Abstract
Introduction:
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a rare but important cause of sudden cardiac death. We investigated the role of cardiac magnetic resonance imaging (CMR) in the evaluation of patients with suspected ARVC referred by a general cardiology service.
Methods:
Ninety-two patients (mean age 48 ± 15, 49% female), referred for CMR assessment of possible ARVC, were reviewed. CMR included both functional and tissue characteristic imaging.
Results:
No patients had ARVC based on the 1994 Task Force Criteria (TFC) prior to CMR, but 4 met proposed Modified TFC; 15% met one major (±1 minor) TFC, 71% 1 or 2 minor TFC, and 14% no TFC. Reasons for CMR referral included symptomatic arrhythmia of likely RV origin (28%), Electrocardiogram/Holter abnormalities (28%), echocardiographic features suspicious of ARVC (19%), and family history of ARVC (8%). CMR findings strongly suggestive of ARVC were found in nine patients (10%), although only three were considered typical. Of these patients two met 1 major TFC and seven met 1 or 2 minor TFC. CMR findings included RV thinning, aneurysm, and diastolic out-pouching, but only 1 patient had definite fatty infiltration of the RV. Incidentally, CMR detected important, previously undiagnosed pathology, including anomalous pulmonary venous drainage (2 patients) and non-ischaemic cardiomyopathy (6%). CMR was normal in 63%, with minor abnormalities in 29%.
Conclusions:
CMR may play an important diagnostic role in the evaluation of possible ARVC. Patients who do not meet TFC for diagnosis may have CMR features typical of ARVC. Additionally CMR may detect other hitherto undiagnosed structural or functional abnormalities that alter patient management. However the majority of patients referred have a low pretest probability of ARVC, and the rate of normal CMR scans is high.
Keywords: cardiac magnetic resonance imaging, cardiomyopathy
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is characterized by fibro-fatty replacement of ventricular myocardium, notably in the right ventricle (RV). It is an important but rare cause of sudden cardiac death, especially in young people and athletes,1 but diagnosis is challenging due to its protean clinical manifestations, ranging from an absence of symptoms to cardiac arrest.2 Definitive diagnosis of ARVC requires histologic confirmation, but myocardial biopsy is insufficiently sensitive due to the segmental nature of the disease.3 The 1994 Task Force Criteria (TFC) (Table 1) aimed to improve diagnostic accuracy and are based on structural, functional, and electrographic abnormalities, along with family history.4 Modified criteria have been proposed to facilitate the diagnosis of ARVC in first degree relatives (Table 2).5 The current TFC lack sensitivity in early disease, leading to a recently published proposal for revision of the criteria.6
Table 1.
Task Force Criteria for the Diagnosis of right ventricular dysplasia*.
| Family history |
| Major: familial disease confirmed at necropsy or surgery. |
| Minor: family history of premature sudden death (>35 years of age) due to suspected right ventricular dysplasia; family history (clinical diagnosis based on present criteria). |
| ECG depolarization/conduction abnormalities |
| Major: epsilon waves or localized prolongation (>110 ms) of QRS complex in right precordial leads (V1–V3). |
| Minor: late potentials on signal-averaged ECG. |
| ECG repolarization abnormalities |
| Minor: inverted T waves in right precordial leads (V2 and V3) in persons > 12 years of age and in the absence of right bundle branch block. |
| Arrhythmias |
| Minor: sustained or non-sustained left bundle branch block-type ventricular tachycardia documented on ECG or Holter monitoring or during exercise testing; frequent ventricular extrasystoles (1000/24 hours on Holter monitoring). |
| Global or regional dysfunction and structural alterations |
| Major: severe dilatation and reduction of right ventricular ejection fraction with no or mild left ventricular involvement; localized right ventricular aneurysms (akinetic or dyskinetic areas with diastolic bulgings); severe segmental dilatation of right ventricle. |
| Minor: mild global right ventricular dilatation or ejection fraction reduction with normal left ventricle; mild segmental dilatation of right ventricle; regional right ventricular hypokinesia. |
| Tissue characteristics of walls |
| Major: fibrofatty replacement of myocardium on endomyocardial biopsy. |
Notes:
From reference 4. The diagnosis of ARVC would be fulfilled in the presence of 2 major criteria or 1 major plus 2 minor or 4 minor criteria from the different groups.
Abbreviation: ECG, electrocardiogram.
Table 2.
