Abstract
DESIGN
We conducted a retrospective cohort study to examine the role played by length of hospital stay in the risk of healthcare-associated bloodstream infection (BSI), independent of demographic and clinical risk factors for BSI.
PATIENTS
We employed data from 113,893 admissions from inpatients discharged between 2006 and 2008.
SETTING
Large tertiary healthcare center in New York City.
METHODS
We estimated the crude and adjusted hazard of BSI by conducting logistic regression using a person-day data structure. The covariates included in the fully adjusted model included age, sex, Charlson score of comorbidity, renal failure, and malignancy as static variables and central venous catheterization, mechanical ventilation, and intensive care unit stay as time-varying variables.
RESULTS
In the crude model, we observed a nonlinear increasing hazard of BSI with increasing hospital stay. This trend was reduced to a constant hazard when fully adjusted for demographic and clinical risk factors for BSI.
CONCLUSION
The association between longer length of hospital stay and increased risk of infection can largely be explained by the increased duration of stay among those who have underlying morbidity and require invasive procedures. We should take caution in attributing the association between length of stay and BSI to a direct negative impact of the healthcare environment.
Hospitalized patients face a higher risk of infections,1 and those who have longer lengths of stay are more likely to develop healthcare-associated infections.2–4 The conventional view is that longer stays result in greater risk because the hospital environment itself harbors infectious agents,5 in which case minimizing length of stay would reduce the risk of infection. An alternative hypothesis is that patients who stay longer in hospitals are at greater risk because underlying conditions2,6,7 and increased use of invasive procedures induce a longer stay, and these factors increase the risk of infection.1,8,9 In this context, minimizing length of stay would have little impact on the daily probability of infection.
The actual mechanism of association likely involves a combination of environmental and clinical factors; however, previous studies have not carefully examined the biological, clinical, and temporal contributions to the association between length of stay and healthcare-associated infections. Studies that have modeled length of stay and other biological or clinical variables have reported that length of stay is an independent risk factor.3,4,10–12 However, they did not statistically account for the time-varying nature of factors such as central venous catheterization, which increases the risk of infection and lengthens hospital stay. Clarifying the role played by length of stay in infection risk is important for informing healthcare policies, as shortening length of stay may not meet the intended effect of reducing one’s risk of infection.
The aim of this study is to explore the relationship between length of hospital stay and risk of healthcare-associated infections conditional on patient and therapeutic factors.
METHODS
Study Setting and Data
This was a retrospective cohort study employing admission data from 190,457 medical records for patients seen at a large healthcare system in New York City between January 1, 2006, and December 31, 2008. We extracted data from the clinical data warehouse, which integrates information from more than 20 electronic sources; the admission, discharge, and transfer system; and the computerized physician and nursing order-entry system. From this compilation of data, we included demographic data on age and sex; International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnoses and procedure codes on medical conditions, including renal failure and malignancy; and time-stamped summaries on administration of invasive procedures, including ventilation and central venous catheterization.13 We also computed a composite score of illness (Charlson score) based on ICD-9-CM codes for conditions present at admission.14
Bloodstream Infections (BSIs)
We used the standard BSI definition as recommended by the Centers for Disease Control and Prevention’s National Healthcare Safety Network and modified the definition where clinical symptoms were indicated, as the data compiled did not include information on symptoms. We therefore defined BSIs as cases presenting with a blood culture positive for any bacterial pathogen and with no culture positive for the same organism at other body sites within 14 days prior. In the case of a common skin contaminant (eg, coagulase-negative staphylococci), we counted only those cases for which 2 or more blood cultures obtained on separate occasions were positive within 2 days of each other. Length of stay at risk in the hospital was defined as time from the day of admission to the day of culture collection for those with infection and as time to discharge or death for those who did not develop an infection. Admission records with a BSI within the first 2 days of admission or with shorter than 3 days of hospital stay were excluded, to minimize the probability that these infections were community acquired.
Statistical Analysis
We summarized the median and interquartile range of length of stay and tested for a difference in the risk of BSI by categorical variables using the Mantel-Haenszel χ2 test and for a difference in length of stay by risk factors using the Wilcoxon rank-sum test or the Kruskal-Wallis test.
