Abstract
This unit presents a combinatorial library method that consists of the synthesis and screening of mixture-based synthetic combinatorial libraries of peptide molecules. The protocols employ peptide libraries to identify peptides recognized by mAbs and T cells. The first protocol uses a positional scanning peptide library made up of hexapeptides to identify antigenic determinants recognized by mAbs. The 120 mixtures in the hexapeptide library are tested for their inhibitory activity in a competitive ELISA. The second protocol uses a decapeptide library to identify T cell peptide ligands. The 200 mixtures of the decapeptide library are tested for their ability to induce T cell activation. Support protocols cover optimization of the assay conditions for each mAb or T cell, to achieve the best level of sensitivity and reproducibility, and preparation of a hexapeptide library, along with deconvolution approaches.
Synthetic combinatorial libraries have been found to be valuable tools for the study of protein-protein interactions. Of the many different combinatorial library methods developed, the one described in this unit consists of the synthesis and screening of mixture-based synthetic combinatorial libraries of peptide molecules. These libraries are systematically arranged into mixtures containing a total of hundreds of thousands to trillions of individual peptides. The understanding of B cell and T cell specificity has been advanced significantly by the development and use of libraries composed of millions of synthetic peptides. The use of peptide libraries has led to the identification of high-affinity ligands for both T cells and mAbs with known and unknown specificities. These peptides can result from the combinations of the amino acids that do not necessarily correspond to sequences in known proteins. In addition, the native ligand and potential cross-reactive sequences can be identified from protein databases using a biometrical analysis. The results from the screening of peptide libraries have identified relevant epitopes that can lead to the design of novel vaccines for infectious diseases and cancer, as well as target autoantigens involved in autoimmune diseases.
Basic Protocols 1 and 2 describe the use of peptide libraries to identify peptides recognized by mAbs and T cells. Basic Protocols 1 uses a positional scanning peptide library made up of hexapeptides to identify antigenic determinants recognized by mAbs. The 120 mixtures in the hexapeptide library are tested for their inhibitory activity in a competitive ELISA. Basic Protocol 2 uses a decapeptide library to identify T cell peptide ligands. The 200 mixtures of the decapeptide library are tested for their ability to induce T cell activation. The optimization of the assay conditions for each mAb (see Support Protocol 1) or T cell, to achieve the best level of sensitivity and reproducibility, is an important consideration for the appropriate and successful use of mixture-based libraries. Support Protocol 2 describes the preparation of a hexapeptide library, along with deconvolution approaches. This Support Protocol is specifically written for persons trained and experienced in solid-phase peptide chemistry. Therefore, it is advantageous to have access to a peptide synthesis facility or be associated with a peptide chemist.
BASIC PROTOCOL 1
SCREENING PEPTIDE LIBRARY FOR ANTIBODY INHIBITION
In this protocol, a positional scanning hexapeptide library (Table 9.5.1) is used to identify specific peptides that inhibit the binding of a MAb to its antigen. The peptide library consists of six positional libraries, each with one position defined by one of 20 natural amino acids and the remaining five positions as mixtures of 19 amino acids (cysteine excluded). Each peptide mixture of the library represents ~2.5 million (195) individual sequences; the entire library is made up of 120 peptide mixtures for a total of ~52 million different hexapeptides. A nonacetylated form of the library can also be used (see Critical Parameters).
Table 9.5.1.
Peptide Sequences for a Positional Scanning Hexapeptide Librarya
| Peptide mixture number | Peptide sequence |
|---|---|
| 1–20 | Ac-O1XXXXX-NH2 |
| 21–40 | Ac-XO2XXXX-NH2 |
| 41–60 | Ac-XXO3XXX-NH2 |
| 61–80 | Ac-XXXO4XX-NH2 |
| 81–100 | Ac-XXXXO5X-NH2 |
| 101–120 | Ac-XXXXXO6-NH2 |
The 120 peptide mixtures of a hexapeptide library are synthesized with specific amino acids at one of six positions in the peptide (O1, O2, O3, O4, O5, or O6) and five random amino acids (X) using the amino acid mixture described in Table 9.5.3. Ac, acetyl; see Table A.1B.1 for single-letter amino acid codes.
Materials
Peptide or protein antigen of interest
1% (w/v) BSA/PBS (see recipe for PBS)
Hexapeptide positional scanning library (Table 9.5.1): 120 peptide mixtures at 5 mg/ml (see Support Protocol 2; for information on the availability of these libraries, contact C. Pinilla at cpinilla@tpims.org)
Monoclonal antibody (MAb) against antigen of interest
Horseradish peroxidase (HRPO)-conjugated anti-mouse antibody specific for the isotype of the MAb (Calbiochem; use in accordance with manufacturer’s instructions)
Developing solution (see recipe)
4 N sulfuric acid
96-well microtiter plates made of high-binding-capacity polystyrene (e.g., A/2 area plates, Costar; or Immunolon 4 plates, Dynatech)
Moist chamber: sealed Tupperware box lined with wet paper towels
96-well microtiter plate reader with 492-nm filter
Additional reagents and equipment for optimizing antigen and antibody concentrations for screening assay (see Support Protocol 1) and peptide synthesis (see Support Protocol 2 and UNIT 9.1)
Prepare antigen-coated microtiter plates
-
1
Determine optimal antigen and MAb concentrations for use in the screening assay by direct and competitive ELISA (see Support Protocol 1) on antigen-antibody interaction.
This step is essential for successful screening results.If an ELISA protocol has been established for a particular antigen-antibody interaction, all conditions must be optimized so that the system is working in a sensitive range in order to use this pre-established protocol. -
2
Add 50 μl optimal-concentration peptide or protein antigen to each well of a 96-well microtiter plate to coat the wells. Incubate in a moist box 2 hr at 37°C or overnight (18 hr) at room temperature.
Three microtiter plates are routinely used to assay the 120 peptide mixtures of the peptide library in duplicate. -
3
Invert plate and shake off liquid. Wash the wells ten times with water. Remove residual water from wells by rapping the plate upside down over paper towels. Do not allow wells to dry completely.
A sprayer attached to a deionized water tap works well for washing the wells. -
4
Add 100 μl of 1% BSA/PBS to each well and incubate in a moist chamber 1 hr at 37°C to block nonspecific binding sites.
Perform competitive ELISA using hexapeptide library and MAb
-
5
Wash wells as described in step 3. Add 25 μl peptide mixture (1 to 5 mg/ml) or antigen control to appropriate wells, using the configuration shown in Figure 9.5.1. Add 25 μl optimal-concentration MAb to each well. Incubate in a moist chamber 18 hr at 4°C or 1 hr at 37°C.