Proposed Modified Task Force Criteria for the Diagnosis of Familial ARVC*.
| Right ventricular cardiomyopathy in a first-degree relative plus one of the following |
| ECG: T-wave inversion in right precordial leads (V2 and V3) |
| Signal-averaged ECG: Late potentials seen on signal-averaged ECG |
| Arrhythmia |
| Left bundle branch block-type ventricular tachycardia on ECG, Holter monitoring, or during exercise testing; >200 extrasystoles over a 24-hour period |
| Structural or functional abnormality of the right ventricle |
| Mild global right ventricular dilatation or reduction in ejection fraction with normal left ventricle; mild segmental dilatation of the right ventricle; regional right ventricular hypokinesia |
Notes:
Applicability is confined to first-degree relatives who do not fulfil the original task force guidelines. From reference 5.
Abbreviation: ECG, electrocardiogram.
Cardiac magnetic resonance imaging (CMR) is the optimal imaging modality for assessing structural and functional abnormalities of the RV, including regional dysfunction, ventricular volumes, morphological abnormalities, and potentially the presence of fatty infiltration.7,8 Studies assessing the accuracy of CMR in the diagnosis of ARVC have been undertaken on highly selected patient populations with a high prevalence of the disease.9–11 In routine clinical practice, CMR is often requested early in the diagnostic assessment of patients with suspected ARVC. The prevalence of ARVC in the population of patients referred for CMR is therefore likely to be considerably lower than that in study populations, and this may limit the accuracy and utility of CMR. We therefore sought to evaluate the value of CMR in assessing patients referred by a general cardiology service for evaluation of possible ARVC.
Methods
Patients
We identified all patients referred by their clinical cardiologists to our institution between June 2003 and December 2007 for the purposes of CMR and evaluation of possible ARVC. Waitemata Health is the sole provider of publicly funded cardiology services for a population of approximately 500,000 people, through two district general hospitals. During the time period of this analysis there was no on-site electrophysiology service, and all patients were referred for CMR by the general cardiologist responsible for their clinical care. Patients were excluded from the analysis if they were unable to undertake diagnostic imaging, and ninety two patients were included. Clinical details and information regarding TFC such as echocardiography, electrocardiograms, and Holter monitor results were obtained from chart review. This retrospective audit was approved by the Knowledge Centre at Waitemata District Health Board. In accordance with local regulations regarding clinical audit, formal ethical approval was not required.
Cardiac MRI
CMR was performed on a Philips Intera 1.5 Tesla magnet using a Synergy Cardiac Coil (Philips, Best). Electrocardiographic gated steady state free precession cine images were acquired using an axial stack, and in 2 and 4 chamber, left ventricular outflow tract and short axis views. The sequence parameters were a flip angle of 65°, TE = 1.64 ms, TR = 3.3 ms, and slice thickness of 8 mm, with a 2 mm gap in the short axis stack. Supplementary cine images of the RV were obtained, including an RV 2 chamber and outflow tract views Additional sequences routinely performed included T1 weighted turbo spin echo and, if deemed necessary, T1 weighted ‘fat sat’ and delayed enhancement inversion recovery imaging. The sequence parameters for the T1 weighted turbo spin echo were a flip angle of 90°, slice thickness of 8 mm, TE = 25 ms, and TR = 1 beat. When undertaken, delayed enhancement imaging was performed after injection of 0.15 mmol/kg gadolinium-based contrast agent (Omniscan, Nycomed Amersham, Oslo). A three dimensional inversion-recovery segmented gradient echo sequence was used in 2 and 4 chamber, left ventricular outflow tract and short axis views. Imaging was commenced 10 minutes after contrast administration using an inversion time optimized to obtain adequate nulling of normal myocardium (260–340 ms). The imaging sequence parameters included an in-plane voxel size of 1.2 − 1.8 × 1.2 − 1.8 mm2, slice thickness of 6 mm, flip angle of 15°, TE = 3–4 ms, and TR = 8–9 ms. A Philips View Forum workstation was used for image analysis. LV end-diastolic volume, LV end-systolic volume, LV ejection fraction, and maximal wall thickness were calculated from the short axis cine images. Right ventricular volumes were analyzed from the short axis stack and compared with published normal values.12 All scans were jointly analyzed and reported by two cardiologists, each with greater than 5 years of experience in CMR. All decisions regarding RV regional wall motion abnormalities were made by consensus.
Statistics
Data are presented as mean ± standard deviation (SD). Analysis was undertaken using SPSS 17.0 (IBM Software, USA).