We first modeled the risk of BSI by length of stay by employing logistic regression of admission-level data, to assess through nonlongitudinal means that length of stay was associated with BSI. This method is inappropriate for examining how length of stay affects BSI because it does not take into account how infection risk may change with time since admission, during which other risk factors for BSI vary. To solve this problem, we employed survival analysis to examine the risk of developing BSI on day x, conditional on not having developed an infection by day x − 1. We estimated the hazard of BSI and its 95% confidence interval at the midpoint of 1-day intervals by the life-table method and plotted the estimates by time. We also plotted the distribution of persons with renal failure and central venous catheterization by time to demonstrate the increasing concentration of patients who were sicker and required more procedures. Next, we modeled the hazard of BSI via a discrete-time hazard model by performing logistic regression with a person-day data structure accounting for within-admission correlation by the generalized estimating equation. We excluded person-time observations beyond 47 days (top 5% of person-time distribution) to exclude influential outliers. We examined the goodness of fit of different hazard functions by regressing the instantaneous probability of BSI by time, by first modeling a first-order time term only and then adding second- and third-order terms sequentially. When we examined the fit of different hazard functions, we found that both first- and second-order terms were significant in a quadratic hazard model (P < .0001 and P = .003, respectively). However, when we included a cubic term, the second- and third-order time terms were not significant (P = .30 and P = .58, respectively). Therefore, we excluded the third-order term from further analyses.
We performed multivariable analyses with sequential adjustment for (1) age and sex; (2) renal failure, malignancy, and Charlson score; (3) central venous catheterization and mechanical ventilation; and (4) intensive care unit (ICU) stay. Central venous catheterization, mechanical ventilation, and ICU stay were treated as time-varying factors. For each model we estimated the coefficient(s) for time and plotted the predicted hazard of BSI conditional on person-time-weighted covariate values. For example, we entered into the model an average “value” for renal failure by taking the sum of person-time experienced by those with renal failure and dividing it by the total number of person-time units in the data. Additionally, we computed the change in the estimated hazard of BSI at 5, 10, 25, and 40 days after admission between the unadjusted and fully adjusted models.
Statistical analyses were conducted in SAS, version 9.3 (SAS Institute), and STATA, version 10.0 (StataCorp).
RESULTS
The final data set included 113,893 hospital records after application of our exclusion criteria. The overall distribution of hospital stay was right-skewed, with a median of 5 days and an interquartile range of 4–8 days. The distributions of length of stay and BSI risk by each covariate value are summarized in Table 1. All variables, including age of 40 or more years, male sex, renal failure, malignancy, Charlson score, central venous catheterization, mechanical ventilation, and ICU stay, were associated with both longer length of stay and increased BSI risk. Nonsurvival analysis of length stay and BSI by logistic regression showed that a 1-day increase in length of stay was positively associated with BSI infection with an odds ratio of 1.025 (95% confidence interval, 1.023–1.027).
TABLE 1.
