Abstract
Evolutionary biologists have postulated that several fitness advantages may be conferred by the maintenance of duplicate genes, including environmental adaptation resulting from differential regulation. We examined the expression and physiological contributions of two redundant operons in the adaptable bacterium Pseudomonas aeruginosa PA14. These operons, phzA1-G1 (phz1) and phzA2-G2 (phz2), encode nearly identical sets of proteins that catalyze the synthesis of phenazine-1-carboxylic acid, the precursor for several phenazine derivatives. Phenazines perform diverse roles in P. aeruginosa physiology and act as virulence factors during opportunistic infections of plant and animal hosts. Although reports have indicated that phz1 is regulated by the Pseudomonas quinolone signal, factors controlling phz2 expression have not been identified, and the relative contributions of these redundant operons to phenazine biosynthesis have not been evaluated. We found that in liquid cultures, phz1 was expressed at higher levels than phz2, although phz2 showed a greater contribution to phenazine production. In colony biofilms, phz2 was expressed at high levels, whereas phz1 expression was not detectable, and phz2 was responsible for virtually all phenazine production. Analysis of mutants defective in quinolone signal synthesis revealed a critical role for 4-hydroxy-2-heptylquinoline in phz2 induction. Finally, deletion of phz2, but not of phz1, decreased lung colonization in a murine model of infection. These results suggest that differential regulation of the redundant phz operons allows P. aeruginosa to adapt to diverse environments.
Gene duplications give rise to genetic redundancy, an unstable condition that would not be expected to persist over time (1). This property is exhibited by diverse genomes (2–4), however, and evolutionary theorists have proposed several mechanisms whereby duplicate genes might provide selective advantages (5); for example, redundancy may be favored when spatial or temporal differences in expression enable tissue-specific variation or survival under varying environmental conditions (6, 7). Products of duplicated genes are involved in crucial cellular processes, including signal transduction, development, and metabolism (8).
The genome of the bacterium Pseudomonas aeruginosa, an opportunistic pathogen that thrives in both soil and host environments, contains two redundant seven-gene operons termed phzA1-G1 (phz1) and phzA2-G2 (phz2). These operons are nearly identical (∼98% similarity at the DNA level), and each encodes the biosynthetic enzymes for phenazine-1-carboxylic acid (PCA) (9). Downstream modifications derivatize this precursor, generating other phenazines (SI Appendix, Fig. S1A). Pseudomonad phenazines are toxic to many other organisms and cell types due to their inherent redox activity (10, 11). Studies conducted in various plant and animal models of infection have implicated phenazines in colonization and pathogenicity (12, 13). In addition, recent studies have elucidated beneficial roles for phenazines in the physiology of P. aeruginosa, which is not negatively affected by the redox toxicity of phenazines (14). These roles are thought to contribute to the dramatic effects of phenazines on biofilm development, where their production induces a switch between wrinkled (rugose) and smooth morphotypes (15).
Advantages conferred by phenazines may support conservation of the phz operon in the more than 57 currently identified phenazine-producing species (16). However, although phz operons display a broad phylogenetic distribution, genomes containing more than one phz operon are rare; of the bacterial genomes sequenced to date, only those belonging to P. aeruginosa and Streptomyces cinnamonensis contain a second, redundant phz operon (16). In P. aeruginosa, the regions surrounding phz1 and phz2 are highly divergent. phz1 is flanked by phzM and phzS, which encode enzymes that convert PCA to pyocyanin (PYO) (SI Appendix, Fig. S1B). phz2 lies ∼2.6 MB away from phz1 and is not flanked by phenazine-modifying enzymes. A third phenazine-modifying enzyme, PhzH, is encoded at a distinct site and converts PCA to phenazine-1-carboxamide. Additional phenazines that have been detected in P. aeruginosa cultures are intermediates and by-products arising from PhzM and PhzS activity or are produced by enzymes with unknown coding genes (17, 18).
The duplicate P. aeruginosa phz operons are preceded by distinct promoter regions (9). The phz1 promoter contains a las box 390 bp upstream of the phzA1 translational start site (19). Located upstream of many genes regulated by quorum sensing, las box motifs recruit the transcriptional regulators LasR and/or RhlR (20, 21). The las box, LasR, and RhlR are required for complete induction of phzA1 (20). The phz1 operon has been shown to be regulated by P. aeruginosa quinolones, and binding of the Pseudomonas quinolone signal (PQS) to the transcriptional regulator PqsR (also known as MvfR) is required for WT PYO production (22, 23). Furthermore, the PQS dependence of phz gene expression has been reported in several RNA array studies and is often attributed to the quinolone signal control of phz1; however, it is unlikely that microarray probes can distinguish between highly similar phz1 and phz2. Thus, the quinolone dependence of phz2 expression has remained an open question. Finally, the GacA-dependent orphan repressor QscR, encoded upstream of phz2, has a negative effect on the expression of both phz operons through an unknown mechanism (24, 25).
In this study, we generated mutants lacking genes involved in phenazine and quinolone biosynthesis and evaluated phenazine production, colony morphogenesis, and pathogenicity. We used promoter–GFP fusions to examine the relative expression levels of phz1 and phz2 during growth in liquid batch cultures and colony development. We tested the contributions of phz1 and phz2 to P. aeruginosa pathogenicity using a murine model of lung infection. Our results demonstrate a major role for phz2 during planktonic growth, and an almost exclusive role for phz2 in P. aeruginosa phenazine production during biofilm development and host infection.
Results
Both phz1 and phz2 Contribute to Phenazine Production During Planktonic Growth.
