Abstract
Paravertebral titanium rod migration represents an unusual and potentially fatal complication of vertebral stabilization surgical procedures. This condition, which requires a prompt and rapid diagnosis, is often mistaken for other more common diseases, or scotomized. We present a case of a 69 years old female affected by a non-Hodgkin lymphoma with evidence of migration of both rods five years after the posterior stabilization procedure for a pathological L3 fracture. Unusual clinical onset was represented by a left S1 radiculopathy without other symptoms. For several months, the symptoms were attributed to a possible radicular infiltration by the lymphoma. We conclude that paravertebral rod migration could happen not only within the spinal canal, but could also rarely damage blood vessels or parenchymal organs. This is generally a long-term complication, probably due to an insufficient fixation. Strict long-term follow-up monitoring is mandatory since this unusual complication can mimic other more common pathological conditions.
Keywords: primary bone lymphoma, spine stabilization, hardware failure, misdiagnosed clinical picture
Introduction
Primary bone lymphoma (PBL) comprises less than 5% of all malignant bone tumors and almost 7% of all extra-nodal lymphomas. Only 1.7% of all PBLs have been reported to involve the vertebrae.1,2 The most frequent spinal localization are lumbar or lower dorsal segments.3,4 Skeletal involvement by lymphoma is more common in males than females.3,4 The usual age of presentation is during the 5th to 6th decade of life.4,5 Occasionally, it may involve the vertebrae and encroach upon the nerve root, causing claudication and radicular syndrome mimicking the symptoms related to common degenerative diseases (ie, spinal stenosis, and degenerative scoliosis).6,7
On imaging, PBL can mimic other more common neoplastic lesions of the mobile spine (ie, chordomas and metastases).1,2,8 Histologically, primary bone tumors have characteristic diagnostic features, except for small cell osteosarcoma and Ewing’s sarcoma. However, immunohistochemistry for CD-99 and positivity for leukocyte common antigen (LCA) can be diagnostic.9 Epidural involvement of spinal cord by the soft tissue rather than vertebral body collapse is more common in spinal lymphomas, most frequently at a single level of involvement. Diffuse large-B-cell lymphoma is the most common type of non-Hodgkin’s lymphoma involving the spine.10,11 The optimal management of PBL is not well defined. Traditionally, radiation therapy alone was the treatment of choice, but it does not produce durable remission for most patients and it is associated with an increased related neurotoxicity, especially in older patients.1,2,12,13 The most recent reports suggest that a combination of radio and chemotherapy should remain the main treatment, and that surgery should be used in combination with them especially in cases of massive neoplastic vertebral derangement, and\or spinal cord compression.1,2,14,15
In this situation, in order to stabilize the pathological skeletal segment the use of titanium fixation devices is needed. Sometimes complications related to these implants can appear, and are often represented by breakage or displacement of screws and rods.16,17
Rarely, a titanium rod migration and the subsequent necessity to remove it could occur, leading to a second surgical procedure aiming at avoiding dangerous injuries to parenchymal organs.16,18–25 In the current paper we report an unusual case of migration of both rods many years after the surgical procedure of lumbar fracture stabilization. Clinical onset was represented only by an S1 radiculopathy, and was underestimated for months.
Case Report
A caucasic woman of 69 years with history of hypertension was admitted to the emergency room in 2005 for severe low back pain. There was neuroradiological (lumbar X-rays and CT scan) evidence of a pathological fracture of the third lumbar vertebral body with subsequent lumbar canal stenosis. Two days later, a surgical procedure of posterior lumbar stabilization with titanium pedicle screws (located at L2 and L4) and rods associated with a decompressive laminectomy was performed, in addition to a biopsy (Fig. 1). After the histological diagnosis of a diffuse non-Hodgkin B-cell lymphoma (Stage IV A), tailored adjuvant chemotherapy (five cycles of cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (CHOP) protocol) and involved field radiotherapy (total dose 30 Gy) were planned.