Results
The clinical characteristics of the patients are summarized in Table 3. The study comprised of 92 patients with a mean age of 48 ± 15 years, 51% of patients being male. The majority of the patients were European (59%), but notably, 18% were of Maori or Pacific Island origin. Symptoms were the norm, the most common primary symptoms being palpitations (37%), and presyncope or syncope (29%). A minority of patients had a family history of confirmed ARVC (10%) or sudden cardiac death (4%). Eighty-nine (97%) had a resting ECG available for review, and the most common findings were frequent ventricular ectopy (30%) or wide complex tachycardia consistent with ventricular tachycardia (20%). Forty (43%) of patients underwent Holter monitoring prior to CMR, and of those forty 24% had frequent ventricular ectopy, while only 5% had wide complex tachycardia consistent with ventricular tachycardia. No patients had signal averaged ECG as there is no onsite electrophysiology service.
Table 3.
Baseline characteristics.
| Number of patients | 92 |
| Age (years) | 48 ± 15 |
| Gender (male) | 51% |
| Ethnicity | |
| New Zealand European | 51 (55%) |
| Maori/Pacific Island | 16 (17%) |
| Other/unspecified | 25 (28%) |
| Primary presenting symptom | |
| None | 10 (11%) |
| Presyncope/syncope | 26 (29%) |
| Palpitations | 34 (37%) |
| Chest pain | 5 (5%) |
| Arrhythmias | 5 (5%) |
| Cardiac arrest | 3 (3%) |
| Other/unspecified | 9 (10%) |
| Family history | |
| None | 68 (74%) |
| ARVC | 9 (10%) |
| Sudden cardiac death | 4 (4%) |
| Cardiomyopathy | 2 (2%) |
| Other/unknown | 9 (10%) |
| Baseline electrocardiogram | |
| Normal sinus rhythm | 35 (38%) |
| Frequent VPCs | 28 (30%) |
| Wide complex tachycardia or VF | 18 (20%) |
| Atrial fibrillation/flutter | 7 (8%) |
| Other/unknown | 4 (4%) |
| Holter monitor findings | |
| Normal sinus rhythm | 12 (13%) |
| Frequent VPCs | 22 (24%) |
| Wide complex tachycardia or VF | 5 (5%) |
| Atrial fibrillation | 1 (1%) |
| Unknown | 52 (57%) |
Note: Values are means ± SD, or numbers of patients (percentages).
The indications for CMR assessment given by the referring cardiologist are shown in Table 4 and include symptomatic arrhythmia of likely RV origin (28%), electrocardiographic/Holter abnormalities (28%), echocardiographic features suspicious of ARVC (19%), and family history of ARVC (8%). Prior to CMR, no patients met the 1994 TFC for ARVC (Table 5); 15% met one major (±1 minor) TFC, 71% had one or two minor TFC, but surprisingly, 14% appeared to have no documented major or minor criteria. Applying the Modified TFC to the patients who had a first degree relative diagnosed with ARVC, a further 4 patients were identified.
Table 4.
Indications for CMR.
| Indication given for CMR scan | |
|---|---|
| Symptomatic arrhythmia of RV origin | 26 (28%) |
| Abnormal ECG or Holter +/− symptoms | 27 (28%) |
| Abnormal echo +/− symptoms | 17 (19%) |
| Resuscitated SCD | 2 (2%) |
| Family history of ARVC | 7 (8%) |
| Family history of SCD or cardiomyopathy | 4 (4%) |
| Syncope | 7 (8% ) |
| Unknown/others | 1 (3%) |
Note: Values are numbers of patients (percentage).
Table 5.
Task Force Criteria Prior to CMR.
| Task Force Criteria | |
|---|---|
| One major | 11 (12%) |
| One major + one minor | 3 (3%) |
| Two minor | 10 (11%) |
| One minor | 55 (60%) |
| None | 13 (14%) |
| Modified Task Force Criteria met | 4 (4%) |
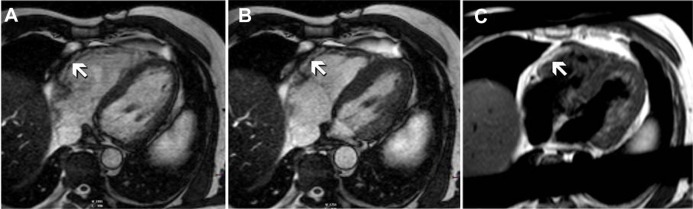
CMR features strongly suggestive of ARVC were found in 10% of the patients (n = 9), although only 3 were considered typical of the condition. The features noted include RV thinning, free wall or outflow tract aneurysm or diastolic pouching. Only one patient had definite fatty infiltration of the RV on CMR (Fig. 1). Sixty-three percent of patients had normal CMR, while 28.5% of the patients had minor abnormalities. The mean RV end diastolic volume (RVEDV) and RV end systolic volume (RVESV) were 157 mL ± 50 mL and 70 ± 3 6 mL respectively. Average RVEF was 59% ± 8%. Only 6 patients (7%) had RVEF < 50%. Overall, when the results of the CMR examination were compared in relation to the TFC, two patients met one major TFC whereas seven met one or two minor criteria.