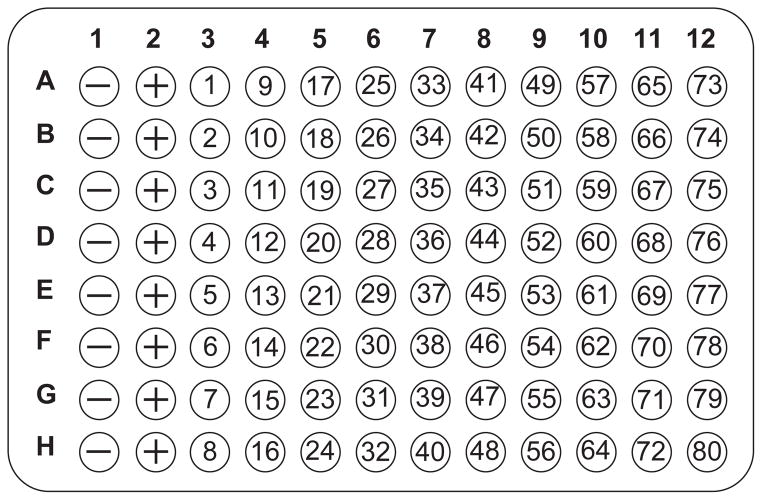
As shown in Figure 9.5.1, wells in the first column of each plate contain no inhibitor and serve as controls for 100% antibody binding to the antigen. Wells in the second column, a 2-fold serial dilution of antigen, serve as competitive controls to check the sensitivity of the assay. The remaining 80 wells are used to screen 80 peptide mixtures.To facilitate using a multichannel pipettor, pipet the peptide library into 1-ml polypropylene tubes and arrange tubes in vertical columns of eight. -
6
Wash wells as described in step 3. Add 50 μl HRPO-conjugated anti-mouse antibody to each well. Incubate in a moist chamber 1 hr at 37°C.
-
7
Wash wells as described in step 3. Add 50 μl developing solution to each well. Incubate in the dark 10 to 15 min at room temperature. Add 25 μl of 4 N sulfuric acid to each well to stop reaction.
Figure 9.5.1.
Layout of plate for the screening of 80 peptide mixtures by competitive ELISA. A 96-well microtiter plate is coated with antigen and incubated with antibody and peptide mixtures to test for inhibitory activity by the mixtures. Peptide mixtures are tested in duplicate on a second plate; wells containing antibody only (− ; column 1) and antibody plus the soluble antigen of interest (+; column 2) are included in each row.
Identify inhibitory peptide mixture(s)
-
8
Determine inhibitory activity of each peptide mixture by reading the A492 in a microtiter plate reader.
Inhibitory peptide mixtures will have a low A492. Alternatively, the % inhibition for each peptide mixture can be calculated relative to the control antigen in column 1 of each plate. -
9
Retest peptide mixtures with >30% to 50% inhibition by competitive ELISA (steps 1 to 8) except use four serial 2-fold dilutions of peptide mixture. For peptide mixtures with >50% inhibition, retest using eight serial 2-fold dilutions.
-
10
Determine concentrations of inhibitory peptide mixtures that give 50% antibody binding (IC50) and select the most active peptide mixture(s) that has the lowest IC50.
Carry out selection and synthesis
-
11
Select the most active peptide mixture(s) for each of the six amino acid positions. Based on the amino acids present in the fixed position of the selected peptide mixtures, synthesize individual peptides that correspond to all possible combinations of those amino acids.
If the sequence of the immunogen is known, the screening results can be used to quickly locate the antigenic determinant recognized by the antibody. Otherwise, this synthesis is carried out to confirm the screening results. -
12
Perform competitive ELISA of individual peptides to confirm screening of the peptide library and identify the specific antigenic determinant or high-affinity ligands.
BASIC PROTOCOL 2
SCREENING A PEPTIDE LIBRARY TO IDENTIFY CD4+ OR CD8+ T CELL LIGANDS
This protocol describes how peptides that activate CD4+ and CD8+ T cell clones can be identified from using a positional scanning peptide library. The extent of T cell activation can be assessed by commonly used methods such as: (1) proliferation assays that measure the incorporation of cell [3H]-thymidine into newly synthesized DNA; (2) [51Cr]- release or fluorescence-based (using Eu as a label) assays that measure cytotoxicity; and (3) quantitative assessments of cytokine production and release measured by standard sandwich ELISA. Positional scanning libraries have been used to identify peptide ligands for T cell clones of known and unknown specificities (see Background Information for specific references). It is assumed that the assay is optimized for each T cell clone (see Critical Parameters).
Materials
T cell clone (TCC)
Decapeptide positional scanning library (prepared in a manner similar to hexapeptide library in Support Protocol 2; for information on the availability of these libraries, contact C. Pinilla at cpinilla@tpims.org)
Antigen presenting cells (APC) and appropriate growth medium
[3H]-thymidine
Na2 51CrO4
ELISA kit to measure cytokine production, such as gamma-interferon or GM-CSF (PharMingen)
96-well U-bottom microtiter plates (Costar)
Microtiter plate reader
Cell harvester
Liquid scintillation counter for proliferation CD4+ assays
Gamma counter for cytotoxicity assays; alternatively a sensitive fluorometer for the detection of released fluorescence from labeled target cells
-
1
Grow TCC to the necessary number of cells.
Approximately 5 to 100 million cells are needed to screen a decapeptide library. -
2
Determine T cell activation in response to each of the 200 mixtures of the decapeptide library.
Five 96-well plates can be used for a decapeptide library, composed of 200 samples; prepare duplicates per sample. -
3
Add peptide mixtures (10 to 25 μl/well, 0.2 mg/ml final concentration; higher concentrations are not recommended due to cell toxicity problems) to each well of 96-well microtiter plates. Add appropriate number of APC and TCC cell suspension to each well (final volume 200 to 250 μl/well). See Table 9.5.2 and Critical Parameters for more information on number of cells used.
A multichannel pipette or automated liquid handler is recommended for dispensing the library to microtiter plates. -
4
Culture cells with decapeptide library for 24 to 72 hr at 37°C.
Alternatively, for the 51Cr-release cytotoxic assay, culture 4 hr at 37°C. -
5
Determine activity of peptide mixtures against TCC that is appropriate for the cell type.
CD4+ T cell proliferation: See UNIT 7.10, Support Protocol. Add 0.25 to 1 μCi [3H]-thymidine to each well during last 8 hr of incubation. Harvest cells and measure incorporated radioactivity by liquid scintillation counting.
51Cr-release assay for CD8+ T cells: See UNIT 3.11, Basic Protocol 2. Transfer 150 μl from each well into counting tubes or plates and assess percent radioactivity released to supernatant.