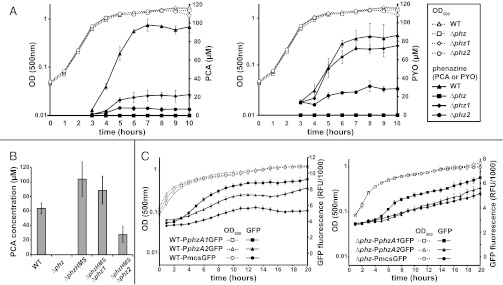
P. aeruginosa liquid batch cultures begin to produce phenazines in early stationary phase. To investigate the relative contributions of the two phz operons to phenazine production in planktonic cultures, we deleted each individual operon in P. aeruginosa PA14. We quantified phenazines in culture supernatants from the phz1 deletion mutant (Δphz1), the phz2 deletion mutant (Δphz2), and a mutant in which both phenazine operons had been deleted (Δphz). Both Δphz1 and Δphz2 produced significantly less PCA compared with WT (Fig. 1A, Left). Δphz1 produced more PCA than Δphz2, but the combined total PCA produced by the two mutants did not reach the WT level. ∆phz1 produced slightly less PYO than WT, whereas ∆phz2 produced approximately one-third of WT (Fig. 1A, Right). The levels of other phenazines were below the detection limit of this study.
Fig. 1.
P. aeruginosa phz1 and phz2 contribute to phenazine production in planktonic culture. (A) Growth of WT, Δphz, Δphz1, and Δphz2 with PCA (Left) and PYO (Right) quantification. (B) PCA in ΔphzHMS, ΔphzHMSΔphz1, and ΔphzHMSΔphz2 culture supernatants measured after 16 h of growth. (C) Expression levels of GFP reporter constructs for phz1 and phz2 operons in WT (Left) and Δphz (Right) backgrounds. Error bars indicate SD of biological triplicates.
PCA modification might have prevented us from accurately quantifying the total PCA produced by each operon. Thus, we created a triple-deletion mutant (ΔphzHMS) that lacks phzH, phzM, and phzS and in this background deleted each phz operon. ΔphzHMS produced more PCA than WT (Fig. 1B); this represents the total PCA production from both operons. This amount was higher than the combined total PCA produced by ∆phz1 and ∆phz2, confirming that the conversion of PCA to other phenazines prevented accurate quantification of total PCA. Deleting phz1 in this background decreased PCA production slightly,whereas deleting phz2 drastically reduced PCA production, indicating that phz2 is responsible for the majority of the PCA produced by planktonic cultures.
To address the possibility that the phz operons are expressed at different levels, we created fluorescent reporter constructs containing the 500-bp promoter regions upstream of each operon fused to gfp. These reporters, PphzA1GFP and PphzA2GFP, were integrated into the chromosome at a neutral site in WT and ∆phz. We found higher expression of PphzA1GFP than of PphzA2GFP in the WT and ∆phz backgrounds (Fig. 1C).
phz2 Is Sufficient for WT Phenazine Production in Colony Biofilms.
Phenazine production has been best characterized using planktonic cultures, but phenazines also have been shown to affect the morphology of different types of biofilms (15, 26). We compared the development of Δphz, Δphz1, and Δphz2 with that of WT in a colony morphology assay. The Δphz mutant exhibited a wrinkled morphology, as described previously (27). Strikingly, the presence of phz2 alone was sufficient for maintenance of the WT phenotype, whereas deletion of phz2 led to a hyper wrinkled morphology much like that of Δphz (Fig. 2A). Biofilms formed by Δphz and Δphz2 exhibited an almost twofold increase in agar surface coverage compared with those formed by the WT (Fig. 2B), whereas loss of phz1 had no effect on colony surface coverage. These results suggest that phz2, but not phz1, is important for phenazine production in biofilms.
Fig. 2.
phz2 is sufficient for maintaining WT colony morphology. Colony development (A) and surface area quantification (B) over the course of 6 d. (Scale bar: 1 cm.) Error bars indicate SD of three independent experiments.