Figure 1.
CMR 4 Chamber images, at end diastole (A) and end systole (B), using cine imaging. Note the focal akinesis of the basal segment of the RV free wall (arrows). The corresponding T1 turbo spin echo sequence is shown in (C) demonstrating fatty infiltration in the same segments (arrows).
Note: Although not diagnostic, based on CMR findings this patient was considered highly likely to have ARVC.
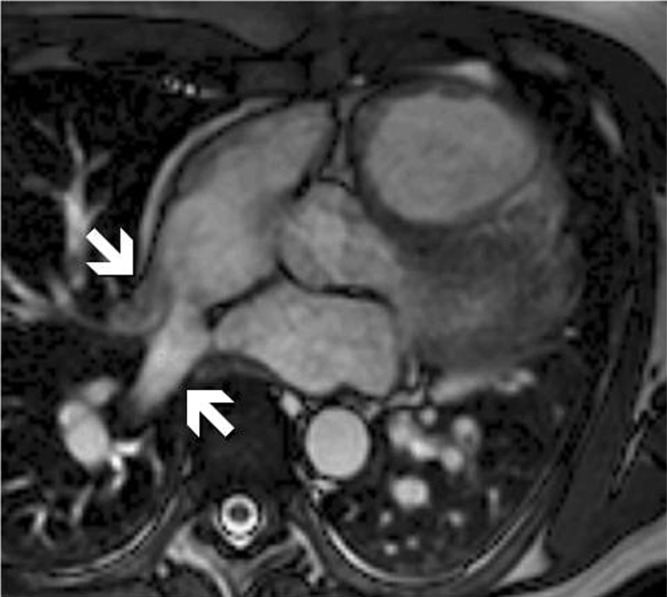
CMR detected important, previously undiagnosed pathology that altered patient management. Two patients had anomalous pulmonary venous drainage (Fig. 2). Both had dilated RV on echocardiography, but the anomalous venous drainage was not identified on either transthoracic or transoesophageal echocardiograms. Six percent of patients were deemed to have a cardiomyopathy on the basis of LV dysfunction not previously identified. A minority of patients underwent delayed enhancement imaging, as literature supporting the diagnostic use of this technique in ARVC was published late in the period reviewed in this report. Three patients had myocardial late gadolinium enhancement (LGE) on CMR. Of these three, 2 were subsequently considered by their clinicians to have had myocarditis and 1 had severe pulmonary hypertension secondary to severe emphysema. None had RV LGE seen including the one patient who had definite fatty infiltration of the RV on CMR.
Figure 2.
CMR cine image showing anomalous pulmonary venous drainage to the right atrium, an incidental finding in a patient referred for possible ARVC based on RV enlargement on echocardiography.
Discussion
This study describes the use of CMR in the evaluation of patients referred for possible ARVC by a general cardiology service. As such, the findings reflect the utility of CMR in a population who likely have a very low prevalence of ARVC. This is an important distinction from prior studies, which have focused on scanning groups of patients with a relatively high prevalence of ARVC, as determined by the TFC.9–11
ARVC remains a significant concern for general cardiologists and referral for CMR may be based largely on clinical suspicion rather than objective evidence such as that required by the TFC. It is readily apparent in our data that the majority of patients referred for CMR did not have a complete evaluation for ARVC as defined in the TFC. CMR appears to have been requested by clinicians prior to undertaking even simple investigations such as Holter monitoring in many cases. Furthermore our data suggest that a normal CMR result ended the evaluation for ARVC, with clinicians treating the result as a “rule out” investigation. As such, we cannot accurately address the true prevalence of Task Force Criteria-defined ARVC in our population, although we think it less likely that patients would meet TFC for ARVC in the setting of an entirely normal CMR.
In our group 60% of referrals had only one minor TFC and 14% had none. It is not surprising therefore that 63% of scans were normal. This high rate of normal results is similar to that reported elsewhere, and has important implications for resource utilization and funding, especially in a tax-payer funded system such as that found in New Zealand. In a series of 162 patients reported by Weissman et al, 51% had normal CMR scans.13 The Appropriateness Criteria deem CMR evaluation for ARVC in patients presenting with syncope or ventricular arrhythmia to be highly appropriate (A, median score 9).14 Many of our patients unfortunately did not meet even these basic clinical criteria. We believe that further education of clinical cardiologists regarding the role of CMR in the evaluation of ARVC should be a priority.