Cytokine production in CD4 + or CD8+ T cells: See UNIT 6.8, Basic Protocol. Transfer the supernatants to microtiter plates that contain bound anti-cytokine monoclonal antibody (an automated liquid handler or multichannel pipette for this step is advantageous). Develop ELISA as specified by kit.
-
6
Confirm results by repeating experiments.
The incorporated radioactivity or optical densities can vary between experiments, however, the rank order of most active peptide mixtures should be fairly consistent between assays. See Critical Parameters for discussion of data analysis. -
7
Design and identify peptides based on library screening results by combining the defined amino acids of the most active mixtures at each position of the library.
These peptides do not necessarily correspond to sequences in known proteins. -
8
Using a biometrical analysis, the data from the library is systematically compared with the peptide sequence in protein databases to identify possible natural and potential cross-reactive ligands (see Background Information).
-
9
Synthesize individual peptides deduced from library results and database searches. Determine activity of individual peptides.
Table 9.5.2.
Examples of Conditions Used in T Cell Activation Assays with Positional Scanning Libraries
| Assay | Clone | No. of cells/well
|
APC:TCC RATIO | APC | Timea | Reference | |
|---|---|---|---|---|---|---|---|
| TCC | APC | ||||||
| Thymidine incorporation | Human/CD4+ | 2.5 × 105 | 1 × 105 | 4 | PBMC | 48 hr | Hemmer et al., 2000 |
| Human/CD4+ | 2 × 104 | 5 × 104 | 2.5 | PBMC | 48 hr | Hemmer et al., 1999 | |
| Human/CD4+ | 2 × 104 | 1 × 105 | 5 | PBMC | 48 hr | Sospedra et al, 2006 | |
| Human/CD4+ | 2 × 104 | 5 × 104 | 2.5 | BLS | 48 hr | Sospedra et al, 2006 | |
| Human/CD4+ | 3 × 104 | 1 × 105 | 3.3 | PBMC | 72 hr | Venturini et al, 2006 | |
| Mouse/CD4+b | 3 × 105c | N/Ad | N/Ad | N/Ad | 48 hr | Wilson et al., 1999; Judkowski et al., 2001 | |
| Chromium release | Human/CD8+ | 1 × 104 | 1 × 103 | 0.1 | T2 line | 4 hr | Pinilla et al., 2001 |
| Human/CD8+ | 1 × 104 | 1 × 103 | 0.1 | T2 line | 4 hr | Rubio-Godoy et al, 2002a | |
| Human/CD8+ | 1 × 104 | 1 × 103 | 0.1 | T2 line | 4 hr | Rubio-Godoy et al, 2002c | |
| Human/CD8+ | 2 × 103 | 1 × 104 | 5 | T2 line | 4 hr | Boggiano et al, 2005 | |
| Gamma interferon production | Mouse/CD8+ | 3 × 104 | 1 × 104 | 0.3 | T2-A2.1/Kb | 20 hr | Lustgarten et al, 2006 |
| GM-CSF production | Human/CD4+ | 2.5 × 104 | 5 × 104 | 2 | LCL | 48 hr | Judkowski et al, 2011 |
Time of incubation with the mixtures from positional scanning library.
TCR-transgenic mice-CD4+.
Whole spleen cells.
Not applicable.
SUPPORT PROTOCOL 1
OPTIMIZING ANTIGEN AND ANTIBODY CONCENTRATIONS FOR SCREENING ASSAY
This protocol describes optimization of the assay conditions for screening a synthetic peptide combinatorial library. A 2-fold serial dilution of peptide or protein antigen is titered against a 2-fold dilution series of MAb by ELISA to determine the optimal concentrations of both reagents. The optimal concentration of antibody is further defined by a competitive ELISA using soluble peptide or protein antigen as a competitive inhibitor. See UNIT 2.1 for a complete discussion of ELISA techniques.
Materials
Peptide or protein antigen—one sample dissolved in PBS and one in bicarbonate buffer
PBS (see recipe)
Bicarbonate buffer (see recipe)
1% (w/v) BSA/PBS (see recipe for PBS)
MAb specific for peptide or protein antigen
Horseradish peroxidase (HRPO)-conjugated secondary antibody specific for isotype of MAb (Calbiochem), diluted according to supplier’s instructions in 1% BSA/PBS
Developing solution (see recipe)
4 N sulfuric acid
96-well microtiter plate made of high-binding-capacity polystyrene (e.g., A/2, Costar; or Immunolon 4, Dynatech)
Moist box: sealed Tupperware box lined with wet paper towels
Microtiter plate reader with 492-nm filter
Titer antigen and antibody by direct ELISA
-
1
Place 50 μl peptide or protein antigen dissolved in PBS in a well in the first column of a 96-well microtiter plate. Repeat for antigen dissolved in bicarbonate buffer. Perform 2-fold serial dilutions of the antigen in the appropriate buffers in duplicate across the plate. Incubate in a moist box 2 hr at 37°C or 18 hr (overnight) at room temperature.
For standard 96-well plate, use 100 μl/well; for a A/2 area 96-well plate, use 50 μl/well. Include controls for nonspecific binding (UNIT 2.1).. Use 100 to 500 ng/ml initial concentration for synthetic peptides 10 to 20 residues in length and ≤25 ng/ml for peptides >20 residues. Peptides <10 residues in length may need to be coupled to a larger protein (UNIT 9.4) in order to carry out ELISA. For protein antigens, use 1 to 10 μg/ml.Results with PBS and bicarbonate buffers should be compared to determine which gives better results.Always incubate plates in moist box to prevent evaporation of reagents. -
2
Shake liquid from wells and wash wells ten times with water. Remove residual water from wells by rapping plates upside down over paper towels. Do not allow wells to dry completely.
-
3
Add 100 μl of 1% BSA/PBS to each well to block plates for nonspecific binding. Incubate plate in moist chamber 1 hr at 37°C.
-
4
Wash wells as described in step 2. Add 50 μl MAb (1 μg/ml) to each well and perform 2-fold serial dilutions in 1% BSA/PBS down the plate. Incubate plate in moist box overnight at 4°C.
For most antigen-antibody combinations examined, assay sensitivity is improved when this incubation is carried out overnight at 4°C. However, incubating 1 hr at 37°C may give reasonable results in some cases. -
5
Wash wells as described in step 2. Add 50 μl HRPO-conjugated secondary antibody per well. Incubate 1 hr at 37°C.