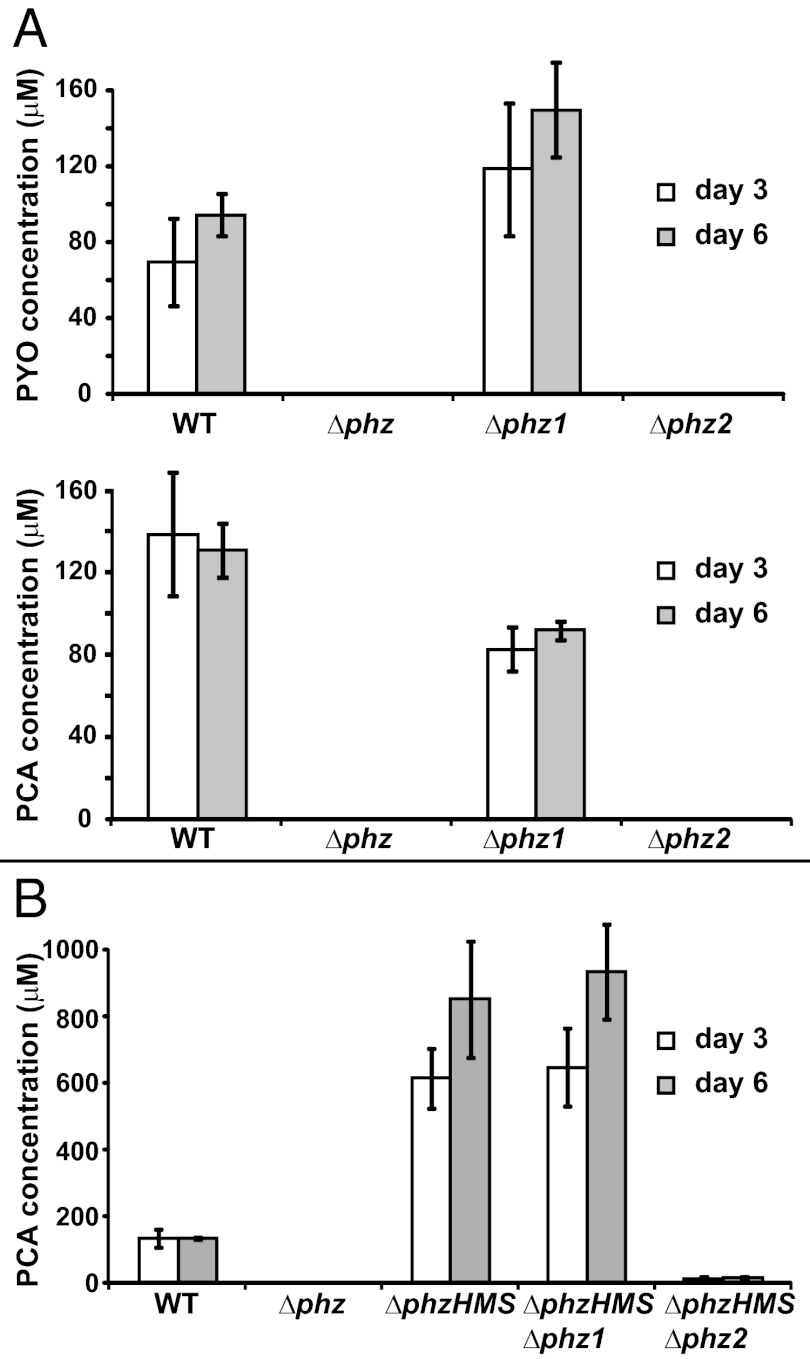
To confirm that the mutant biofilm phenotypes were consistent with their phenazine production profiles, we extracted and quantified phenazines from the agar on which the biofilms were grown. Δphz1 produced ∼60% of the PCA produced by WT, but generated ∼60% more PYO (Fig. 3A). The combined total of PYO and PCA produced by the Δphz1 was comparable to that produced by the WT biofilm (SI Appendix, Fig. S2). HPLC analysis detected no PCA or PYO from Δphz2 (SI Appendix, Fig. S3). Deletion of phzH, phzM, and phzS in the Δphz1 and Δphz2 backgrounds confirmed that all of the detectable phenazines produced by the WT colony could be produced by phz2 alone (Fig. 3B). Complementation with phz2 restored phenazine production and the WT phenotype (SI Appendix, Fig. S4).
Fig. 3.
The total PCA pool in biofilms is produced by phz2. (A) Quantification of PYO (Upper) and PCA (Lower) produced by WT, Δphz, Δphz1, and Δphz2 colonies grown on agar plates. (B) Quantification of PCA produced by ΔphzHMS, ΔphzHMSΔphz1, and ΔphzHMSΔphz2 strains grown on agar plates. Error bars indicate SD of three independent experiments.
Recently, Huang et al. (28) reported temperature-dependent regulation of phenazine production in P. aeruginosa M18 and PAO1. We grew P. aeruginosa PA14 colonies at 25 °C and 37 °C to test whether phenazine production would be affected by temperature. Similar to what Huang et al. described for liquid cultures, we found increased PYO production at 37 °C for PA14 WT and ∆phz1 colonies. The higher growth temperature did not significantly alter the phenazine production phenotypes that we observed, however; at both 25 °C and 37 °C, ∆phz2 colonies produced no detectable amounts of phenazines (SI Appendix, Fig. S5).
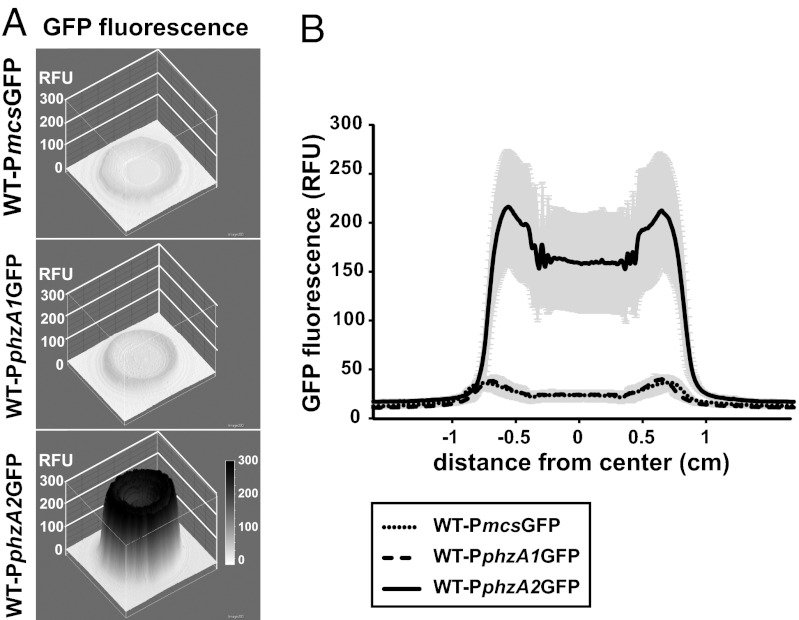
We next evaluated phz operon expression in biofilms. We quantified fluorescence across the midsection of colonies grown from strains containing PphzA1GFP and PphzA2GFP. Fluorescence levels for PphzA2GFP were significantly higher than the background in a colony containing the GFP gene cloned without a promoter (PmcsGFP) (Fig. 4). Fluorescence for the PphzA1GFP colony was indistinguishable from background. Quantification of colony midsection fluorescence gave rise to a “Batman”-shaped plot, likely related to an increased cell concentration at the colony perimeter resulting from the “coffee ring” effect (29) when a cell suspension is first spotted onto an agar surface.