The incremental value of CMR in evaluating patients with unexplained RV abnormalities on echocardiography is underscored by the finding of 2 patients with previously undiagnosed anomalous pulmonary venous drainage. Both patients had RV dilation on transthoracic echocardiography without pulmonary hypertension or a visible shunt, and transoesophageal echocardiography had been unrevealing. We believe that CMR has an important role in evaluating patients with unexplained RV enlargement, independent of the possibility of ARVC. Of equal importance is the new diagnosis of cardiomyopathy with LV dysfunction in 5 patients (6%), confirmation of which would ensure those patients receive appropriate therapy.
CMR with LGE can detect intramyocardial fibrosis and this finding can precede functional abnormalities, potentially allowing for better detection of disease at an early stage than with TFC.9,15 However the finding of LGE is well described in a wide variety of pathologies, including dilated cardiomyopathy, in acute and chronic myocarditis, in pulmonary hypertension and, of course, in ischaemic heart disease.16–20 This is reflected in our study by 3 patients who had LGE on CMR and had diagnoses other than ARVC. CMR with LGE is therefore valuable in evaluating patients with unexplained ventricular dysrhythmias, but may not assist with the diagnosis of ARVC.
We suspect that many CMR services receive similar referrals for assessing patients with possible ARVC. Indeed the results of our series are remarkably similar to those reported by Roghi et al from Milan in an oral abstract presentation to the Society of Cardiac Magnetic Resonance meeting in 2009 (otherwise unpublished).21 They presented results from a group of 91 patients referred for possible ARVC. Fifty-two percent had frequent ventricular ectopy (compared with 48% in our series), 69% were considered to have a low pre-test probability of the disease, and only 3 were considered to have ARVC prior to CMR. The imaging results allowed a further 4 patients to be considered as likely to have ARVC. Interestingly, two patients were found to have anomalous pulmonary venous drainage. Such concordance in reported data suggests our experience is typical of other CMR centers where the population studied has a low prevalence of the condition.
The TFC has represented a guide to ARVC diagnosis over the past 16 years. Although highly specific, they lack sensitivity for early disease. The proposed “Modified TFC” addresses this issue, but incompletely. The assessment of structural and functional abnormalities of the right ventricle is a challenging aspect of diagnosis. The unmatched ability of CMR to evaluate both global function and focal wall motion abnormalities in the RV, coupled with the potential for non-invasive characterization of intramyocardial fatty infiltration, have led to a keen interest from clinicians in using this technique to assess patients for ARVC.7 Attempts have been made to clarify the sensitivity, specificity and predictive value of CMR imaging in patients with ARVC, but the populations tested have a much higher prevalence of the condition than can be expected in routine clinical practice. Individual components of the imaging data have been assessed. Tandri et al noted that finding an EF% < 50 was 73% sensitive and 95% specific in a population consisting of 40 patients who were confirmed as having ARVC by TFC.10 There were regional abnormalities in this study of RV-free wall function in 80% of patients, and 60% had fatty infiltration. Interestingly there was no fat seen in segments with normal contractile function. The sensitivity of fatty infiltration alone was investigated by the same group in 2006,11 with the presence of fat having a sensitivity of 84% and a specificity of 79%, this time in 40 patients of whom 15 were confirmed by TFC. Sen-Chowdhry et al carefully reviewed the operating characteristics of CMR in a population of patients of which 58% fulfilled either the original or Modified TFC.9 While diagnostic or strongly suspicious CMR findings were found to be 100% sensitive in patients who were TFC-proven, specificity was only 29%, and positive predictive value 35%. However, of the 119 apparent “false positive” CMR scans, it is important to note that 50% fulfilled the Modified TFC. This, coupled with results from a subgroup of genotype positive patients, led the authors to suggest that CMR is in fact detecting much earlier forms of the disease. This may explain the discrepancy between RV angiography and CMR reported by White et al, who found CMR to be 60% specific, compared with 100% for RV angiography.22 Our data are consistent with the hypothesis put forward by Sen-Chowdhry et al. Nine patients had CMR scans with findings consistent with ARVC, three of whom were deemed typical of the condition, but none of the 9 were TFC-confirmed prior to scanning. Increasingly there is an acceptance by clinicians that some patients will be diagnosed with ARVC without fulfilling TFC. A recent review of cases in the Auckland region by Boddington et al suggested that 50% of patients carrying a diagnosis of ARVC did not meet strict TFC.23 The recently published proposed TFC incorporate cut-off values for RVEF and RVEDV on CMR, which permits classification of severity and differentiation from normality (Table 7).6 Previous diagnostic reliance on subjective assessment of RV wall thinning, wall motion abnormalities, and fatty infiltration of the myocardium by CMR has proven problematic. Recognition of significant fatty involvement without concomitant fibrosis of the RV in normal individuals makes this unique capability of CMR of limited value. Using the proposed RV imaging criteria, the sensitivity of the CMR improved from 79% to 89% for major criteria and 68% to 78% for minor criteria.6
Table 7.