For monoclonal antibodies that are IgG specific, use HRPO-conjugated anti-mouse antibody that is both light- and heavy-chain-specific for IgG. For monoclonal antibodies of a different isotype, use the appropriate secondary antibody. -
6
Wash wells as described in step 2. Add 50 μl developing solution to each well and develop in the dark 10 to 15 min at room temperature. Add 25 μl of 4 N sulfuric acid to each well to terminate the reaction.
-
7
Read A492 on a microtiter plate reader.
-
8
Identify the concentrations of antibody and antigen that give the optimum results—i.e., the lowest antigen and antibody concentrations that yield A492 = 1.5 to 2.0.
Determine optimal antibody concentration by competitive ELISA
-
9
Coat wells of a 96-well microtiter plate with 50 μl peptide or protein antigen diluted in PBS or bicarbonate buffer to the concentration determined in step 8. Incubate in moist box overnight (18 hr) at 25°C or 2 hr at 37°C.
-
10
Wash wells as described in step 2. Add 100 μl of 1% BSA/PBS to block nonspecific binding. Incubate in moist box 1 hr at 37°C.
-
11
Wash wells as described in step 2. Add 25 μl of 1% BSA/PBS to each well. Add 25 μl per well of control antigen (containing same amount of control antigen coating the plate) to wells in columns 1 to 3 of the top row. Repeat with 25 μl per well control antigen at 100× and 1000× for wells of the top row in columns 4 to 6 and 7 to 9, respectively. Perform 2-fold serial dilutions down the plate.
-
12
Add 25 μl MAb at a dilution to give final optimal concentration (determined in step 8) to each well of columns 1, 4, 7, and 10. Repeat using antibody at concentrations 2× optimal in columns 2, 5, 8, and 11 and 0.5× optimal in columns 3, 6, 9, and 12. Incubate in moist box 18 hr at 4°C.
Columns 10 to 12 contain no inhibitor and represent 100% antibody binding. For the best sensitivity in competitive ELISA, use the lowest antibody concentration that yields A492 = 1.5 to 2.0. -
13
Wash wells as described in step 2. Add 50 μl HRPO-conjugated secondary antibody. Incubate in moist box 1 hr at 37°C.
-
14
Develop and read plates as in steps 6 and 7.
-
15
Determine the concentration of antigen that gives 50% antibody binding (IC50) for the three antibody concentrations. Choose the antibody concentration that gives the lowest IC50 with a signal-to-noise ratio ≥10:1. Use these conditions to screen the peptide library (see Basic Protocols 1 and 2).
SUPPORT PROTOCOL 2
PREPARING A POSITIONAL SCANNING PEPTIDE LIBRARY
Peptide mixtures making up either the nonacetylated or the acetylated hexapeptide positional scanning library (Table 9.5.1) are prepared using the chemical mixture approach (i.e., coupling with a mixture of amino acids in a specific ratio; Table 9.5.3) in conjunction with simultaneous multiple peptide synthesis (SMPS; Houghten, 1985) using methylbenzhydrylamine (MBHA) polystyrene resin and t-Boc chemistries (UNIT 9.1). A complete hexapeptide library consists of six positional libraries, each of which has a defined amino acid composition at one position of the peptide and a random composition at the other five positions—120 peptide mixtures containing a total of ~50 million different hexapeptides. Hexapeptide libraries are particularly suited for determining antigenic determinants for MAbs raised against peptides and linear sequences in proteins. HPLC analysis is used to identify the approximately equimolar concentration of each amino acid using the physically divided, coupled, and recombined mixture resins as controls. This protocol is designed for a person trained and experienced in peptide chemistry (Stewart and Young, 1984; Atherton and Sheppard, 1989).
Table 9.5.3.
Composition of Mixture for Chemical Ratio Synthesis
| Code | Amino acid | Ratioa |
|---|---|---|
| A | Alanine | 0.95 |
| D | Aspartate | 0.90 |
| E | Glutamate | 0.95 |
| F | Phenylalanine | 0.81 |
| G | Glycine | 1.00 |
| H | Histidine | 0.85 |
| I | Isoleucine | 1.16 |
| K | Lysine | 1.05 |
| L | Leucine | 1.08 |
| M | Methionine | 0.89 |
| N | Asparagine | 1.20 |
| P | Proline | 0.96 |
| Q | Glutamine | 1.20 |
| R | Arginine | 1.42 |
| S | Serine | 1.30 |
| T | Threonine | 1.60 |
| V | Valine | 1.14 |
| W | Tryptophan | 0.89 |
| Y | Tyrosine | 1.26 |
Ratio of each amino acid derivative necessary for approximately equimolar coupling when using a 6-fold excess of amino
Materials
Methylbenzhydrylamine (MBHA) polystyrene resin (Peninsula)
N-α-t-Boc-amino acids with side-chain protecting groups
Polypropylene mesh pockets (Synthesis Division)
Additional reagents and equipment for peptide synthesis (UNITS 9.1 & 9.6) and ninhydrin test (UNIT 9.6)
-
1
Number 120 polypropylene mesh packets and add 400 mg MBHA polystyrene resin to each.
-
2
Use SMPS methodology to synthesize peptides. At the first coupling step, couple the 20 amino acids individually to bags 101 to 120 and 19-amino-acid mixture as described in Table 9.5.1 to bags 1 to 100.
Resin in bags 101 to 120 has a defined amino acid at position 6 (see Table 9.5.1). Resin in the other bags has a mixture of amino acids in a ratio that is known to yield approximately equimolar distribution (Ostresh et al., 1994; see Table 9.5.3).The ninhydrin test described in UNIT 9.6, Kaiser’s ninhydrin test (Kaiser et al., 1970), Gisin’s picric acid procedure (Gisin, 1972), or Lebl’s bromphenol blue procedure (Krchnak et al., 1989) can be used to monitor the reaction. -
3
At the second step, couple 20 amino acids individually to resin in bags 81 to 100. Couple 19-amino-acid mixture to resin in bags 1 to 80 and 101 to 120.
Resin in bags 81 to 100 has a defined amino acid at position 5 (see Table 9.5.1). -
4
Repeat in this manner through the sixth coupling.
-
5
Acetylate the N-terminal of the peptide mixtures, if desired.
-
6
Deprotect and cleave the peptide mixture from the resin using the low-high hydrogen fluoride method (Tam et al., 1983; Houghten et al., 1986). Extract the peptide mixtures from the resin using 95% acetic acid and lyophilize 3 times, reconstituting powders in acetic acid and a final time in 50% acetonitrile in water. Weigh out each mixture and add water to make aliquots of 5 mg/ml.
Sonication is often used to solubilize peptide mixtures containing hydrophobic amino acids (phenylalanine, isoleucine, leucine, and tryptophan) in the defined positions. Maintain sonicator water bath temperature <10°C.