Fig. 4.
phz2 is expressed at higher levels than phz1 in colony biofilms. A 3D surface fluorescence intensity plot (A) and quantification of fluorescence (B) of GFP reporter constructs for phz1 and phz2 operons. Fluorescence quantification was performed using the surface plot analysis across the middle of the colony with ImageJ. Shading indicates the SD of biological triplicates.
Quinolone-Dependent Regulation of the phz2 Operon.
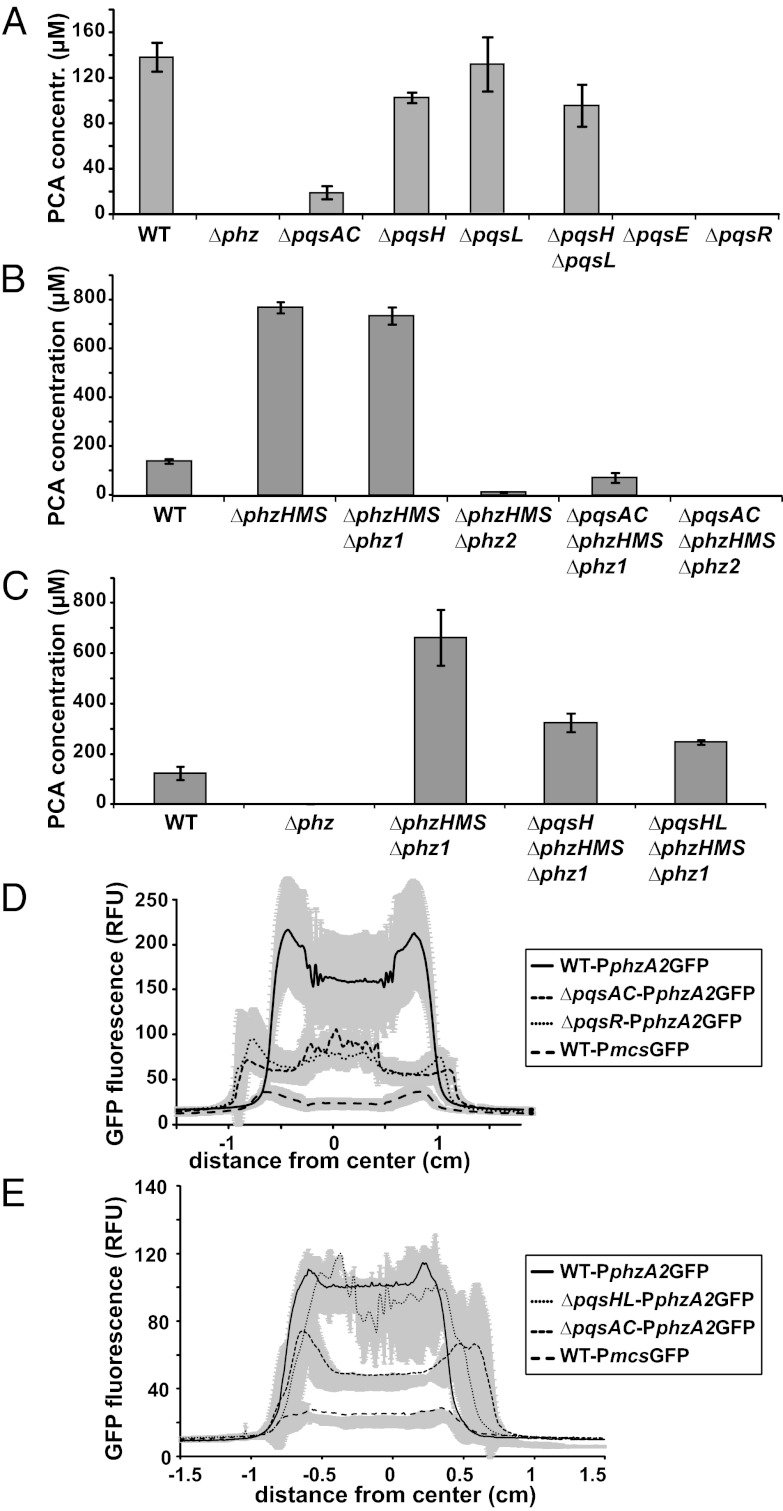
Although signaling mechanisms controlling the expression of phz1 have been identified (30), little is known about the regulation of phz2. We sought to test whether quinolones are required for phz2 induction in biofilms. P. aeruginosa produces three major types of alkyl quinolones: PQS, 4-hydroxy-2-heptylquinoline (HHQ), and 4-hydroxy-2-heptylquinoline-N-oxide (HQNO) (31, 32). We generated a mutant lacking the genes pqsABC (ΔpqsAC), which is unable to produce quinolones (33). We also created individual deletions of the genes encoding PqsL and PqsH, which catalyze the formation of HQNO and PQS, respectively (32, 34). ΔpqsAC showed the most significant defect in PCA production, whereas ∆pqsL was unaffected (Fig. 5A). ΔpqsHL had PCA production similar to that of ΔpqsH, suggesting that HHQ, not HQNO, is responsible for the majority of PCA production. Of note, mutants lacking PqsR (MvfR) or the PQS response protein PqsE did not produce PCA, consistent with previous work indicating that these proteins are required for phenazine synthesis (35).