Revised Taskforce Criteria.
| Global or regional dysfunction and structural alterations |
| *Major |
| By 2D echo |
| • Regional RV akinesia, dyskinesia, or aneurysm and 1 of the following (end diastole): |
| – PLAX RVOT ≥ 32 mm (corrected for body size [PLAX/BSA] ≥19 mm/m2) |
| – PSAX RVOT ≥ 36 mm (corrected for body size [PSAX/BSA] ≥21 mm/m2) or fractional area change ≤33% |
| By MRI |
| • Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and 1 of the following: |
| – Ratio of RV end-diastolic volume to BSA ≥ 110 mL/m2 (male) or ≥100 mL/m2 (female) or RV ejection fraction ≤40% |
| By RV angiography |
| • Regional RV akinesia, dyskinesia, or aneurysm |
| *Minor |
| By 2D echo |
| • Regional RV akinesia or dyskinesia and 1 of the following (end diastole): |
| – PLAX RVOT ≥ 29 to <32 mm (corrected for body size [PLAX/BSA] ≥16 to <19 mm/m2) |
| – PSAX RVOT ≥ 32 to <36 mm (corrected for body size [PSAX/BSA] ≥18 to <21 mm/m2) or fractional area change > 33% to ≤40% |
| By MRI |
| • Regional RV akinesia or dyskinesia or dyssynchronous RV contraction and 1 of the following: |
| – Ratio of RV end-diastolic volume to BSA ≥ 100 to <110 mL/m2 (male) or ≥90 to <100 mL/m2 (female) or RV ejection fraction > 40% to ≤45% |
| Tissue characterization of wall |
| *Major |
| Residual myocytes < 60% by morphometric analysis (or <50% if estimated), with fibrous replacement of the RV free wall myocardium in ≥1 sample, with or without fatty replacement of tissue on endomyocardial biopsy |
| *Minor |
| Residual myocytes 60% to 75% by morphometric analysis (or 50% to 65% if estimated), with fibrous replacement of the RV free wall myocardium in ≥1 sample, with or without fatty replacement of tissue on endomyocardial biopsy |
| Repolarization abnormalities |
| *Major |
| Inverted T waves in right precordial leads (V1, V2, and V3) or beyond in individuals >14 years of age (in the absence of complete right bundle-branch block QRS ≥ 120 ms) |
| *Minor |
| Inverted T waves in leads V1 and V2 in individuals >14 years of age (in the absence of complete right bundle-branch block) or in V4, V5, or V6 |
| Inverted T waves in leads V1, V2, V3, and V4 in individuals >14 years of age in the presence of complete right bundle-branch block |
| Depolarization/conduction abnormalities |
| *Major |
| Epsilon wave (reproducible low-amplitude signals between end of QRS complex to onset of the T wave) in the right precordial leads (V1 to V3) |
| *Minor |
| Late potentials by SAECG in ≥1 of 3 parameters in the absence of a QRS duration of ≥110 ms on the standard ECG |
| Filtered QRS duration (fQRS) ≥114 ms |
| Duration of terminal QRS < 40 μV (low-amplitude signal duration) ≥38 ms |
| Root-mean-square voltage of terminal 40 ms ≤ 20 μV |
| Terminal activation duration of QRS ≥ 55 ms measured from the nadir of the S wave to the end of the QRS, including R′, in V1, V2, or V3, in the absence of complete right bundle-branch block |
| Arrhythmias |
| *Major |
| Nonsustained or sustained ventricular tachycardia of left bundle-branch morphology with superior axis (negative or indeterminate QRS in leads II, III, and aVF and positive in lead aVL) |
| *Minor |
| Nonsustained or sustained ventricular tachycardia of RV outflow configuration, left bundle-branch block morphology with inferior axis (positive QRS in leads II, III, and aVF and negative in lead aVL) or of unknown axis |
| >500 ventricular extrasystoles per 24 hours (Holter) |
| Family history |
| *Major |
| ARVC confirmed in a first-degree relative who meets current Task Force criteria |
| ARVC confirmed pathologically at autopsy or surgery in a first-degree relative |
| Identification of a pathogenic mutation† categorized as associated or probably associated with ARVC in the patient under evaluation |
| *Minor |
| History of ARVC in a first-degree relative in whom it is not possible or practical to determine whether the family member meets current Task Force criteria |
| Premature sudden death (<35 years of age) due to suspected ARVC in a first-degree relative |
| ARVC confirmed pathologically or by current Task Force Criteria in second-degree relative |
| Diagnostic terminology for revised criteria: definite diagnosis: 2 major or 1 major and 2 minor criteria or 4 minor from different categories; borderline: 1 major and 1 minor or 3 minor criteria from different categories; possible: 1 major or 2 minor criteria from different categories |
Note: PLAX indicates parasternal long-axis view.