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see APPENDIX 2; for suppliers, see APPENDIX 5.
Bicarbonate buffer, pH 9.6
5.6 g NaHCO3 (67 mM final)
3.5 g Na2CO3 (33 mM final)
900 ml H2O
Adjust pH to 9.6 with 1 M NaOH
Add H2O to 1 liter
Store 1 to 2 months at room temperature
Developing solution
Dissolve one 10-mg tablet o-phenylenediamine·2HCl (Sigma) in 6 ml water (0.33 M final). Add 25 μl of 3% hydrogen peroxide (0.012% final). Prepare fresh.
Phosphate-buffered saline (PBS)
0.78 g NaH2PO4 (7 mM final)
3.8 g Na2HPO4 (27 mM final)
8.5 g NaCl (145 mM final)
Add 900 ml H2O.
Adjust pH to 7.0 with 1 M NaOH.
Add H2O to 1 liter.
Store 1 to 2 months at 4°C.
COMMENTARY
Background Information
Synthetic peptide combinatorial libraries have been found to be valuable tools for the study of protein-protein interactions. The development of such libraries is an extension of parallel synthetic approaches presented in the 1980s. The methods used to synthesize these libraries include parallel arrays, one-bead one-compound, and mixture-based approaches. The protocol presented here for the synthesis of a positional scanning hexapeptide library (see Support Protocol 2) is based on the latter approach. Instead of testing individual peptide analogs, millions of peptides can be tested in a single assay in order to define the specificity of a mAb or T cell receptor. Of the various possible peptide library approaches, synthetic combinatorial libraries offer an advantage of working with free peptides in solution, which allows the testing of libraries at various peptide concentrations to accommodate a particular assay system. Also, since they are not attached to a solid support or displayed on phage, synthetic combinatorial libraries can be used in any established assay. Reviews of the synthesis and use of mixture-based libraries for the identification of novel compounds have been presented (Houghten et al., 1999; Pinilla et al., 2003; Houghten et al., 2008). These reviews also present a discussion on the practical aspects relevant to the screening and deconvolution of mixture-based libraries. Furthermore, reviews on studies using synthetic peptide combinatorial libraries to explore immunological specificity have also been reported (Pinilla et al., 1999; Borras et al., 2002; Nino-Vasquez et al., 2004).
The deconvolution of positional scanning libraries for the identification of individual peptides is straightforward. The screening results should be analyzed by rank ordering each position by activity. In most cases, there will be clear differences in activity between active and inactive mixtures. Since the combination of amino acids identified from the active mixtures leads to active peptides, it is important to be very selective with respect to how many amino acids are chosen for each position. For example, if there were two active mixtures at each position of the hexapeptide library, then one would synthesize 64 (26) individual sequences. These peptides are tested to identify the most active sequences.
The deconvolution of decapeptide positional scanning libraries screened against T cells follows the same approach. However, with more positions the number of individual peptides to synthesize from the combination of active mixtures becomes very large (often >100). In this case, certain amino acids that share chemical characteristics with each other at a defined position can be narrowed down to one. For example, most T cell screens yield expected activity at known MHC anchor positions, and amino acids such as valine, alanine, isoleucine, and leucine often have similar activities at a given position. This redundancy can be used to minimize the number of individual peptides that need to be made.
A complementary strategy, termed biometrical analysis, has been developed to systematically compare the results obtained from screening a positional scanning peptide library composed of millions to trillions of sequences with the millions of sequences within protein databases. This approach is based on the assumption that each amino acid in a peptide epitope or ligand in a protein/protein interaction contributes independently and additively to recognition, or, in general terms, strength of interaction. Hence, the stimulatory value of each amino acid in a given position can be added to that of the amino acid in the next position throughout the length of the peptide. Consequently, a stimulatory score can be calculated for each peptide. A scoring matrix is generated by transforming the screening data of each of the 20 amino acids defined in each position of the library. Individual peptides are given a score calculated by adding the individual activities of the amino acids for the length of the library. This matrix is then used to score all the overlapping peptides of a given length in the protein databases and thus identify the sequences with the highest score. From this list a number of sequences with the highest scores can be synthesized and tested to confirm the activities (Hemmer et al., 1999; Zhao et al., 2001a, 2001b). The authors have used both the traditional deconvolution and the biometrical analysis for T cell clones of known and unknown specificities. When T cell ligands are known, they are found within the set of peptides with the highest scores (Pinilla et al., 2001; Zhao et al., 2001). Table 9.5.4. provides a short summary of T cell specificity studies using positional scanning libraries. It can be seen that successful studies have been reported for human and mouse CD4+ and CD8+ cells.
Table 9.5.4.
T Cell Specificity Studies using Positional Scanning Libraries
| Organism | Tcc | Research Area | Antigen | Method a | Reference |
|---|---|---|---|---|---|
| CD4 | |||||
| Human | CSF-3 | Infectious diseases | Unknown | Biometrical | Hemmer et al., 1999 |
| Human | TL3A6 | Autoimmunity | MBP 87-99 | Traditional Biometrical |
Hemmer et al., 2000 Zhao et al, 2001a |
| Human | MN b | Autoimmunity | Unknown | Biometrical | Sospedra et al, 2005 |
| Human | GP5F11 | Infectious diseases | Flu HA 306-318 | Biometrical and Traditional | Markovic-Plese et al, 2005 |
| Human | Clone 6 | Infectious diseases | Gag 272-281 | Biometrical | Venturini et al, 2006 |
| Human | X8 | Infectious diseases | Unknown | Biometrical | Luneman et al, 2007 |
| Human | VRC b | Infectious diseases | Unknown | Biometrical | Judkowski et al, 2011 |
| Mouse | Tg (BAND) | Model system | PCC | Traditional | Wilson et al., 1999 |
| Mouse | Tg (BDC2.5) | Autoimmunity | Unknown | Traditional Biometrical |
Judkowski et al, 2001 Judkowski et al, 2004 |
| CD8 | |||||
| Human | LAU 203/1.5 | Cancer | Melan-A 26-35 | Traditional Biometrical |
Pinilla et al., 2001 Rubio-Godoy et al, 2002b |
| Human | 3-3F4 | Infectious diseases | pp65 495-503 | Traditional | La Rosa et al, 2001 |
| Human | LAU 50/4D7 | Cancer | Unkown | Biometrical and Traditional | Rubio-Godoy et al, 2002a |
| Human | LAU Tyr/34 | Cancer | Tyrosinase 368-376 | Biometrical and Traditional | Rubio-Godoy et al, 2002c |
| Human | N1216 | Infectious diseases | Tax 11-19 | Biometrical | Nino-Vasquez et al, 2004 |
| Human | 161jAX14 | Infectious diseases | Gag 77-85 | Biometrical and Traditional | Boggiano et al, 2005 |
| Mouse | Tg 3K-36 | Model system | Unkown | Traditional | Huseby et al, 2005 |
| Mouse | p773-782 | Cancer | Her-2 neu 773-782 | Traditional | Lustgarten et al, 2006 |
Method used for the identification of peptides. Traditional refers to peptides derived from the combination of amino acids based on activity of positional scanning library. Biometrical refers to the identification of peptides in protein databases derived from the positional scanning based biometrical analysis.
