.175.6.1751727. [DOI] [PubMed] [Google Scholar]
- 35.Morales E, Pineda C, Martinez-Lavin M. Takayasu's arteritis in children. J Rheumatol. 1991;18:1081–4. [PubMed] [Google Scholar]
- 36.Ishikawa K, Maetani S. Long-term outcome for 120 Japanese patients with Takayasu's disease. Clinical and statistical analyses of related prognostic factors. Circulation. 1994;90:1855. doi: 10.1161/01.cir.90.4.1855. [DOI] [PubMed] [Google Scholar]
- 37.Liang P, Tan-Ong M, Hoffman GS. Takayasu's arteritis: Vascular interventions and outcomes. J Rheumatol. 2004;31:102–6. [PubMed] [Google Scholar]
- 38.Shigemura K, Tanaka K, Arakawa S, Hara I, Kawabata G, Fujisawa M. Malignant pheochromocytoma with IVC thrombus. Int Urol Nephrol. 2007;39:103–6. doi: 10.1007/s11255-005-4969-4. [DOI] [PubMed] [Google Scholar]
- 39.Dossett LA, Rudzinski ER, Blevins LS, Chambers EP., Jr Malignant pheochromocytoma of the organ of Zuckerkandl requiring aortic and vena caval reconstruction. Endocr Pract. 2007;13:493–7. doi: 10.4158/EP.13.5.493. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji Y, Inoue T, Murakami H, Hino Y, Matsuda H, Okita Y. Deep vein thrombosis caused by congenital interruption of the inferior vena cava – A case report. Angiology. 2001;52:721–5. doi: 10.1177/000331970105201010. [DOI] [PubMed] [Google Scholar]
- 41.Giordano P, Weber K, Davis M, Carter E. Acute thrombus of the inferior vena cava. Am J Emerg Med. 2006;24:640–6. doi: 10.1016/j.ajem.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 42.Park JH, Lee JB, Han MC, Choi BI, Im CK, Chang KH, et al. Sonographic evaluation of inferior vena caval obstruction: correlative study with vena cavography. AJR Am J Roentgenol. 1985;145:757–2. doi: 10.2214/ajr.145.4.757. [DOI] [PubMed] [Google Scholar]
- 43.Soler R, Rodriguez E, Lopez MF, Marini M. MR imaging in inferior vena cava thrombosis. Eur J Radiol. 1995;19:101–7. doi: 10.1016/0720-048x(94)00587-3. [DOI] [PubMed] [Google Scholar]
- 44.Yamada N, Ishikura K, Ota S, Tsuji A, Nakamura M, Ito M, et al. Pulse-spray pharmacomechanical thrombolysis for proximal deep vein thrombosis. Eur J Vasc Endovasc Surg. 2006;31:204–11. doi: 10.1016/j.ejvs.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Robbins MR, Assi Z, Comerota AJ. Endovascular stenting to treat chronic long-segment inferior vena cava occlusion. J Vasc Surg. 2005;41:136–40. doi: 10.1016/j.jvs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Dean SM, Tytle TL. Acute right lower limb extremity iliofemoral deep venous thrombosis secondary to an anomalous inferior vena cava: A report of two cases. Vasc Med. 2006;11:165–9. doi: 10.1177/1358863x06074829. [DOI] [PubMed] [Google Scholar]
- 47.Thompson JF, Clifford PC, McEwan JA, Chant AD. Phaeochromocytoma and abdominal aneurysm: confirmation of diagnosis after aortic surgery using mIBG imaging. Eur J Vasc Surg. 1989;3:457–9. doi: 10.1016/s0950-821x(89)80056-8. [DOI] [PubMed] [Google Scholar]
- 48.Ehata T, Karasawa F, Watanabe K, Satoh T. Unsuspected pheochromocytoma with abdominal aortic aneurysm-a case report. Acta Anaesthesiol Sin. 1999;37:27–8. [PubMed] [Google Scholar]
- 49.Spanos C, Moros I, Spanos G, Drakotou I, Mavromanolis H, Spanos P. Elective resection of pheochromocytoma with concomitant abdominal aortic aneurysm repair: report of a case. Surg Today. 2006;36:741–3. doi: 10.1007/s00595-005-3233-y. [DOI] [PubMed] [Google Scholar]
- 50.Creager MA, Loscalzo J. Diseases of Aorta. In: Fauci AS, Kasper DL, Longo DL, Braunwald E, Hauser SL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008. pp. 1563–8. [Google Scholar]
- 51.The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352:1649–55. [PubMed] [Google Scholar]