Abbreviations: RVOT, RV outflow tract; BSA, body surface area; PSAX, parasternal short-axis view; aVF, augmented voltage unipolar left foot lead; aVL, augmented voltage unipolar left arm lead.
LGE on CMR permits myocardial tissue characterization in the LV. It can be difficult to be certain of LGE for characterization of RV myocardium because of the thin wall of the RV and possible confusion with fat. No provision for this information is presently included in the modified guidelines.
Limitations
This is a small study population and the assessment of the presence or absence of TFC prior to CMR was clearly not uniform. With more meticulous investigation for all TFC, it is possible more patients may have been confirmed with ARVC on this basis prior to CMR. As with any retrospective analysis there are issues of potential referral bias and inconsistent data collection. However, we do not believe that these potential problems detract importantly from the key messages highlighted by our data.
Conclusions
CMR is a potentially useful tool for the evaluation of patients with suspected ARVC and patients who do not meet TFC may have CMR features consistent with a diagnosis of ARVC. Importantly, CMR may detect unsuspected important abnormalities which may account for the original presentation (eg, anomalous pulmonary venous drainage) and which result in a change in therapeutic approach. However it appears that for general cardiology practice the majority of patients referred for CMR have a very low pretest probability of ARVC, and the rate of normal CMR scans is high. The clinical challenge remains in making an accurate assessment using the TFC, understanding the role of CMR in this condition, and continuing to educate referring clinicians in the evolving understanding of this area.
Table 6.
CMR findings.
| Imaging findings | |
|---|---|
| RVEDV (mL) | |
| Normal | 157 ± 50 |
| Male | 190 ± 33 |
| Female | 148 ± 35 |
| RVESV (mL) | |
| Normal | 70 ± 36 |
| Male | 78 ± 20 |
| Female | 56 ± 18 |
| RVEF (%) | 59 ± 8 |
| Patients with RVEF% < 50% | 6 (7%) |
| Normal CMR scan | 58 (63%) |
| CMR findings consistent with ARVC | 9 (10%) |
| Focal RV dysfunction | 9 (9%) |
| Fatty infiltration | 1 (1%) |
| Other CMR findings | |
| Dilated LV | 3 (3%) |
| LV dysfunction | 5 (6%) |
| Anomalous pulmonary veins | 2 (2%) |
| Other minor abnormalities | 5 (5%) |
Note: Values are means ± SD, or numbers of patients (percentages).
Abbreviations: RV, right ventricle; RVEDV, right ventricular end diastolic volume; RVESV, right ventricular end systolic volume; RVEF, right ventricular ejection fraction.