Multiple clones.
Critical Parameters
Whether screening in a mAb or T cell system, assay optimization is beneficial for obtaining clear results from the library screening. An example of how to optimize antigen-antibody interactions is described in Support Protocol 1. A similar approach should be used to evaluate the assay parameters for the relevant T cell assay in order to optimize the T cell activation signals. These include the determination of optimal cell culture conditions and the number of cells in which the lowest concentration of a specific antigen or crude antigen preparation would give a clear reproducible signal. An example of the number of cells in various T cell activation assays is shown for a number of T cell clones in Table 9.5.2. In general, even if the signal of activation generated by the most active mixtures is low (i.e., 2 to 3; activation is measured as a stimulation index, which results from the readout of mixtures plus cells divided by the readout of cells without mixtures), but clearly reproducible in more than one assay, relevant antigens can be effectively identified (Wilson et al., 1999; Hemmer et al., 2000; Judkowski et al., 2001).
In most cases, it is difficult to predict which library will give the best results. Thus, in the past 10 years, the authors have synthesized a number of different peptide libraries to choose from that can be screened against each mAb or T cell. These libraries range in length from tetra- to dodecapeptides and vary in N- and C-terminal ending groups. Also, the authors have peptide libraries containing L-, D-, and unusual amino acids, as well as libraries made up of only D-amino acids. Table 9.5.5 shows many of the peptide libraries that have been synthesized and are available for screening. In order to choose which library will generate the best results, sampler libraries made up of one or two positional sublibraries of some or all of the positional scanning libraries can be assembled. The information from the screening of these samplers can be used to select the complete library or libraries to be screened. It is important to note that not every library will contain active peptides for a given mAb or T cell assay. One should not limit the search for new peptide antigens using only one library. The greater number of libraries screened increases the chances of finding new ligands that were previously unknown.
Table 9.5.5.
Positional Scanning Synthetic Combinatorial Libraries
| Composition | Library | N-terminus | C-terminus | no. samples |
|---|---|---|---|---|
| L-amino acids | Hexapeptide | Ac- | CONH2 | 120 |
| H- | CONH2 | 120 | ||
| Hexapeptide (dual position) | Ac- | CONH2 | 1200 | |
| H- | CONH2 | 1200 | ||
| Nonapeptide | Ac- | CONH2 | 180 | |
| H- | CONH2 | 180 | ||
| Ac- | COOH | 180 | ||
| H- | COOH | 180 | ||
| Decapeptide | Ac- | CONH2 | 200 | |
| H- | CONH2 | 200 | ||
| Ac- | COOH | 200 | ||
| H- | COOH | 200 | ||
| Dodecapeptide | Ac- | CONH2 | 240 | |
| H- | CONH2 | 240 | ||
| D-amino acids | Tripeptide | Ac- | CONH2 | 60 |
| Hexapeptide | Ac- | CONH2 | 120 | |
| Hexapeptides (dual position) | Ac- | CONH2 | 400 | |
| H- | CONH2 | 400 | ||
| Decapeptide | Ac- | CONH2 | 200 | |
| H- | CONH2 | 200 | ||
| L-,D- and unnatural amino acids | Tetrapeptide | H- | CONH2 | 240 |
| Ac- | CONH2 | 240 |
We have reported on the advantages of evaluating a large panel of cytokines for vaccinia specific T cell lines and clones to select the cytokines to be monitored instead of only measuring the standard cytokines of T cell activation, such as IFN-γ, IL-2 or TNF-α (Judkowski et al., 2011). Furthermore, we found that the quantification of GM-CSF in response to mixtures of positional scanning libraries is a very sensitive readout. The analysis of the production of five cytokines (IL-2, IL-4, IL-13, IFN-γ, GM-CSF and TNF-α) in response to different peptide concentrations and collected at different times revealed that GM-CSF is produced in large amounts and it accumulates in the supernatant even when low antigen concentrations are used for the stimulation.
Another parameter to be considered for the optimization of the use of positional scanning libraries for the elucidation of T cell specificity is the choice of antigen presenting cells. A report for a highly cross-restricted T cell clone showed that using bare lymphocyte syndrome (BLS) cells transfected with a single HLA class II molecule can significantly improve the prediction capacity of the library in comparison to using autologous PBMC (Sospedra et al., 2010). This is explained by the fact that the response of a cross-restricted T cell clone to the mixtures can be “masked” by an overlay of different responses. Another example of the advantage of using BLS cells expressing a single MHC class II molecule was found for a vaccinia specific clone (VRC19-122). For this clone, while very low responses to the mixtures of a decapeptide positional scanning library were detected when using autologous B lymphoblastoid cell lines (LCL) as antigen presenting cells, a clear screening profile that led to the identification of a peptide and its corresponding protein antigen was obtained when using BLS cells (unpublished observation). It is important to note that this T cell clone showed clear cytokine production in response to the peptide, both when presented by BLS and LCL cells. However, lower concentrations of peptide were required for the production of cytokines when presented by BLS than LCL cells, which is in agreement with the results of the screening of the positional scanning library and suggests that the BLS cells used have higher levels of HLA DRB5*0101 than LCL cells. All together these findings suggest that “tuning” the sensitivity and ability of inducing T cell activation clearly contributes to the detection of T cell responses to the mixtures of a positional scanning library. The continued understanding of factors involved in T cell activation and the development of tools that can augment the detection of T cell responses will facilitate the widespread utility of positional scanning libraries for the elucidation of T cell specificity.
Anticipated Results
Upon synthesis and testing of individual peptides derived from the screening of a positional scanning library, one should be able to confirm the activity of the selected mixtures. It is important to note that it is the activity of the individual peptides within a mixture that results in the observed activity, and not the amino acid in the defined position of the mixture. If no activity is found within the set of individual peptides, then either more peptides need to be prepared that include amino acids that were not initially selected or an iteration can be prepared using the most active mixture as a starting point. Upon iterating an active mixture and defining more positions of the library with amino acids, the resulting mixtures are less complex since they contain less peptides, and this should result in an increase in activity relative to the original mixtures from the library. The use of this strategy in which a biased positional scanning library was prepared based on the initial screening results of a decapeptide positional scanning library has been reported (Judkowski et al., 2001). This strategy is most useful when the initial library screening results do not reveal significantly active mixtures at a majority of the positions.
