Abstract
Objective:
To determine the proportion of polycystic ovarian syndrome (PCOS) patients who have normal body mass index (BMI) and to compare the clinical, hormonal, and metabolic profile between lean and overweight patients of PCOS.
Materials and Methods:
One hundred consecutive infertile women with PCOS were studied and divided into lean (BMI between 18.5 and 23) and overweight (BMI ≥ 23). Metabolic and hormonal profile (serum FSH, LH, testosterone, prolactin, TSH on days 2–3 of menstrual cycle; serum progesterone premenstrually; serum insulin—fasting and 2 hours postglucose, glucose tolerance test, and fasting serum lipid profile) was performed along with pelvic sonogropahy; and clinical features, viz. waist hip ratio, hirsutism, acne, acanthosis nigricans, and clitoromegaly were recorded.
Results:
42% of the PCOS subjects had normal BMI. Average age, hirsutism (80.9% vs. 89.7%), irregular cycles (92.8% vs. 96.6%), acne (9.5% vs. 15.5%), clitoromegaly (2.3% vs. 3.4%), endometrial thickness >4 mm (9.5% vs. 15.5%), and hormonal profile were similar in the lean and overweight PCOS groups. Family history of diabetes (9.5% vs. 24.1%), abnormal glucose tolerance test (GTT) (4.7% vs. 10.3%), deranged lipid profile (14.2% vs. 31%), and 2-hour postprandial insulin levels were higher in the overweight PCOS (P < 0.05). Insulin resistance was observed in 83.3% of lean PCOS but was still lower than 93.1% seen in overweight PCOS (P < 0.05).
Conclusion:
42% of the PCOS had normal BMI, but clinical and hormonal profile was similar to PCOS patients with elevated BMI (overweight/obese). However, insulin resistance is observed in 83.3% of lean PCOS. Family history of diabetes, impaired GTT, deranged lipid profile, and insulin resistance were more prevalent in overweight PCOS.
Keywords: Metabolic syndrome, obesity, overweight, polycystic ovary syndrome
INTRODUCTION
Polycystic ovarian syndrome (PCOS) affects 4–12% of women of reproductive age. It is a conglomeration of symptoms with varied presentations such as hirsutism, acne, alopecia, anovulatory cycles, and obesity. According to ESHRE/ASRM consensus[1] workshop at Rotterdam in 2003, the diagnosis of PCOS is based on the presence of any two of (1) chronic anovulation, (2) clinical/ biochemical parameters for hyperandrogenism, and (3) polycystic ovaries on ultrasonography. According to the AE PCOS (Androgen Excess and PCOS Society) criteria, PCOS should be defined by the presence of hyperandrogenism (clinical and/or biochemical), ovarian dysfunction (oligo- anovulation and/or polycystic ovaries), and the exclusion of related disorders. Overweight or obesity is not an essential criterion, although menstrual irregularities, obesity, and hypertension are considered as clinical indicators of PCOS. Recent studies have shown that insulin resistance is a key feature in the evolution of PCOS and predisposes to type 2 diabetes mellitus in the long run. Although a major proportion of PCOS patients are overweight or obese, some of them may be having normal or lower body mass index.
This study aimed to determine the proportion of PCOS subjects who are not overweight/obese and to compare the clinical, hormonal, and metabolic profile of lean [normal body mass index (BMI)] and overweight/obese PCOS women.
MATERIALS AND METHODS
One hundred consecutive women with age range 20- 38 years presenting with infertility over a 3-year period and diagnosed to have PCOS according to ESHRE/ASRM criteria[1] were included in the study after obtaining an informed written consent. Patients with BMI <23 kg/ m2 were included in the lean PCOS and those with BMI ≥23 kg/ m2 were considered as overweight PCOS.[2]
A detailed history including menstrual pattern, personal, past, family, obstetric, and treatment history was recorded. A complete physical examination including general, systemic, breast, and pelvic examination was also performed, and subjects were screened for signs of hyperandrogenism such as hirsutism, acne, acanthosis nigricans, clitoromegaly, and alopecia. Hirsutism scoring was done according to Ferriman Gallwey score and women with a score >7 were considered hirsute.[3] Height and weight were recorded by the standard methods. Waist circumference was measured at the level of the umbilicus without clothing and in standing position. Hip circumference was measured at the level of ischial tuberosity. Subjects with known thyroid disorder (including those with subclinical hypothyroidism), hypothalamic, pituitary or adrenal disease, neoplastic disease, hepatic/ renal/cardiovascular disorders, and tuberculosis were not included for the study.