Fig. 5.
Quinolones regulate the phz2 operon in biofilms. (A–C) Quantification of PCA from strains containing deletions in various biosynthetic genes involved in H-alkyl-quinolone (HAQ) and phenazine production. (D and E) Expression levels of the phz2 operon in strains containing deletions in HAQ and phenazine biosynthetic genes in representative experiments. Error bars in A–C and shading in D and E indicate SD of biological triplicates.
To investigate whether quinolones affect PCA production by modulating phz2 expression, we deleted the pqsABC genes in the ΔphzHMSΔphz1 and ΔphzHMSΔphz2 backgrounds and assayed for phenazine production from biofilms. ΔpqsACΔphzHMSΔphz1 showed a 90% reduction in PCA production compared with ΔphzHMSΔphz1 (Fig. 5B), demonstrating quinolone-dependent regulation of phz2. Removing quinolones also abolished PCA production from phz1 (i.e., the ΔpqsACΔphzHMS∆phz2 mutant). Finally, to evaluate which quinolones are responsible for phz2 induction, we generated the mutants ΔpqsHΔphzHMSΔphz1 and ΔpqsHΔpqsLΔphzHMSΔphz1. PCA production in these mutants was half that in the ΔphzHMSΔphz1 mutant, suggesting that both PQS and its precursor HHQ positively regulate phenazine production from phz2 (Fig. 5C).
To further verify that quinolones affect phz2 expression, we introduced PphzA2GFP into the ΔpqsAC, ΔpqsR, and ΔpqsHL mutants and compared fluorescence among the strains. phz2 expression was significantly reduced in the ΔpqsAC-PphzA2GFP and ΔpqsR-PphzA2GFP strains in biofilms (Fig. 5D), demonstrating that PqsR-mediated quinolone signaling regulates phz2 expression; however, phz2 expression in ΔpqsHL-PphzA2GFP was similar to that in WT (Fig. 5E). These observations were recapitulated in planktonic cultures (SI Appendix, Fig. S6). These results indicate that quinolones can positively regulate transcription of phz2, and that HHQ in particular plays a more significant role in induction of phz2 than in induction of phz1.
PQS production requires molecular oxygen as a substrate for the monooxygenase PqsH, suggesting that expression of phz1, but not of phz2, is oxygen-dependent. We tested this by growing WT, ∆phz, Δphz1, and Δphz2 colonies in an anaerobic chamber on a medium containing 40 mM nitrate (an alternate electron acceptor for P. aeruginosa respiration). Indeed, extracts from agar on which WT or Δphz1 colonies were grown contained comparable amounts of PCA, whereas extracts from media supporting ∆phz or Δphz2 colonies contained no phenazines (SI Appendix, Fig. S7).
phz2 Operon Is Required for Lung Colonization in a Murine Model of Infection.
Phenazine production contributes to virulence in diverse infection models (13, 36, 37). Characterizations of the bacterial populations associated with infections have suggested that P. aeruginosa assumes a biofilm-like lifestyle during host colonization (38). Based on our observation that the phz2 operon is required for phenazine production in biofilms, we tested whether phz2 is required for respiratory infection in a murine model (37). Clearance of Δphz2 and Δphz bacteria was significantly greater than that of WT bacteria (Fig. 6). Clearance was similar for Δphz1 and WT. These results suggest that the phz2 operon is required for P. aeruginosa virulence.
Fig. 6.
The phz2 operon is necessary for full virulence in a murine lung infection model. CFU counts of WT, Δphz, and Δphz2 strains from mouse lungs. Mice were inoculated with 0.3–1 × 105 CFU of P. aeruginosa WT, Δphz1, or Δphz2 and euthanized at 18 h after infection. CFU counts were performed by dilution and plating of whole lung homogenates. Straight lines within the data points indicate average CFU/mL. P values between data are shown.
Discussion
Differential expression of highly similar genes may confer selective advantages and increase complexity (5). We evaluated the expression and physiological roles of two nearly identical operons, phz1 and phz2, encoded by the P. aeruginosa genome. We hypothesized that the nonhomologous promoter regions of these redundant operons allow for condition-dependent regulation of PCA biosynthesis in diverse environments. To test this, we generated mutants with deletions in each of these operons and created fluorescent reporters for monitoring operon expression. We also deleted genes required for downstream conversion of PCA to PYO and phenazine-1-carboxamide, enabling accurate assessment of PCA production from each operon.
We found that when P. aeruginosa was grown in liquid cultures, phz1 was expressed at higher levels than phz2 (Fig. 1C, Left). However, both operons contributed significantly to PCA production, with phz2 making a greater contribution (Fig. 1B), suggesting that factors other than transcriptional regulation control the amount of PCA produced planktonically. In contrast, phz2 was the sole operon expressed and responsible for all of the phenazine production in colonies (Figs. 3 and 4). phz2 was also required for WT colony morphogenesis.
We then evaluated the quinolone dependence of phz operon expression and phenazine production, and found that a mutation that abolished all quinolone production led to a much greater reduction in PCA compared with a mutation that abolished production of PQS alone. Previous studies have demonstrated that phz1 induction is highly dependent on PQS (22, 23). Our observations indicate that quinolone-dependent regulation of phz2 differs from that of phz1, in that HHQ is the major signal controlling phz2 expression. However, like phz1 induction, phz2 induction is PqsR- and PqsE-dependent (Fig. 5). Intriguingly, neither phz operon promoter contains an identifiable PqsR-binding motif, and additional regulators may be required for their induction (39). We also detected some quinolone-independent expression of phz2, given that removing quinolone biosynthesis genes did not abolish PCA production completely (Fig. 5B).