Antibodies raised against proteins often recognize discontinuous antigenic determinants. Because the peptide libraries described here are composed of linear hexapeptide sequences, it may be assumed that such libraries are not applicable for identifying discontinuous determinants. However, a number of such antigen-antibody interactions have been examined and specific sequences identified (Pinilla et al., 1999). The sequences identified are probably only a part of a much larger antigenic determinant.
Time Considerations
For either a hexapeptide or decapeptide positional scanning library, the screening can be carried out on several microtiter plates. The data analysis for this number of samples may be difficult at first, but spreadsheet templates can be designed to expedite the process. Screening thus takes 1 to 3 days depending on the nature of the assay. If active mixtures are found, the complete library should be screened at least once more followed by further dose-response experiments carried out on the most active mixtures. Analysis for the selection of which individual peptides to make will be dependent on the complexity of the screening data. Synthesis of the individual peptides can take 2 to 4 weeks. Individual peptides should be tested using serial dilutions to determine their activities.
Literature Cited
- Atherton E, Sheppard RC. Solid Phase Peptide Synthesis: A Practical Approach. IRL Press; Oxford: 1989. [Google Scholar]
- Boggiano C, Moya R, Pinilla C, Bihl F, Brander C, Sidney J, Sette A, Blondelle SE. Discovery and characterization of highly immunogenic and broadly recognized mimics of the HIV-1 CTL epitope Gag(77-85) Eur J Immunol. 2005;35:1428–1437. doi: 10.1002/eji.200425903. [DOI] [PubMed] [Google Scholar]
- Borras E, Martin R, Judkowski V, Shukaliak J, Zhao Y, Rubio-Godoy V, Valmori D, Wilson D, Simon R, Houghten R, Pinilla C. Findings on T cell specificity revealed by synthetic combinatorial libraries. J Immunol Methods. 2002;267:79–97. doi: 10.1016/s0022-1759(02)00142-4. Reviews of the use of combinatorial libraries to study antibody, MHC, and T cell specificity. [DOI] [PubMed] [Google Scholar]
- Gisin BF. The monitoring of reactions in solid-phase peptide synthesis with picric acid. Anal Chim Acta. 1972;58:248–249. doi: 10.1016/S0003-2670(00)86882-8. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, Kondo T, Cortese I, Bielekova B, Straus SE, McFarland HF, Houghten R, Simon R, Pinilla C, Martin R. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nature Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Pinilla C, Gran B, Vergelli M, Ling N, Conlon P, McFarland HF, Houghten R, Martin R. Contribution of individual amino acids within MHC molecule or antigenic peptide to TCR ligand potency. J Immunol. 2000;164:861–871. doi: 10.4049/jimmunol.164.2.861. [DOI] [PubMed] [Google Scholar]
- Houghten RA. General method for the rapid solid-phase synthesis of large numbers of peptides: Specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci USA. 1985;82:5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten RA, Bray MK, De Graw ST, Kirby CJ. Simplified procedure for carrying out simultaneous multiple hydrogen fluoride cleavages of protected peptide resins. Int J Pept Protein Res. 1986;27:673–678. doi: 10.1111/j.1399-3011.1986.tb01064.x. [DOI] [PubMed] [Google Scholar]
- Houghten RA, Pinilla C, Appel JR, Blondelle SE, Dooley CT, Eichler J, Nefzi A, Ostresh JM. Mixture-based synthetic combinatorial libraries. J Med Chem. 1999;42:3743–3778. doi: 10.1021/jm990174v. Reviews of the synthesis and use of mixture-based combinatorial libraries. [DOI] [PubMed] [Google Scholar]
- Houghten RA, Pinilla C, Giulianotti MA, Appel JR, Dooley CT, Nefzi A, Ostresh JM, Yu Y, Maggiora GM, Medina-Franco JL, Brunner D, Schneider J. Strategies for the Use of Mixture-Based Synthetic Combinatorial Libraries: Scaffold Ranking, Direct Testing In Vivo, and Enhanced Deconvolution by Computational Methods. J Comb Chem. 2008;10:3–19. doi: 10.1021/cc7001205. Reviews of the synthesis and use of mixture-based combinatorial libraries. [DOI] [PubMed] [Google Scholar]
- Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, Marrack P, Kappler JW. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Judkowski V, Pinilla C, Schroder K, Tucker L, Sarvetnick N, Wilson DB. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- Judkowski VA, Allicotti GM, Sarvetnick N, Pinilla C. Peptides from common viral and bacterial pathogens can efficiently activate diabetogenic T-cells. Diabetes. 2004;53:2301–2309. doi: 10.2337/diabetes.53.9.2301. [DOI] [PubMed] [Google Scholar]
- Judkowski V, Bunying A, Ge F, Appel JR, Law K, Sharma A, Raja-Gabaglia C, Norori P, Santos RG, Giulianotti MA, Slifka MK, Douek DC, Graham BS, Pinilla C. GM-CSF Production Allows the Identification of Immunoprevalent Antigens Recognized by Human CD4+ T Cells Following Smallpox Vaccination. PLoS ONE. 2011;6:e24091. doi: 10.1371/journal.pone.0024091. Identification of peptides from immunoprevalent vaccinia proteins recognized by CD4+ T cells derived from smallpox vaccinated human subjects. GM-CSF is found to be a sensitive readout to measure the activation of T cells in response to mixtures of positional scanning libraries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser ET, Colescott RL, Blossinger CD, Cook PI. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Krchnak V, Vagner J, Eichler J, Lebl M. Color monitored solid phase peptide synthesis. In: Jung G, Bayer E, editors. Peptides 1988. Walter de Gruyter; Berlin: 1989. pp. 232–234. [Google Scholar]
- La Rosa C, Krishnan R, Markel S, Schneck JP, Houghten R, Pinilla C, Diamond D. Enhanced immune activity of cytotoxic T-lymphocyte epitope analogues derived from positional scanning synthetic combinatorial libraries. Blood. 2001;97:1776–1786. doi: 10.1182/blood.v97.6.1776. [DOI] [PubMed] [Google Scholar]