Distribution of Length of Stay and Bloodstream Infections (BSIs) by Covariate
| Covariate | No. | Length of stay, median (IQR) | P | BSI, no. (%) | P |
|---|---|---|---|---|---|
| Age category | <.0001 | .001 | |||
| <18 years | 20,421 | 5 (4–8) | 457 (2.2) | ||
| 18–39 years | 25,270 | 5 (4–7) | 237 (0.9) | ||
| 40–59 years | 25,270 | 6 (5–11) | 554 (2.2) | ||
| 60–79 years | 18,715 | 7 (5–11) | 680 (2.4) | ||
| ≥80 years | 14,217 | 7 (5–11) | 247 (1.7) | ||
| Sex | <.0001 | <.0001 | |||
| Male | 49,118 | 4 (4–9) | 1,233 (2.5) | ||
| Female | 64,775 | 7 (5–11) | 942 (1.5) | ||
| Renal failure | <.0001 | <.0001 | |||
| Yes | 18,642 | 8 (6–15) | 927 (5.0) | ||
| No | 95,251 | 6 (4–9) | 1,248 (1.3) | ||
| Malignancy | <.0001 | <.0001 | |||
| Yes | 12,390 | 7 (5–12) | 447 (3.6) | ||
| No | 101,503 | 6 (4–9) | 1,728 (1.7) | ||
| Charlson score | <.0001 | <.0001 | |||
| 0 | 56,707 | 5 (4–8) | 631 (1.1) | ||
| 1 | 20,487 | 6 (5–10) | 382 (1.9) | ||
| 2 | 14,675 | 7 (5–11) | 385 (2.6) | ||
| 3 | 22,024 | 8 (5–13) | 777 (3.5) | ||
| Intensive care unit | <.0001 | <.0001 | |||
| Yes | 17,944 | 11 (7–20) | 1,011 (5.6) | ||
| No | 95,949 | 5 (4–8) | 1,164 (1.2) | ||
| Central venous catheter | <.0001 | <.0001 | |||
| Yes | 11,801 | 13 (8–23) | 893 (7.6) | ||
| No | 102,092 | 6 (4–9) | 1,282 (1.3) | ||
| Mechanical ventilation | <.0001 | <.0001 | |||
| Yes | 5,503 | 17 (9–30) | 569 (10.3) | ||
| No | 108,390 | 6 (4–9) | 1,606 (1.5) |
NOTE. IQR, interquartile range.
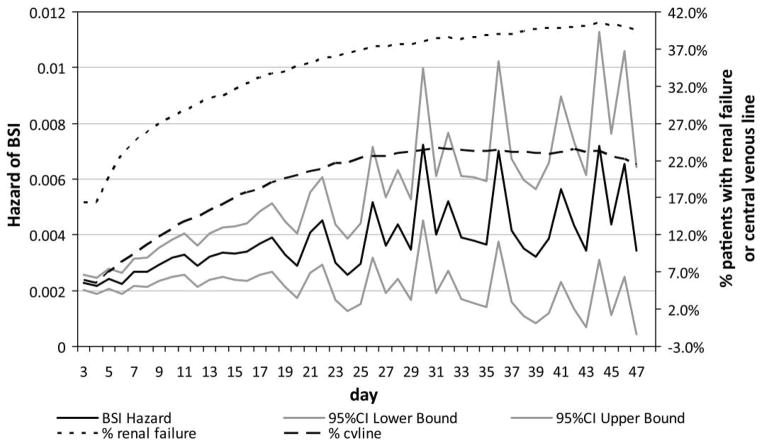
A plot of the hazard of BSI over time, as well as the changing distribution of people with renal failure and central venous catheterization, is illustrated in Figure 1. The hazard of BSI increased with time during the first 3 weeks, simultaneous to the increasing proportion of people with renal failure and central venous catheterization. The results shown in this figure and in Table 1 highlight that while subjects who stay in the hospital longer have an increasing hazard of infection, they are also more likely to be sicker and undergo invasive procedures that may independently increase infection risk.
FIGURE 1.
Plot of the unadjusted hazard of bloodstream infection (BSI) and the changing distribution of persons with renal failure or central venous catheter over time. CI, confidence interval.
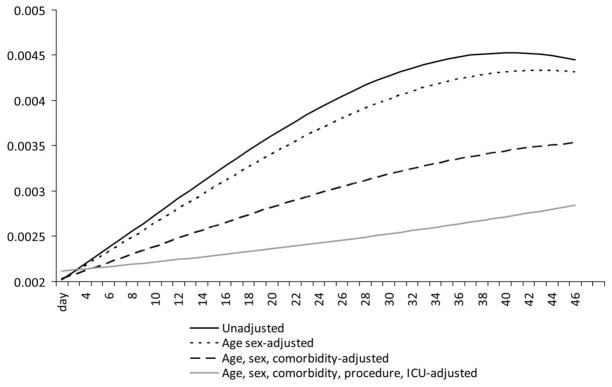
Consistent with Figure 1, we found that adjusting for known risk factors for BSI substantially attenuated the hazard of infection. The coefficients for both the linear and the quadratic terms for time were significant in an unadjusted model (Table 2) but decreased with subsequent adjustment for age, sex, comorbid factors, invasive procedures, and ICU stay. When we included all the covariates, the coefficients for both the first-order and the second-order time terms were not statistically significantly different from 0 (Table 2). Figure 2 plots the change in the predicted hazard of BSI given covariate values weighted by person-time contributed. The adjusted hazard of BSI is shifted lower as we sequentially adjust for other factors. In the fully adjusted model, although the hazard still increased slightly over time, this trend was not statistically significant. The magnitude of these differences are quite substantial: 5 days after admission the estimated hazard of BSI in the fully adjusted model was 2.6% lower than that of the unadjusted model, and at days 10, 25, and 40 the estimated probability was 17%, 38%, and 40% lower, respectively, than that of the unadjusted model.