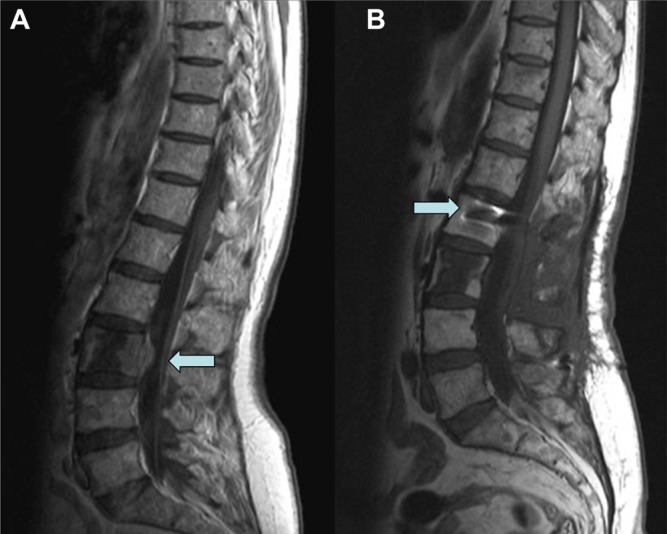
Figure 1.
A preoperative lumbosacral MRI showed a L3 involvement by lymphomatous tissue with evidence of intracanalar diffusion as indicated by arrow (A). A postoperative MRI with evidence of surgical procedure of decompressive laminectomy and stabilization with titanium pedicle screws (see arrow) and rods (B).
The postoperative course was favorable until April 2011 when an acute S1 left radiculopathy (without referred low back pain) and paresthesias appeared, which was unresponsive to intensive rehabilitation and the use of painkillers. The magnetic resonance imaging (MRI) results were initially misinterpreted and the clinical condition was attributed to a possible neoplastic nerve sheath involvement.
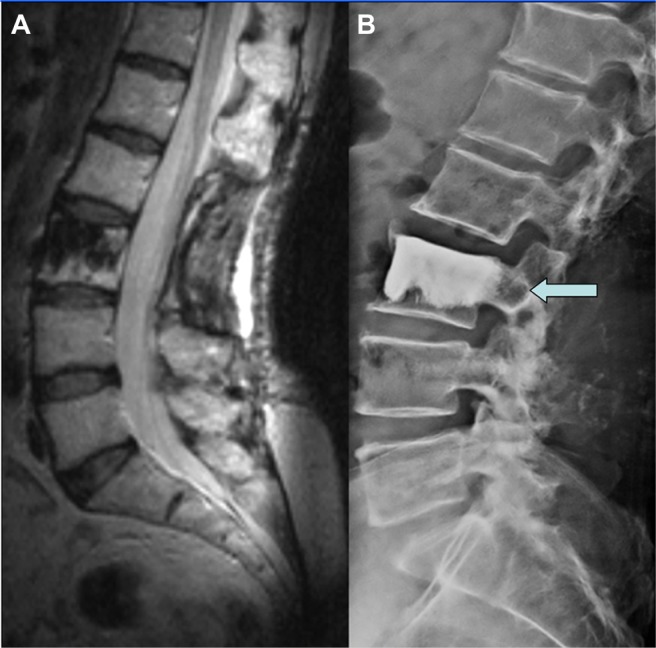
In August 2011, because of the worsening of the S1 left radiculopathy and the appearance of a motor deficit in foot flexion, a lumbar X-ray and a new MRI finally showed an unusual migration of both rods. The left one was distally migrated to the pre-sacral region adjacent to the sacral foramen of the exit of the S1 root (Fig. 2) and the right one was positioned on the upper level of the second lumbar vertebral body (Figs. 2 and 3). A critical retrospective analysis of a previous MRI demonstrated that, in some slices, the migration had already been evident (Fig. 3).
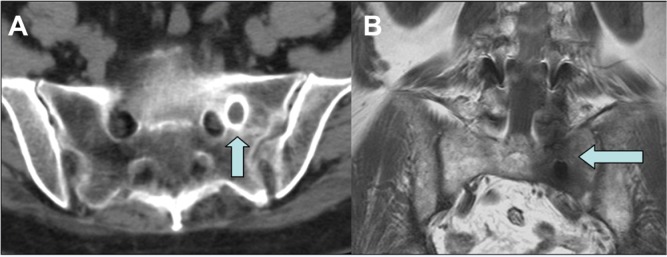
Figure 2.
Preoperative lumbar CT-scan (A) and MRI (B) showed the proximity of migrated rod to the foramen of the exit of the left S1 root and the intensive inflammatory reaction of the surrounding bone, each indicated by arrows.
Figure 3.