- Lunemann JD, Gelderblom H, Sospedra M, Quandt JA, Pinilla C, Marques A, Martin R. Cerebrospinal fluid-infiltrating CD4+ T cells recognize Borrelia burgdorferi lysine-enriched protein domains and central nervous system autoantigens in early lyme encephalitis. Infect Immun. 2007;75:243–251. doi: 10.1128/IAI.01110-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustgarten J, Dominguez AL, Pinilla C. Identification of cross-reactive peptides using combinatorial libraries circumvents tolerance against Her-2/neu-immunodominant epitope. J Immunol. 2006;176:1796–1805. doi: 10.4049/jimmunol.176.3.1796. [DOI] [PubMed] [Google Scholar]
- Markovic-Plese S, Hemmer B, Zhao Y, Simon R, Pinilla C, Martin R. High level of cross-reactivity in influenza virus hemagglutinin-specific CD4+ T-cell response: Implications for the initiation of autoimmune response in multiple sclerosis. J Neuroimmunol. 2005;169:31–38. doi: 10.1016/j.jneuroim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Nino-Vasquez JJ, Allicotti G, Borras E, Wilson DB, Valmori D, Simon R, Martin R, Pinilla C. A powerful combination: the use of positional scanning libraries and biometrical analysis to identify cross-reactive T cell epitopes. Mol Immunol. 2004;40:1063–1074. doi: 10.1016/j.molimm.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Ostresh JM, Winkle JH, Hamashin VT, Houghten RA. Peptide libraries: Determination of relative reaction rates of protected amino acids in competitive couplings. Biopolymers. 1994;34:1684–1689. doi: 10.1002/bip.360341212. [DOI] [PubMed] [Google Scholar]
- Pinilla C, Martin R, Gran B, Appel JR, Boggiano C, Wilson DB, Houghten RA. Exploring immunological specificity using synthetic peptide combinatorial libraries. Curr Opin Immunol. 1999;11:193–202. doi: 10.1016/s0952-7915(99)80033-8. Reviews of the use of combinatorial libraries to study antibody, MHC, and T cell specificity. [DOI] [PubMed] [Google Scholar]
- Pinilla C, Rubio-Godoy V, Dutoit V, Guillaume P, Simon R, Zhao Y, Houghten R, Cerottini JC, Romero P, Valmori D. Combinatorial peptide libraries as an alternative approach to the identification of ligands for tumor reactive cytolytic T lymphocytes. Cancer Res. 2001;61:5153–5160. [PubMed] [Google Scholar]
- Pinilla C, Appel JR, Borras E, Houghten RA. Advances in the use of synthetic combinatorial chemistry: Mixture-based libraries. Nat Med. 2003;9:118–122. doi: 10.1038/nm0103-118. [DOI] [PubMed] [Google Scholar]
- Rubio-Godoy V, Ayyoub M, Dutoit V, Servis C, Schink A, Rimoldi D, Romero P, Cerottini JC, Simon R, Zhao Y, Houghten RA, Pinilla C, Valmori D. Combinatorial peptide library based identification of peptide ligands for tumor-reactive cytolytic T lymphocytes of unknown specificity. Eur J Immunol. 2002a;32:2292–2299. doi: 10.1002/1521-4141(200208)32:8<2292::AID-IMMU2292>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rubio-Godoy V, Dutoit V, Zhao Y, Simon R, Guillaume P, Houghten R, Romero P, Cerottini JC, Pinilla C, Valmori D. Positional scanning-synthetic peptide library-based analysis of self- and pathogen-derived peptide cross-reactivity with tumor-reactive Melan-A-specific CTL. J Immunol. 2002b;169:5696–5707. doi: 10.4049/jimmunol.169.10.5696. [DOI] [PubMed] [Google Scholar]
- Rubio-Godoy V, Pinilla C, Dutoit V, Borras E, Simon R, Zhao Y, Cerottini JC, Romero P, Houghten RA, Valmori D. Towards synthetic combinatorial peptide libraries in positional scanning format (PS-SCL)-based identification of CD8+ tumor-reactive T-cell ligands: A comparative analysis of PS-SCL recognition by a single tumor-reactive CD8+ CTL. Cancer Res. 2002c;62:2058–2063. [PubMed] [Google Scholar]
- Sospedra M, Zhao Y, zur Hausen H, Muraro PA, Hamashin C, de Villiers EM, Pinilla C, Martin R. Recognition of conserved amino acid motifs of common viruses and its role in autoimmunity. PLoS Pathog. 2005;1:e41. doi: 10.1371/journal.ppat.0010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Muraro PA, Stefanova I, Zhao Y, Chung K, Li Y, Giulianotti M, Simon R, Mariuzza RA, Pinilla C, Martin R. Redundancy in antigen-presenting function of the HLA-DR and -DQ molecules in the multiple sclerosis-associated HLA-DR2 haplotype. J Immunol. 2006;176:1951–1961. doi: 10.4049/jimmunol.176.3.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Zhao Y, Giulianotti M, Simon R, Pinilla C, Martin R. Combining positional scanning peptide libraries, HLA-DR transfectants and bioinformatics to dissect the epitope spectrum of HLA class II cross-restricted CD4+ T cell clones. J Immunol Methods. 2010;353:93–101. doi: 10.1016/j.jim.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Young JD. Solid Phase Peptide Synthesis. 2. Pierce Chemical Co; Rockford, Ill: 1984. [Google Scholar]
- Tam JP, Heath WF, Merrifield RB. SN2 deprotection of synthetic peptides with a low concentration of HF in dimethyl sulfide: Evidence and application in peptide synthesis. J Am Chem Soc. 1983;105:6442–6455. [Google Scholar]
- Venturini S, Allicotti G, Zhao Y, Simon R, Burton DR, Pinilla C, Poignard P. Identification of peptides from human pathogens able to cross-activate an HIV-1-gag-specific CD4(+) T cell clone. Eur J Immunol. 2006;36:27–36. doi: 10.1002/eji.200425767. [DOI] [PubMed] [Google Scholar]
- Wilson DB, Pinilla C, Wilson DH, Schroder K, Boggiano C, Judkowski V, Kaye J, Hemmer B, Martin R, Houghten RA. Immunogenicity. I Use of peptide libraries to identify epitopes that activate clonotypic CD4+ T cells and induce T cell responses to native peptide ligands. J Immunol. 1999;163:6424–6434. [PubMed] [Google Scholar]
- Zhao Y, Gran B, Pinilla C, Markovic-Plese S, Hemmer B, Tzou A, Whitney LW, Biddison WE, Martin R, Simon R. Combinatorial peptide libraries and biometric score matrices permit the quantitative analysis of specific and degenerate interactions between clonotypic T-cell receptors and MHC-peptide ligands. J Immunol. 2001a;167:2130–2141. doi: 10.4049/jimmunol.167.4.2130. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Grover L, Simon R. TEST: A web-based T-cell epitope search tool. Proceedings of the 14th IEEE symposium on computer-based medical systems; 2001b. pp. 493–497. [Google Scholar]