The differences in phz1 and phz2 expression with respect to quinolone dependence and growth mode (i.e., planktonic vs. biofilm) are consistent with a role for oxygen as a major environmental cue for P. aeruginosa lifestyle transitions. P. aeruginosa produces HHQ, but not PQS, under anaerobic conditions (40). Cells in the crowded interior of biofilms and aggregates formed during infection experience anoxia. The findings that HHQ is more important for phz2 expression, and that phz2 expression is the source of PCA in biofilms, suggest that phz2 is regulated in response to oxygen indirectly via inhibition of PqsH. Accordingly, the dependence of phz1 expression on PQS is consistent with the higher expression levels in aerobically grown liquid batch cultures. Another monooxygenase gene, phzS, is located adjacent to phz1 in the genome, and both loci may be controlled by the same promoter; thus, oxygen availability may signal transitions between planktonic and biofilm lifestyles, allowing P. aeruginosa to fine-tune phenazine production in response (Fig. 7). Additional studies are needed to examine the microscale distribution of oxygen and its correlation with phz operon expression in biofilms.
Fig. 7.

Model for environment-dependent expression of phz1 and phz2. (Left) In shaken planktonic cultures, the presence of oxygen leads to the production of PQS. PQS positively regulates the expression of phz1 and phz2. (Right) In biofilms, oxygen-dependent production of PQS is decreased owing to the presence of micro aerobic and anaerobic environments. HHQ positively regulates the expression of phz2 (as also suggested by ref. 49), and phz2 is expressed at higher levels than phz1.
We also observed that phz2 is important for host colonization in a murine model of infection (Fig. 6). Although the contributions of phenazines to P. aeruginosa pathogenicity via host damage are well recognized (41, 42), we and others have recently reported beneficial roles for phenazines in P. aeruginosa physiology, including intercellular signaling, redox balancing, and enhanced iron acquisition (27, 43, 44). These diverse effects likely act together to support host colonization and infection. Furthermore, phenazines have dramatic effects on the morphological development of P. aeruginosa colonies. Whether biofilms form in our particular infection model is not known, but airway obstruction by bacterial aggregates has been reported in a similar model (37). The fact that phz2 is the relevant operon for pathogenicity in an acute infection model suggests that P. aeruginosa experiences oxygen limitation and initiates aggregate/biofilm formation even in a short time period.
The maintenance of redundant genes is paradoxical, because the presence of a duplicate gene relaxes selection pressure, allowing rapid divergence (1). Redundant genes can persist when they retain overlapping activities, yet diverge sufficiently to give rise to individualized functions (2). Alternatively, changes in the regulation of duplicated genes, in the absence of major changes at the coding sequence level, also can give rise to unique “functions,” in that the localization or timing of their expression can alter their physiological roles or ultimate effects (5, 8, 45, 46). Surveys examining genetic redundancy in yeast and bacterial genomes have identified duplicate genes that exhibit such subfunctionalization (3, 47, 48), but most of these gene pairs show relatively low sequence similarity, and their functional redundancy is usually inferred. The P. aeruginosa phz1 and phz2 operons are exceptional in their high degree of sequence similarity and redundancy. Here we have presented evidence that their specialization derives from their differential expression. Complementary regulation of the two phz operons appears to confer an advantage over regulation of a single operon, such that these nearly identical gene clusters are maintained (Fig. 7).
Materials and Methods
Bacterial Strains and Growth Conditions.
For cloning and strain construction, bacterial cultures were routinely grown at 37 °C in LB broth. For all planktonic phenazine production and gene expression experiments, strains (listed in SI Appendix, Table S1) were grown in 1% tryptone at 37 °C. Colony biofilms were grown on 1% tryptone/1% agar plates at room temperature (22–25 °C) and >95% humidity. Coomassie blue (20 µg/mL) and Congo red (40 µg/mL) were added to the plates used for morphology assays (60 mL medium; 10-cm square plates). Biofilm phenazine quantification assays were performed with 40 mL of 1% tryptone/1% agar medium without dye in 10-cm round Petri dishs. The primers used in this study are listed in SI Appendix, Table S2, and protocols for the generation of mutants and fluorescent reporter constructs are described in SI Appendix.
Quantification of Phenazines from Biofilms and Liquid Cultures.
For biofilm phenazine quantification, precultures were grown overnight in LB. Then 10 µL from these stationary-phase cultures was spotted on 1% tryptone/1% agar plates (described above) in technical replicates (10 per preculture). On day 3 or 6, five of these colonies were scraped from the plate, and half of the solid growth substrate was transferred to a 50-mL conical tube with 3 mL of water and nutated overnight to extract phenazines. Filtered extract was then loaded directly onto a Waters Symmetry C18 reverse-phase column (4.6 × 250 mm; 5 mm particle size) in a Beckman SystemGold high-performance liquid chromatograph with a photodiode array detector. Phenazines were separated following a protocol and under conditions described previously (27). Standards at known concentrations were used to calculate conversion factors for PYO and PCA (8 × 10−6 mM/AU and 9.5 × 10−6 mM/AU, respectively).
For liquid cultures grown in 1% tryptone, 200-µL samples were taken after ∼16 h of growth. These were then filtered and analyzed by HPLC as described above.
GFP Fluorescence Quantification.