AP and LL lumbar x-ray performed after an S1 left radiculopathy onset showed the migration of both paravertebral rods, with the left one migrated in the pre-sacral region and the right one positioned at the upper level of second lumbar vertebral body (A and B). A coronal and sagittal plane of preoperative MRI allowed the visualization of the bone sacral groove created by the migrated left rod as marked by arrows (C and D).
Afterwards, the patient was submitted to a second surgical procedure. This procedure involved removal of screws and rods with concomitant L3 vertebroplasty, without hardware replacement. There was no hardware replacement because of the posterolateral arthrodesis process was adequate and complete (Figs. 4 and 5).
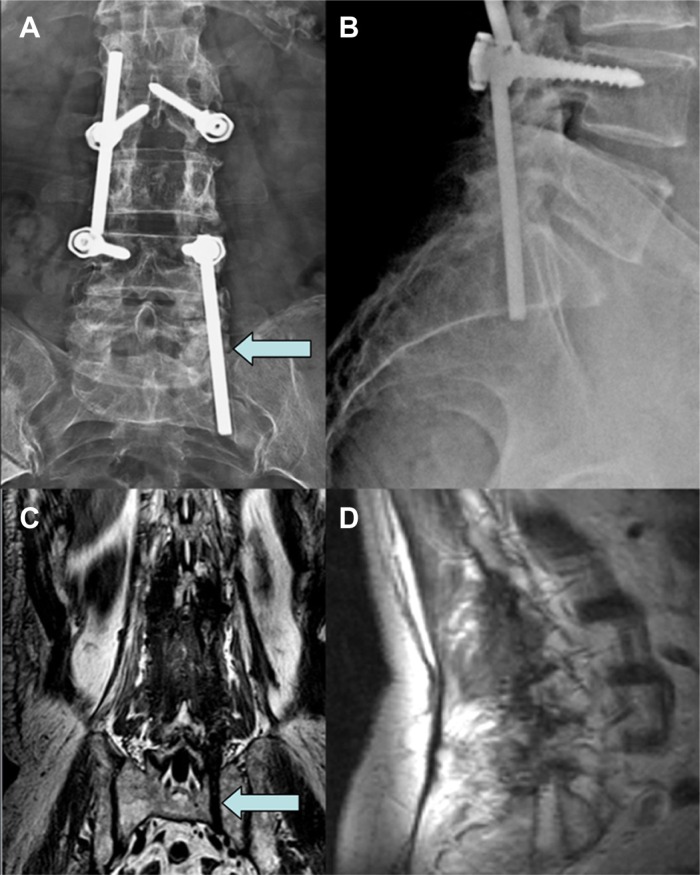
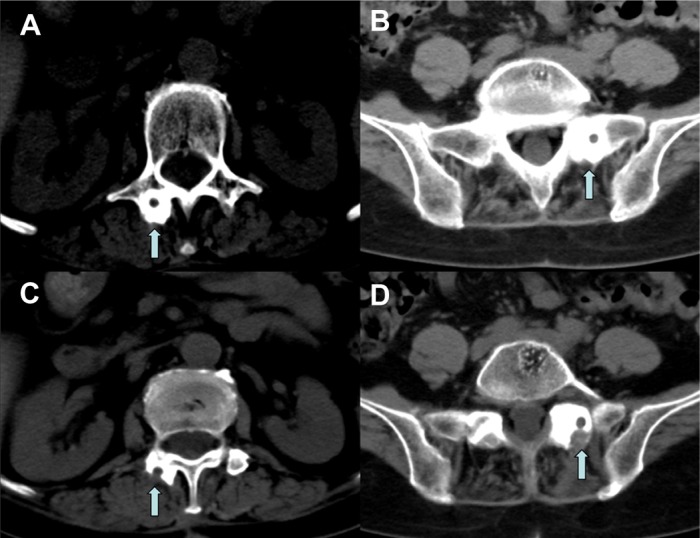
Figure 4.
Lumbar preoperative CT scan with evidence of bone formation around the migrated rods probably due to micro movements (A and B). The arrows show the strong bone reaction that during the second surgical procedure needed bone drilling to grant rods removal (C and D).
Figure 5.
Postoperative lumbar MRI (A) and LL X-ray (B) with evidence of screws and rods removal, and L3 vertebroplasty (see arrow) with preservation of spine stability.
The S1 radiculopathy and paresthesias disappeared in one month. The postoperative neuroimaging control (lumbar dynamic X-ray and MRI) showed no complications, while confirming the L3 vertebroplasty without any sign of spinal instability (Fig. 5). The patient was discharged four days after the operation and she is still in good condition at one-year follow up, without any sign of clinical or radiological spine instability.