A set of blood investigations was performed for each subject which comprised complete blood count with ESR (erythrocyte sedimentation rate), fasting lipid profile, hormonal profile (day 2 or 3 of menstruation) including LH, FSH, total testosterone, prolactin, and TSH. Serum progesterone was measured on days 21–23 of menstrual cycle. Blood sugar values and serum insulin values were determined during a 75 g oral glucose tolerance test (GTT). Values of GTT were assessed according to ADA, 1997.[4] Presence of insulin resistance was defined by fasting or postload insulin levels >25 and >41 μU/ Ml, respectively[5,6] (DRG diagnostic instrument, GmbH, Germany). Hormonal estimation was done by chemiluminescence immunoassay. Lipid profile was estimated by using enzymatic colorimetric technique and criteria adopted were in consonance with NCEP-ATP III guidelines.[7] Deranged lipid profile was considered if any of the cholesterol, triglycerides, and HDL-cholesterol fractions were abnormal, the individual cutoffs taken were ≥200, ≥150, and <50 mg/ dL, respectively. Abnormal waist hip circumference was taken as ≥0.8.
Sonography of the pelvis with transvaginal probe (7 MHz) was done on day 2 of menstrual cycle or withdrawal bleed to evaluate day 2 endometrial thickness. Polycystic ovaries were defined as an ovary with 12 or more follicles measuring 2–9 mm in diameter and/or increased ovarian volume more than 10 cm3 along with stromal hyperplasia.
Data are presented as mean ± SD. The various clinical, metabolic, and hormonal characteristics were tabulated in the two groups and compared using unpaired, two-tailed Student's t-test. Significance was set at P < 0.05. All statistical analyses were performed by using MS Office 97 Excel.
RESULTS
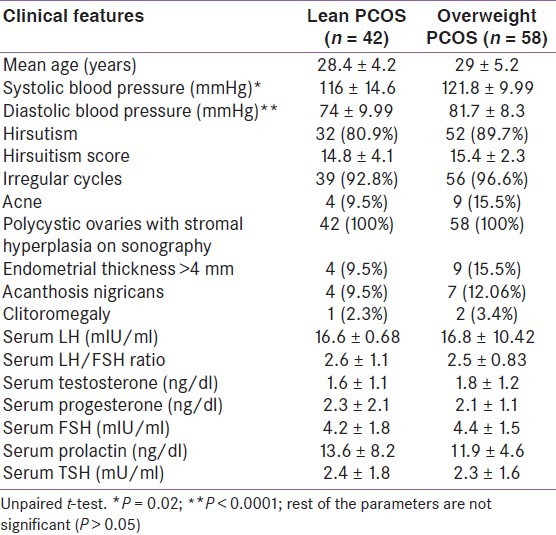
The study group had 58 subjects with BMI ≥23, i.e., 58% were overweight or obese PCOS with infertility, the rest had normal weight. In fact, 28% were obese with a BMI ≥27. The clinical features in both the groups are shown in Table 1. Average age was 28.4 ± 4.2 years in the lean PCOS group (n = 42) and 29 ± 5.2 years in the overweight PCOS group (n = 58). Presence of hirsutism, hirsutism score, irregular menstrual cycles, endometrial thickness of >4 mm, and acanthosis nigricans were comparable in both the groups. The hormonal profile was similar in both the groups, with serum LH, LH/FSH ratio and testosterone levels higher and serum progesterone levels lower denoting anovulation in both the groups. The average systolic as well as diastolic blood pressures were significantly higher in the overweight or obese subjects [Table 1].