Footnotes
Author Contributions
Conceived and designed the study: JPC, CE, HH. Analysed the data: KLL, JC. Wrote the first draft of the manuscript: KLL. Contributed to the writing of the manuscript: JPC, CE. Agree with the manuscript results and conclusions: KLL, CE, HH, JPC. Jointly developed the structure and arguments for the paper: KLL, CE, HH, JPC. Made critical revisions an approved the final version: JPC, KLL. All authors reviewed and approved the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Marcus F, Fontaine G, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–98. doi: 10.1161/01.cir.65.2.384. [DOI] [PubMed] [Google Scholar]
- 2.Sen-Chowdhry S, Lowe MD, Sporton SC, McKenna WJ. Arrhythmogenic right ventricular cardiomyopathy: Clinical presentation, diagnosis, and management. Am J Med. 2004 Nov 1;117(9):685–95. doi: 10.1016/j.amjmed.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Angelini A, Basso C, Nava A, Thiene G. Endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy. Am Heart J. 1996 Jul;132(1 Pt 1):203–6. doi: 10.1016/s0002-8703(96)90416-0. [DOI] [PubMed] [Google Scholar]
- 4.McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994 Mar;71(3):215–8. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid MS, Norman M, Quraishi A, et al. Prospective evaluation of relatives for familial arrhythmogenic right ventricular cardiomyopathy/dysplasia reveals a need to broaden diagnostic criteria. J Am Coll Cardiol. 2002 Oct 16;40(8):1445–50. doi: 10.1016/s0735-1097(02)02307-0. [DOI] [PubMed] [Google Scholar]
- 6.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Br Heart J. 1994 Mar;71(3):215–8. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayser HWM, van der Wall EE, Sivananthan MU, Plein S, Bloomer TN, de Roos A. Diagnosis of Arrhythmogenic Right Ventricular Dysplasia: A Review. Radiographics. 2002;22:639–48. doi: 10.1148/radiographics.22.3.g02ma07639. [DOI] [PubMed] [Google Scholar]
- 8.Corrado D, Basso C, Thiene G. Arrhythmogenic right ventricular cardiomyopathy: diagnosis, prognosis, and treatment. Heart. 2000;83:588–95. doi: 10.1136/heart.83.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sen-Chowdhry S, Prasad SK, Syrris P, et al. Cardiovascular magnetic resonance in arrhythmogenic right ventricular cardiomyopathy revisited: comparison with task force criteria and genotype. J Am Coll Cardiol. 2006;48:2132–40. doi: 10.1016/j.jacc.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 10.Tandri H, Macedo R, Calkins H, et al. Role of magnetic resonance imaging in arrhythmogenic right ventricular dysplasia: Insights from the North American arrhythmogenic right ventricular dysplasia (ARVD/C) study. Am Heart J. 2008;155:147–53. doi: 10.1016/j.ahj.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Tandri H, Castillo E, Ferrari VA, et al. Magnetic resonance imaging of arrhythmogenic right ventricular dysplasia: sensitivity, specificity, and observer variability of fat detection versus functional analysis of the right ventricle. J Am Coll Cardiol. 2006;48:2277–84. doi: 10.1016/j.jacc.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 12.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–82. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 13.Weissman G, Calloway NH, AR F. Findings by magnetic resonance imaging in patients evaluated for arrhythmogenic right ventricular cardiomyopathy. Circulation. 2006;114 II_382-d-3. [Google Scholar]
- 14.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 Appropriateness Criteria for Cardiac Computed Tomography and Cardiac Magnetic Resonance Imaging: A Report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006 Oct 3;48(7):1475–97. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Tandri H, Saranathan M, Rodriguez ER, et al. Noninvasive detection of myocardial fibrosis in arrhythmogenic right ventricular cardiomyopathy using delayed-enhancement magnetic resonance imaging. J Am Coll Cardiol. 2005;45:98–103. doi: 10.1016/j.jacc.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 16.Blyth KG, Groenning BA, Martin TN, et al. Contrast enhanced-cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur Heart J. 2005 Oct;26(19):1993–9. doi: 10.1093/eurheartj/ehi328. Epub May 17, 2005. [DOI] [PubMed] [Google Scholar]
- 17.McCrohon JA, Moon JCC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–9. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–22. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 19.De Cobelli F, Pieroni M, Esposito A, et al. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol. 2006;47:1649–54. doi: 10.1016/j.jacc.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Abdel-Aty H, Kriedemann I, et al. Contrast-Enhanced Cardiovascular Magnetic Resonance Imaging of Right Ventricular Infarction. J Am Coll Cardiol. 2006;48:1969–76. doi: 10.1016/j.jacc.2006.05.078. [DOI] [PubMed] [Google Scholar]
- 21.Roghi A, Pedretti S, Pedrotti P, S D. Incremental value of cardiac magnetic resonance in the characterization of unselected patients referred to exclude arrhythmogenic right ventricular cardiomyopathy. J Cardiovasc Magn Reson. 2009;11:062. [Google Scholar]
- 22.White JB, Razmi R, Nath H, Kay GN, Plumb VJ, Epstein AE. Relative utility of magnetic resonance imaging and right ventricular angiography to diagnose arrhythmogenic right ventricular cardiomyopathy. J Interv Card Electrophysiol. 2004 Feb;10(1):19–26. doi: 10.1023/B:JICE.0000011480.66948.c3. [DOI] [PubMed] [Google Scholar]
- 23.Boddington D, Smith W, Occleshaw C, Hood M, Lever N. Review of the arrhythmogenic right ventricular cardiomyopathy patients in the auckland region database. Heart Lung Circ. 2007;16(Suppl 2):S106–7. [Google Scholar]