Discussion
PBL is defined as a lymphomatous osseous involvement without any systemic neoplastic diffusion within 6 months from the first diagnosis. The incidence is less than 1% in primary non-Hodgkin lymphoma. The lesion is commonly located in a short bone, such as the ilium, scapula, or vertebra. PBLs involving a single vertebra comprise 1.7% of all PBLs.6,7,17,26 The clinical presentation is usually represented by back pain, eventually associated with neurological deficits related to the vertebral level involved. There are also general symptoms such as weight loss, night sweats, and fever. Neuroradiological evaluation allows the visualization of bone segments involved and eventual spinal cord or roots compression by neoplastic tissue. These clinical and radiological presentations are not specific and can mimic other more common medical conditions like infective, degenerative or traumatic diseases.18–25 This makes the definitive diagnosis difficult and often leads to a significant delay in initiation of effective treatment.1,2,27,28
The correct treatment for patients affected by PBL is not standardized. For example, many researchers have sustained that in case of spinal cord or nerve roots compression, a surgical procedure of posterior decompressive laminectomy and stabilization followed by adjuvant local radiotherapy and chemotherapy (CHOP-based chemotherapy) is mandatory.1,2,27,29,30
Other researchers have reported that the sole combination of chemo and radiotherapy, without any surgical procedure, could rapidly reduce compression of the involved neurologic structures. This is even recommended in cases of spinal cord compression by pathologic tissue. Radiation therapy could be performed alone or with chemotherapy, but chemotherapy is performed alone only rarely.1,2,27,30 Despite the absence of a standardization, a surgical procedure is generally recommended in cases of progressive neurological deficits due to compression by a tumor and\ or vertebral fragments. In these cases, it is important to obtain a satisfactory spinal cord decompression as well as to maintain spine stability. This is why the surgical procedure often consists of a combination of decompressive laminectomy and titanium pedicle screws and rods hardware stabilization.
In this context, the most frequent instrument failures are screws and rods breakage or displacement, while an unusual complication is represented by a paravertebral rods migration. This mobilization could be distal to, proximal to, or within the spinal canal, and is often not an acute postoperative problem but a long-term one.16,19 Generally, distal or proximal rod migration is minimal without any clinical disturbances.20 In some cases, this complication provokes only symptoms related to spinal instability because of the absence of a valid arthrodesis process for incorrect stabilization by the system. Rarely, rod migration can result in potentially fatal outcomes, because of blood vessels or bowel injuries.21–23 For example, Fitchett and colleagues19 reported a rod migration in abdomen in a woman submitted to a scoliosis correction with acute onset of pain in iliac fossa and evidence of bowel lesion. Al-Binali and colleagues23 reported an acute abdominal bleeding secondary to a rod migration with laceration of the internal iliac artery. Dhatt and colleagues25 reported an unusual case of anterior spinal rod migration from the dorsolumbar spine to the knee. All of these authors stressed the unusual presentation of this complication.
According to literature, a rod migration is probably provoked by a fatigue-fracture of the rod, caused by excessive unbalanced motion at the operated levels. It can also be caused by a slipping of the instrument due to a screws loosening, probably due to an insufficient torque-tightening.21 In our case, the rods migration could have been provoked by an inappropriate fixation, but it is quite strange that it happened in every dices. Hence, a progressive dynamic overstress on the stabilization system caused by micro movements is hypothesized to have contributed to migration. However, it is to be stressed that a careful fixation should be performed to avoid any rod migration.
A further unusual event of our case report is the evidence of opposite migration of both rods, probably due not only to the usual flexion and extension movements, but mainly to the lateral bending ones. Beside rods mobilization, a post-operative CT-scan showed a complete arthrodesis process around the rods, theoretically due to device micro movements that induced solid bone formation (Fig. 4). This bone formation provided adequate arthrodesis and spine stability, but did not prevent the rods migration inside the bony tunnel. This condition justifies the clinical picture because the patient did not complain about postural low back pain, but rather had radiculopathy because of compression by the migrated rod. The presence of a presumable adequate arthrodesis induced us to remove the fixation system. For safety we performed only a minimally invasive vertebroplasty without hardware replacement, since spine stability had already been achieved (Figs. 4 and 5).