Table 1.
Clinical and hormonal profi le in the lean and overweight polycystic ovarian syndrome subjects
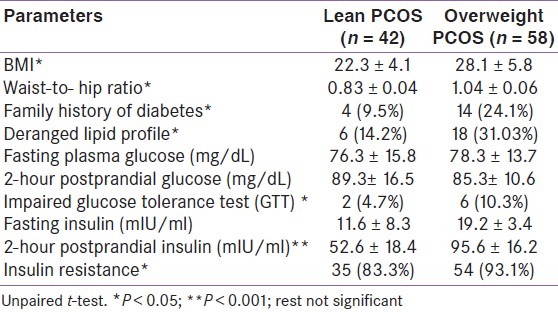
Metabolic parameters in both the groups are shown in Table 2. The BMI and waist-to-hip ratio were significantly higher in the overweight group denoting the prevalence of android obesity. Number of patients with impaired GTT and higher postprandial insulin were more in the overweight PCOS group. Family history of diabetes, deranged lipid profile, 2-hour postprandial insulin, and insulin resistance were significantly more in overweight patients. However, fasting and the postprandial glucose levels were comparable. Insulin resistance was present in a large number of the study subjects irrespective of whether they were lean or obese. 83.3% of lean subjects and 93.1% of overweight subjects had insulin resistance [Table 2].
Table 2.
Anthropometric and metabolic parameters in the two groups
DISCUSSION
Women with PCOS are generally overweight or obese; however, 42% in the present study had a normal BMI. This study outlines the fact that patients with PCOS, irrespective of their weight, demonstrate clinical manifestations such as irregular cycles, acne, hirsutism; hormonal abnormalities such as higher LH, LH/FSH, and testosterone levels. Metabolic disturbances, viz. impaired GTT, abnormal lipid profile, higher fasting/2-hour insulin levels, and higher insulin resistance, are also witnessed in the PCOS patients, but were markedly higher in PCOS with elevated BMI. Similar findings have been observed in previous studies.[5,6,8]
This study indicates that irrespective of weight, PCOS patients (lean as well as overweight or obese), are inherently insulin resistant with compensatory hyperinsulinemia and this plays a central role in the pathogenesis of PCOS.[9–11] Hyperinsulinemia probably acts at the level of hypothalamic–pituitary axis and stimulates LH secretion which leads to anovulation with irregular cycles. In the liver, it decreases production of sex hormone-binding protein and IGF-1-binding protein which results in an increase in free androgen in the blood and an increase in free IGF-1 in the ovary. Within the ovary, it promotes formation of androstenedione and testosterone which is seen clinically as hirsutism and acne. Increased androgens are converted to estrones and are responsible for causing endometrial hyperplasia and potentiates LH secretion.
Insulin resistance and hyperinsulinemia are presently considered risk factors in the development of atherosclerosis raising serum triglycerides and lowering the level of high-density lipoprotein cholesterol, and this may play an etiological role in the development of hypertension.[12,13] It also elevates the level of plasminogen activator inhibitor, the major inhibitor of fibrinolysis, which is an independent risk factor for atherosclerosis. Hyperinsulinemia increases the risk of cardiovascular diseases. Thus, the metabolic disorders associated with PCOS along with their long-term sequelae have to be closely evaluated in both lean and obese PCOS patients.[13]
Presence of obesity makes these patients susceptible to deranged lipid profile, impaired GTT and a greater degree of insulin resistance. Recently, obesity has been reported to be a significant predictor for development of metabolic syndrome within the PCOS subjects.[14] Two-hour postprandial insulin levels and insulin resistance and acanthosis nigricans (marker of insulin resistance) were much higher in the overweight group as compared to the lean PCOS patients in this study. Family history of diabetes was also more common in overweight as compared to lean, and this may indicate a genetic predisposition. Glucose tolerance test is considered better than simply the fasting and postprandial values in the evaluation of PCOS patients.[6]
To conclude, insulin resistance is inherent to PCOS, irrespective of whether the subject is lean or obese. Metabolic abnormalities are more frequently observed when obesity is associated in PCOS. It is suggested that all PCOS subjects should be considered at risk of atherosclerosis and its manifestations in view of the insulin resistant state; periodic monitoring of blood pressure, lipids, and an annual OGTT specially for obese PCOS patients should be performed.