For planktonic cultures, strains were grown in LB overnight, then diluted in 1% tryptone to an OD500 of 0.05 into 96-well plates (Costar; Corning). The plates were incubated at 37 °C with continuous shaking at the “low” speed in a BioTek Synergy 4 plate reader equipped with Gen5 data analysis software. The excitation wavelength was 480 nm, and emission was measured at 510 nm. For biofilms, strains were grown on 90 mL of solid media (1% tryptone, 1% agar in 10-cm square plates). Images were acquired using a Typhoon Trio variable mode scanner (GE Healthcare) after 3 d of growth. The excitation wavelength was 488 nm, and emission was measured at 520 nm. Fluorescence data were processed by surface plot analysis in ImageJ.
In Vivo Infection Model.
P. aeruginosa strains were grown in LB liquid precultures at 37 °C. Lung infections of P. aeruginosa were performed using 8-wk-old C57BL/6J mice. Mice were anesthetized with 100 mg/kg of ketamine and 5 mg/kg of xylazine and then inoculated with 0.3–1 × 105 CFU of organism before being euthanized at 18 h after infection. Bacterial CFU were assayed by homogenizing the whole lung and plating dilutions of the resuspended tissue on LB agar. All mouse infections were performed in accordance with the guidelines of Columbia University’s Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank D. Hogan for the ∆pqsH and ∆pqsR strains and Chinweike Okegbe and Ana Santiago del Toro for help with the experiments. This work was supported by startup funding from Columbia University (to L.E.P.D.), a National Science Foundation Integrative Graduate Education and Research Traineeship (to H.S.), a Parker B. Francis Fellowship (to T.S.C.), and National Institutes of Health Grant R01 HL073989 (to A.S.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213901109/-/DCSupplemental.
References
- 1.Ohno S. Evolution by Gene Duplication. New York: Springer; 1970. p. 160. [Google Scholar]
- 2.Thomas JH. Thinking about genetic redundancy. Trends Genet. 1993;9(11):395–399. doi: 10.1016/0168-9525(93)90140-d. [DOI] [PubMed] [Google Scholar]
- 3.Gevers D, Vandepoele K, Simillon C, Van de Peer Y. Gene duplication and biased functional retention of paralogs in bacterial genomes. Trends Microbiol. 2004;12(4):148–154. doi: 10.1016/j.tim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Tirosh I, Barkai N. Comparative analysis indicates regulatory neofunctionalization of yeast duplicates. Genome Biol. 2007;8(4):R50. doi: 10.1186/gb-2007-8-4-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuckerkandl E. Intrinsically driven changes in gene interaction complexity, I: Growth of regulatory complexes and increase in number of genes. J Mol Evol. 2001;53(4-5):539–554. doi: 10.1007/s002390010244. [DOI] [PubMed] [Google Scholar]
- 6.Riehle MM, Bennett AF, Long AD. Genetic architecture of thermal adaptation in Escherichia coli. Proc Natl Acad Sci USA. 2001;98(2):525–530. doi: 10.1073/pnas.021448998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Núñez MA, Pérez-Rueda E, Gutiérrez-Ríos RM, Merino E. New insights into the regulatory networks of paralogous genes in bacteria. Microbiology. 2010;156(Pt 1):14–22. doi: 10.1099/mic.0.033266-0. [DOI] [PubMed] [Google Scholar]
- 8.Kafri R, Levy M, Pilpel Y. The regulatory utilization of genetic redundancy through responsive backup circuits. Proc Natl Acad Sci USA. 2006;103(31):11653–11658. doi: 10.1073/pnas.0604883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavrodi DV, et al. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183(21):6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell CC, et al. Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. Am J Pathol. 2009;175(6):2473–2488. doi: 10.2353/ajpath.2009.090166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ran H, Hassett DJ, Lau GW. Human targets of Pseudomonas aeruginosa pyocyanin. Proc Natl Acad Sci USA. 2003;100(24):14315–14320. doi: 10.1073/pnas.2332354100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahme LG, et al. Common virulence factors for bacterial pathogenicity in plants and animals. Science. 1995;268(5219):1899–1902. doi: 10.1126/science.7604262. [DOI] [PubMed] [Google Scholar]
- 13.Starkey M, Rahme LG. Modeling Pseudomonas aeruginosa pathogenesis in plant hosts. Nat Protoc. 2009;4(2):117–124. doi: 10.1038/nprot.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassett DJ, Charniga L, Bean K, Ohman DE, Cohen MS. Response of Pseudomonas aeruginosa to pyocyanin: Mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect Immun. 1992;60(2):328–336. doi: 10.1128/iai.60.2.328-336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietrich LE, Teal TK, Price-Whelan A, Newman DK. Redox-active antibiotics control gene expression and community behavior in divergent bacteria. Science. 2008;321(5893):1203–1206. doi: 10.1126/science.1160619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mavrodi DV, et al. Diversity and evolution of the phenazine biosynthesis pathway. Appl Environ Microbiol. 2010;76(3):866–879. doi: 10.1128/AEM.02009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byng GS, Eustice DC, Jensen RA. Biosynthesis of phenazine pigments in mutant and wild-type cultures of Pseudomonas aeruginosa. J Bacteriol. 1979;138(3):846–852. doi: 10.1128/jb.138.3.846-852.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansford GS, Holliman FG, Herbert RB. Pigments of Pseudomonas species, IV: In vitro and in vivo conversion of 5-methylphenazinium-1-carboxylate into aeruginosin A. J Chem Soc Perkin Trans 1. 1972;1:103–105. doi: 10.1039/p19720000103. [DOI] [PubMed] [Google Scholar]