TABLE 2.
Coefficient for Time and Hazard of Bloodstream Infection in Sequentially Adjusted Models
| Model | First-order time term, t | P | Second-order time term, t2 | P |
|---|---|---|---|---|
| Unadjusted | 0.0454 | <.0001 | −0.00055 | .003 |
| Adjusted for age and sex | 0.0400 | <.0001 | −0.00045 | .015 |
| Adjusted for age, sex, and comorbiditya | 0.0235 | .002 | −0.00023 | .024 |
| Adjusted for age, sex, comorbidity,a indwelling devices,b and ICU stay | 0.0055 | .48 | 0.00024 | .90 |
NOTE. ICU, intensive care unit.
Comorbidity includes renal failure, malignancy, and Charlson score.
Indwelling devices include mechanical ventilation and central venous catheterization.
FIGURE 2.
Plot of the hazard of bloodstream infection estimated from models with sequential adjustment for risk factors for bloodstream infection. ICU, intensive care unit.
DISCUSSION
In this analysis of 113,893 hospital records, we observed a nonlinear increase in the hazard of BSI that occurred simultaneous to the increasing proportion of sicker individuals who required invasive procedures. When we adjusted for demographic factors, comorbidity, and time-varying indwelling devices, we found that the estimated hazard was attenuated and not significantly different from a constant hazard, suggesting that the association between length of stay and BSI observed in conventional nonlongitudinal analysis could partially be explained by underlying morbidity, use of invasive procedures, and the greater period of observation among those who require longer stays. While our results downplay the role played by the hospital environment in infection risk, residual risk of BSI still remained. The magnitude of this residual risk, however, is difficult to determine in the absence of a control group without hospital exposure. Although not examined in our study, it would also be important to determine how the hazard of infection varies after discharge, which would allow us to investigate whether the relatively constant hazard of infection in the hospital remains stable outside the hospital setting or whether infection probability falls off. In addition, our study did not consider competing risks due to death and discharge, which are not independent of risk of infection.15
Although we controlled for numerous potential risk factors, our study was limited by potential unmeasured confounders that may be associated with both length of stay and BSI, such as time-varying conditions unaccounted for by catheter administration and ICU stay. Since we found that the risk of infection as related to length of stay is significantly diminished when controlling for the measured confounding variables, it is plausible that controlling for additional unmeasured confounders would have reinforced the null association we found. Furthermore, use of an electronic algorithm to define infections may have misclassified symptomatic infections for which cultures were not obtained. However, the use of a laboratory-based definition allowed for specific identification of a bacterial infection and likely reduced bias due to an assessor’s subjective judgment.16 Nevertheless, our inability to record clinical signs and symptoms of infection was a limitation of the study.
Despite these limitations, we were able to explore the role played by length of stay as an independent risk factor for BSI in more detail than previous studies, describing the hazard of infection while also paying attention to time-varying risk factors. This study was also well powered to detect a nonlinear hazard of infection in the crude analysis.
In conclusion, we observed a nonlinear increase in the hazard of BSI in the healthcare setting, which was attenuated to a constant hazard of BSI after adjusting for risk factors for BSI. Hence, the association between longer length of hospital stay and increased risk of infection can largely be explained by the increased duration of stay among those who had underlying morbidity and those who required invasive procedures. We should take caution in attributing the association between length of stay and BSI to a direct negative impact of the healthcare environment.