Footnotes
Source of Support: The work has been partially funded from a grant received from the Department of Science and Technology (DST)
Conflict of Interest: No.
REFERENCES
- 1.The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 2.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2002;360:235. doi: 10.1016/S0140-6736(02)09512-0. [DOI] [PubMed] [Google Scholar]
- 3.Falsetti L, Gambera A, Andrico S, Sartori E. Acne and hirsutism in polycystic ovary syndrome: Clinical, endocrine-metabolic and ultrasonographic differences. Gynecol Endocrinol. 2002;16:275–84. doi: 10.1080/gye.16.4.275.284. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 5.Saxena P, Prakash A, Nigam A. Effect of Metformin therapy on 2-hour post-glucose insulin levels in patients of PCOS. J Hum Reprod Sci. 2010;3:139–42. doi: 10.4103/0974-1208.74156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxena P, Prakash A, Nigam A. Efficacy of 2-hour post glucose insulin levels in predicting insulin resistance in polycystic ovarian syndrome. J Hum Reprod Sci. 2011;4:20–2. doi: 10.4103/0974-1208.82355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong PC, Kong AP, So WY, Yang X, Ho CS, Ma RC, et al. The usefulness of the International Diabetes Federation and the National Cholesterol Education Program's Adult Treatment Panel III (NCEP ATP III) definitions of the metabolic syndrome in predicting coronary heart disease in subjects with type 2 diabetes. Diabetes Care. 2007;30:1206–11. doi: 10.2337/dc06-1484. [DOI] [PubMed] [Google Scholar]
- 8.Fux Otta C, Wior M, Iraci GS. Clinical, metabolic, and endocrine parameters in response to metformin and lifestyle intervention in women with polycystic ovary syndrome: A randomized, double-blind, and placebo control trial. Gynecol Endocrinol. 2009;8:1–6. doi: 10.3109/09513590903215581. [DOI] [PubMed] [Google Scholar]
- 9.Bhathena RK. Insulin resistance and the long-term consequences of polycystic ovary syndrome. J Obstet Gynaecol. 2011;31:105–10. doi: 10.3109/01443615.2010.539722. [DOI] [PubMed] [Google Scholar]
- 10.Ciampelli M, Fulghesu AM, Cucinelli F, Pavone V, Ronsisvalle E, Guido M, et al. Impact of insulin and body mass index on metabolic and endocrine variables in polycystic ovary syndrome. Metabolism. 1999;48:167–72. doi: 10.1016/s0026-0495(99)90028-8. [DOI] [PubMed] [Google Scholar]
- 11.Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Ciampelli M, Fulghesu AM, Lanzone A. Comment on “Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome”. J Clin Endocrinol Metab. 1999;84:2974–5. doi: 10.1210/jcem.84.8.5939-3. [DOI] [PubMed] [Google Scholar]
- 13.Elting MW, Korsen TJ, Bezemer PD, Schoemaker J. Prevalence of diabetes mellitus, hypertension and cardiac complaints in a follow-up study of a Dutch PCOS population. Hum Reprod. 2001;16:556–60. doi: 10.1093/humrep/16.3.556. [DOI] [PubMed] [Google Scholar]
- 14.Wijeyaratne CN, Seneviratne Rde A, Dahanayake S, Kumarapeli V, Palipane E, Kuruppu N, et al. Phenotype and metabolic profile of South Asian women with polycystic ovary syndrome (PCOS): Results of a large database from a specialist Endocrine Clinic. Hum Reprod. 2011;26:202–13. doi: 10.1093/humrep/deq310. [DOI] [PubMed] [Google Scholar]