Considering previous issues, any clinical disturbances should not be underestimated because they could be related to an instrumentation failure, even many years after the operation. This is especially true for patients submitted to spinal stabilization with metal devices, and, in general, all patients with prostheses. A further concern is that quite often this complication is misdiagnosed. It can be attributed to the basic disease, or to other more common disorders (eg, degenerative spine diseases). The rod migration responsible for the S1 radiculopathy was misdiagnosed for a long time because the physicians’ attention was directed to the theoretically more frequent lymphoma diffusion through the nerve sheath, rather than the rare instrumentation failure. Consequently, an MRI was chosen, as a neuroradiological study was presumably indicated. Even if, at a retrospective reevaluation of the MRI, the rod migration was evident in some slices, a simple lumbar spine X-ray would allow us to make the definitive diagnosis (Fig. 3).
There are several lessons learned from this case study. One is to always follow patients in the long term, especially those who implant prostheses of every type. Do not superficially relate a symptom to the age or to other more common or basic diseases, but rather think about potential unusual complications. It is also worth noting that sometimes less sophisticated diagnostic exams can be much more useful than more modern and sophisticated exams.
Footnotes
Author Contributions
Conceived and designed the experiments: MI, AD, NN. Analysed the data: LA, MG, MD. Wrote the first draft of the manuscript: MI, RC, NH. Contributed to the writing of the manuscript: MI, AD, NN, LA, MG, RC, NH, MD, MS. Agree with manuscript results and conclusions: MI, AD, NN, LA, MG, RC, NH, MD, MS. Jointly developed the structure and arguments for the paper: MI, NN, NH. Made critical revisions and approved final version: MI, AD, NN, LA, MG, RC, NH, MD, MS. All authors reviewed and approved of the final manuscript.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Ramadan KM, Shenkier T, Sehn LH, Gascoyne RD, Connors JM. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: a population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann Oncol. 2007;18:129–35. doi: 10.1093/annonc/mdl329. [DOI] [PubMed] [Google Scholar]
- 2.Beal K, Allen L, Yahalom J. Primary bone lymphoma: treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer. 2006;106:2652–6. doi: 10.1002/cncr.21930. [DOI] [PubMed] [Google Scholar]
- 3.Haddad P, Thaell JF, Kiely JM, Harrison EG, Miller RH. Lymphoma of the spinal extradural space. Cancer. 1976;38:1862–6. doi: 10.1002/1097-0142(197610)38:4<1862::aid-cncr2820380467>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Lyons MK, O’Neill BP, Marsh WR, Kurtin PJ. Primary spinal epidural non-Hodgkin’s lymphoma: report of eight patients and review of the literature. Neurosurgery. 1992;30:675–80. doi: 10.1097/00006123-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Rathmell AJ, Gaspodarowicz MK, Sutcliffe SB, Clark RM. Localized extradural lymphoma: survival, relapse pattern and functional outcome. The Princess Margaret Hospital Lymphoma Group. Radiother Oncol. 1992;24:14–20. doi: 10.1016/0167-8140(92)90348-x. [DOI] [PubMed] [Google Scholar]
- 6.Ebus SC, Bernsen HJ, Norel Van GJ, Donk R. Primary non-Hodgkin’s lymphoma in multiple vertebrae presenting as a lumbar radicular syndrome: A case report. Spine. 2002;27:271–3. doi: 10.1097/00007632-200205150-00025. [DOI] [PubMed] [Google Scholar]
- 7.Travlos J, du Toit G. Primary spinal epidural lymphoma mimicking lumbar spinal stenosis. Spine. 1991;16:377–9. doi: 10.1097/00007632-199103000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Liu JK, Kan P, Schmidt MH. Diffuse large B-cell lymphoma presenting as a sacral tumor. Report of two cases. Neurosurg Focus. 2003;15:1–5. doi: 10.3171/foc.2003.15.2.10. [DOI] [PubMed] [Google Scholar]
- 9.Llombart-Bosch A, Blache R, Peydro-Olaya A. Round-cell sarcomas of bone and their differential diagnosis (with particular emphasis on Ewing’s sarcoma and reticulosarcoma). A study of 233 tumors with optical and electron microscope techniques. Pathol Annu. 1982;17:113–45. [PubMed] [Google Scholar]