Acknowledgments
Financial support. This work was supported by grant R01 NR010822 (“Distribution of the Costs of Antimicrobial-Resistant Infections”) from the National Institute of Nursing Research, National Institutes of Health.
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article. All authors submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and the conflicts that the editors consider relevant to this article are disclosed here.
References
- 1.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6(4):428–442. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman J, McGowan JE., Jr Methodologic issues in hospital epidemiology. III. Investigating the modifying effects of time and severity of underlying illness on estimates of cost of nosocomial infection. Rev Infect Dis. 1984;6(3):285–300. doi: 10.1093/clinids/6.3.285. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen-Van-Tam SE, Nguyen-Van-Tam JS, Myint S, Pearson JC. Risk factors for hospital-acquired urinary tract infection in a large English teaching hospital: a case-control study. Infection. 1999;27(3):192–197. doi: 10.1007/BF02561527. [DOI] [PubMed] [Google Scholar]
- 4.Carr BG, Kaye AJ, Wiebe DJ, Gracias VH, Schwab CW, Reilly PM. Emergency department length of stay: a major risk factor for pneumonia in intubated blunt trauma patients. J Trauma. 2007;63(1):9–12. doi: 10.1097/TA.0b013e31805d8f6b. [DOI] [PubMed] [Google Scholar]
- 5.Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care–associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38(5 suppl 1):S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 6.Kramer AA, Zimmerman JE. A predictive model for the early identification of patients at risk for a prolonged intensive care unit length of stay. BMC Med Inform Decis Mak. 2010;10:27. doi: 10.1186/1472-6947-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins TL, McGee WT, Steingrub JS, Rapoport J, Lemeshow S, Teres D. Early indicators of prolonged intensive care unit stay: impact of illness severity, physician staffing, and pre-intensive care unit length of stay. Crit Care Med. 2003;31(1):45–51. doi: 10.1097/00003246-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Vilins M, Blecher S, Silva MA, Rosenthal VD, Barker K, Salomao R. Rate and time to develop first central line–associated bloodstream infections when comparing open and closed infusion containers in a Brazilian hospital. Braz J Infect Dis. 2009;13(5):335–340. doi: 10.1590/S1413-86702009000500004. [DOI] [PubMed] [Google Scholar]
- 9.Engelhart ST, Hanses-Derendorf L, Exner M, Kramer MH. Prospective surveillance for healthcare-associated infections in German nursing home residents. J Hosp Infect. 2005;60(1):46–50. doi: 10.1016/j.jhin.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 10.El-Masri MM, Hammad TA, McLeskey SW, Joshi M, Korniewicz DM. Predictors of nosocomial bloodstream infections among critically ill adult trauma patients. Infect Control Hosp Epidemiol. 2004;25(8):656–663. doi: 10.1086/502457. [DOI] [PubMed] [Google Scholar]
- 11.Wang FD, Chen YY, Chen TL, Lin YT, Fung CP. Risk factors and mortality of nosocomial infections of methicillin-resistant Staphylococcus aureus in an intensive care unit. J Crit Care. 2011;26(1):82–88. doi: 10.1016/j.jcrc.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Graves N, Tong E, Morton AP, et al. Factors associated with health care–acquired urinary tract infection. Am J Infect Control. 2007;35(6):387–392. doi: 10.1016/j.ajic.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Apte M, Neidell M, Furuya EY, Caplan D, Glied S, Larson E. Using electronically available inpatient hospital data for research. Clin Transl Sci. 2011;4(5):338–345. doi: 10.1111/j.1752-8062.2011.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. 2009;7(4):357–363. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnett AG, Batra R, Graves N, Edgeworth J, Robotham J, Cooper B. Using a longitudinal model to estimate the effect of methicillin-resistant Staphylococcus aureus infection on length of stay in an intensive care unit. Am J Epidemiol. 2009;170(9):1186–1194. doi: 10.1093/aje/kwp249. [DOI] [PubMed] [Google Scholar]
- 16.Lin MY, Bonten MJ. The dilemma of assessment bias in infection control research. Clin Infect Dis. 2012;54(9):1342–1347. doi: 10.1093/cid/cis016. [DOI] [PMC free article] [PubMed] [Google Scholar]