- 19.Whiteley M, Greenberg EP. Promoter specificity elements in Pseudomonas aeruginosa quorum-sensing–controlled genes. J Bacteriol. 2001;183(19):5529–5534. doi: 10.1128/JB.183.19.5529-5534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiteley M, Lee KM, Greenberg EP. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96(24):13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuster M, Urbanowski ML, Greenberg EP. Promoter specificity in Pseudomonas aeruginosa quorum sensing revealed by DNA binding of purified LasR. Proc Natl Acad Sci USA. 2004;101(45):15833–15839. doi: 10.1073/pnas.0407229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Déziel E, et al. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: Multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol Microbiol. 2005;55(4):998–1014. doi: 10.1111/j.1365-2958.2004.04448.x. [DOI] [PubMed] [Google Scholar]
- 23.Xiao G, et al. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol Microbiol. 2006;62(6):1689–1699. doi: 10.1111/j.1365-2958.2006.05462.x. [DOI] [PubMed] [Google Scholar]
- 24.Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP. A distinct QscR regulon in the Pseudomonas aeruginosa quorum-sensing circuit. J Bacteriol. 2006;188(9):3365–3370. doi: 10.1128/JB.188.9.3365-3370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chugani SA, et al. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2001;98(5):2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos I, Dietrich LE, Price-Whelan A, Newman DK. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res Microbiol. 2010;161(3):187–191. doi: 10.1016/j.resmic.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich LE, Price-Whelan A, Petersen A, Whiteley M, Newman DK. The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol Microbiol. 2006;61(5):1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, et al. Temperature-dependent expression of phzM and its regulatory genes lasI and ptsP in rhizosphere isolate Pseudomonas sp. strain M18. Appl Environ Microbiol. 2009;75(20):6568–6580. doi: 10.1128/AEM.01148-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deegan RD, et al. Contact line deposits in an evaporating drop. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 2000;62(1 Pt B):756–765. doi: 10.1103/physreve.62.756. [DOI] [PubMed] [Google Scholar]
- 30.Déziel E, et al. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci USA. 2004;101(5):1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lépine F, Milot S, Déziel E, He J, Rahme LG. Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa. J Am Soc Mass Spectrom. 2004;15(6):862–869. doi: 10.1016/j.jasms.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher LA, McKnight SL, Kuznetsova MS, Pesci EC, Manoil C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J Bacteriol. 2002;184(23):6472–6480. doi: 10.1128/JB.184.23.6472-6480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman JP, et al. Pseudomonas aeruginosa PqsA is an anthranilate-coenzyme A ligase. J Bacteriol. 2008;190(4):1247–1255. doi: 10.1128/JB.01140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Argenio DA, Calfee MW, Rainey PB, Pesci EC. Autolysis and autoaggregation in Pseudomonas aeruginosa colony morphology mutants. J Bacteriol. 2002;184(23):6481–6489. doi: 10.1128/JB.184.23.6481-6489.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farrow JM, 3rd, et al. PqsE functions independently of PqsR-Pseudomonas quinolone signal and enhances the rhl quorum-sensing system. J Bacteriol. 2008;190(21):7043–7051. doi: 10.1128/JB.00753-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apidianakis Y, Rahme LG. Drosophila melanogaster as a model host for studying Pseudomonas aeruginosa infection. Nat Protoc. 2009;4(9):1285–1294. doi: 10.1038/nprot.2009.124. [DOI] [PubMed] [Google Scholar]
- 37.Lau GW, Ran H, Kong F, Hassett DJ, Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect Immun. 2004;72(7):4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worlitzsch D, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109(3):317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, et al. Regulatory feedback loop of two phz gene clusters through 5′-untranslated regions in Pseudomonas sp. M18. PLoS ONE. 2011;6(4):e19413. doi: 10.1371/journal.pone.0019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schertzer JW, Brown SA, Whiteley M. Oxygen levels rapidly modulate Pseudomonas aeruginosa social behaviours via substrate limitation of PqsH. Mol Microbiol. 2010;77(6):1527–1538. doi: 10.1111/j.1365-2958.2010.07303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau GW, Hassett DJ, Britigan BE. Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol. 2005;13(8):389–397. doi: 10.1016/j.tim.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Rada B, Lekstrom K, Damian S, Dupuy C, Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181(7):4883–4893. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price-Whelan A, Dietrich LE, Newman DK. Pyocyanin alters redox homeostasis and carbon flux through central metabolic pathways in Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189(17):6372–6381. doi: 10.1128/JB.00505-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, et al. Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J Bacteriol. 2011;193(14):3606–3617. doi: 10.1128/JB.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154(1):459–473. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kafri R, Springer M, Pilpel Y. Genetic redundancy: New tricks for old genes. Cell. 2009;136(3):389–392. doi: 10.1016/j.cell.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 47.Gu X, Huang W. Testing the parsimony test of genome duplications: A counterexample. Genome Res. 2002;12(1):1–2. doi: 10.1101/gr.214402. [DOI] [PubMed] [Google Scholar]
- 48.Gu X, Zhang Z, Huang W. Rapid evolution of expression and regulatory divergences after yeast gene duplication. Proc Natl Acad Sci USA. 2005;102(3):707–712. doi: 10.1073/pnas.0409186102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ha DG, et al. 2-Heptyl-4-quinolone, a precursor of the Pseudomonas quinolone signal molecule, modulates swarming motility in Pseudomonas aeruginosa. J Bacteriol. 2011;193(23):6770–6780. doi: 10.1128/JB.05929-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.