- 10.The 2007 WHO classification of tumours of the central nervous system. Louis DN, Ohgaki H, Wiestler OD, et al., editors. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green ST, Ng JP, Hart IK, Bone I. Non-Hodgkin’s lymphoma presenting with isolated cauda equina compression. Q J Med. 1987;65:1005–7. [PubMed] [Google Scholar]
- 12.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281–8. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- 13.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG):RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 14.Liu BL, Cheng JX, Zhang X, Zhang W, Cheng H. Limited role of surgery in the management of primary central nervous system lymphoma. Oncol Rep. 2009;22:439–49. doi: 10.3892/or_00000455. [DOI] [PubMed] [Google Scholar]
- 15.Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–95. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 16.De Wald CJ, Stanley T. Instrumentation-related complication of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine. 2006;31:S144–51. doi: 10.1097/01.brs.0000236893.65878.39. [DOI] [PubMed] [Google Scholar]
- 17.Becker J, Venbrocks R. Primary non-Hodgkin lymphoma of the spine. Arch Orthop Trauma Surg. 1998;117:299–401. doi: 10.1007/s004020050278. [DOI] [PubMed] [Google Scholar]
- 18.Habal P, Malek V, Novotny J. Case: unusual migration of osteosynthetic material. Acta Medica. 2005;48:49–52. [PubMed] [Google Scholar]
- 19.Fitchett J, Williams GL, McKain ES, Stephenson BM. A hard object in the right iliac fossa. Ann R Coll Surg Engl. 2008;90:W10–1. doi: 10.1308/147870808X257247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yablon IG, Cowan S, Mortara R. The migration of a Harrington rod after cervical fusion. Spine. 1993;18:356–8. doi: 10.1097/00007632-199303000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Wood KE, Fitch RD, Burton DC, Keiger CJ. Anterior scoliosis rod migration to the lower extremity. Spine J. 2009;9:e9–12. doi: 10.1016/j.spinee.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Banit DM, Iwinski HJ, Jr, Talwalkar V, Johnson M. Posterior spinal fusion in paralytic scoliosis and myelomeningocele. J Pediatr Orthop. 2001;21:117–25. doi: 10.1097/00004694-200101000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Al-Binali AM, Sigalet D, Goldstein S, Al-Garni A, Robertson M. Acute lower gastrointestinal bleeding as a late complication of spinal instrumentation. J Pediatr Surg. 2001;36:498–500. doi: 10.1053/jpsu.2001.21623. [DOI] [PubMed] [Google Scholar]
- 24.Hirano K, Deguchi M, Kanamono T. The migration of a broken luque rod: a case report. J Spinal Disord Tech. 2007;20:176–9. doi: 10.1097/BSD.0b013e31802c2a62. [DOI] [PubMed] [Google Scholar]
- 25.Dhatt S, Kumar S, Arora N, Dhillon M, Tripathy SK. Migration of anterior spinal rod from the dorsolumbar spine to the knee: an unusual complication of spinal instrumentation. Spine. 2010;35:E270–2. doi: 10.1097/BRS.0b013e3181c5d4da. [DOI] [PubMed] [Google Scholar]
- 26.Boukobza M, Mazel C, Touboul E. Primary vertebral and spinal epidural non-Hodgkin’s lymphoma with spinal cord compression. Neuroradiology. 1996;38:333–7. doi: 10.1007/BF00596582. [DOI] [PubMed] [Google Scholar]
- 27.Monnard V, Sun A, Epelbaum R, et al. Primary spinal epidural lymphoma: patients’ profile, outcome, and prognostic factors: a multicenter Rare Cancer Network study. Int J Radiat Oncol Biol Phys. 2006;65:817–23. doi: 10.1016/j.ijrobp.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Iacoangeli M, Roselli R, Pagano L, et al. Intrathecal chemotherapy for treatment of overt meningeal leukemia: comparison between intraventricular and traditional intralumbar route. Ann Oncol. 1995;6:377–82. doi: 10.1093/oxfordjournals.annonc.a059187. [DOI] [PubMed] [Google Scholar]
- 29.Spinazzé S, Caraceni A, Schrijvers D. Epidural spinal cord compression. Crit Rev Oncol Hematol. 2005;56:397–406. doi: 10.1016/j.critrevonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Kahl C, Hirt C, Decker S, et al. Multimodal therapy for localized spinal epidural follicular lymphoma. Onkologie. 2010;33:381–4. doi: 10.1159/000315769. [DOI] [PubMed] [Google Scholar]