Abstract
Bromodomain and extra terminal domain (BET) proteins function as epigenetic signaling factors that associate with acetylated histones and facilitate transcription of target genes. Inhibitors targeting the activity of BET proteins have shown potent antiproliferative effects in hematological cancers through the suppression of c-MYC and downstream target genes. However, as the epigenetic landscape of a cell varies drastically depending on lineage, transcriptional coactivators such as BETs would be expected to have different targets in cancers derived from different cells of origin, and this may influence the activity and mechanism of action of BET inhibitors. To test this hypothesis, we treated a panel of lung adenocarcinoma (LAC) cell lines with the BET inhibitor JQ1 and found that a subset is acutely susceptible to BET inhibition. In contrast to blood tumors, we show that LAC cells are inhibited by JQ1 through a mechanism independent of c-MYC down-regulation. Through gene expression profiling, we discovered that the oncogenic transcription factor FOSL1 and its targets are suppressed by JQ1 in a dose-dependant manner. Knockdown of BRD4 also decreased FOSL1 levels, and inhibition of FOSL1 phenocopied the effects of JQ1 treatment, suggesting that loss of this transcription factor may be partly responsible for the cytotoxic effects of BET inhibition in LAC cells, although ectopic expression of FOSL1 alone did not rescue the phenotype. Together, these findings suggest that BET inhibitors may be useful in solid tumors and that cell-lineage–specific differences in transcriptional targets of BETs may influence the activity of inhibitors of these proteins in different cancer types.
Keywords: drug development, chromatin
Chromatin remodeling is a key epigenetic mechanism for regulating gene expression that contributes to both normal cell phenotypes and cellular transformation. This dynamic process is controlled through the posttranslational modification of histones by distinct families of enzymes such as histone deacetylases, protein methytransferases, and lysine demethylases that add or remove functional groups at a variety of residues on histone tails (1). The resulting “marks” are then recognized by discrete classes of “reader” proteins that interpret the information and assemble into complexes that facilitate chromatin remodeling (1). This recognition step is critical in the regulation of gene expression, as the nuclear reader proteins recruit the necessary factors that initiate mRNA transcription and support mRNA elongation (2).
Members of the bromodomain and extra terminal domain (BET) family of proteins (BRD2, BRD3, BRD4, and the testis-specific BRDT) function as important reader molecules that associate with acetylated histones and govern the assembly of chromatin complexes and transcription activators at specific promoter sites (3). For example, bromodomain-containing protein 4 (BRD4) recruits the positive transcription elongation factor b (P-TEFb) complex to defined genomic locations in mitotic chromatin, promoting the phosphorylation and activation of RNA Pol II (4). Recently, BETs have been shown to control the expression of numerous genes involved in cell cycle, cell growth, inflammation, and cancer, suggesting that they function as epigenetic signaling proteins that regulate transcription from specific promoters in a cell context-dependent manner (5–9). Thus, these proteins provide potential therapeutic targets for modulating gene expression programs associated with various human diseases.
Selective inhibitors of BET proteins have recently been developed that competitively occupy the acetyl-binding pockets of the closely related bromodomains, resulting in release from active chromatin and the suppression of downstream signal transduction events to RNA polymerase (8, 10). These compounds have shown potent inhibitory activity against a range of cell lines derived from hematological malignancies, including multiple myeloma (MM), acute myeloid leukemia (AML), Burkitt’s lymphoma (BL), and mixed-lineage leukemia (MLL) (8, 10–13). Importantly, the efficacy of these compounds has been attributed mainly to their ability to suppress c-MYC (v-myc myelocytomatosis viral oncogene homolog) expression and downstream transcriptional targets, upon which these cancers are dependent for their sustained growth (8, 13). c-MYC is known to contribute to the pathogenesis of certain cancer types, so these unique findings provide a therapeutic avenue for pharmacologically inhibiting c-MYC. However, the activity of BET inhibitors in other cancers, especially carcinomas, remains largely unexplored.
The epigenetic landscape of a cell varies drastically depending on its lineage and differentiation state. Thus, it is reasonable to assume that chromatin binding factors such as BET proteins will have different transcriptional targets in cancers derived from different cells of origin, which in turn will influence the consequences and mechanism of action of BET inhibition. To test this hypothesis, we used lung adenocarcinoma (LAC) as a representative carcinoma and treated a large panel of cell lines with the BET inhibitor JQ1. We establish that a subset of these cell lines are acutely susceptible to BET inhibition and that, in contrast to hematological malignancies, this sensitivity is not dependent on c-MYC down-regulation. Furthermore, we found that the oncogenic transcription factor FOS-like antigen 1 (FOSL1) and its downstream expression targets are suppressed by JQ1 treatment and knockdown of BRD4, implying that FOSL1 may play a role in drug response in LAC. Together, these findings suggest that BET protein inhibitors may be effective across a wide range of cancers, with the mechanism of action dependent on the epigenetic status of the cell.
Results
Subset of LAC Cell Lines Is Sensitive to BET Protein Inhibition.
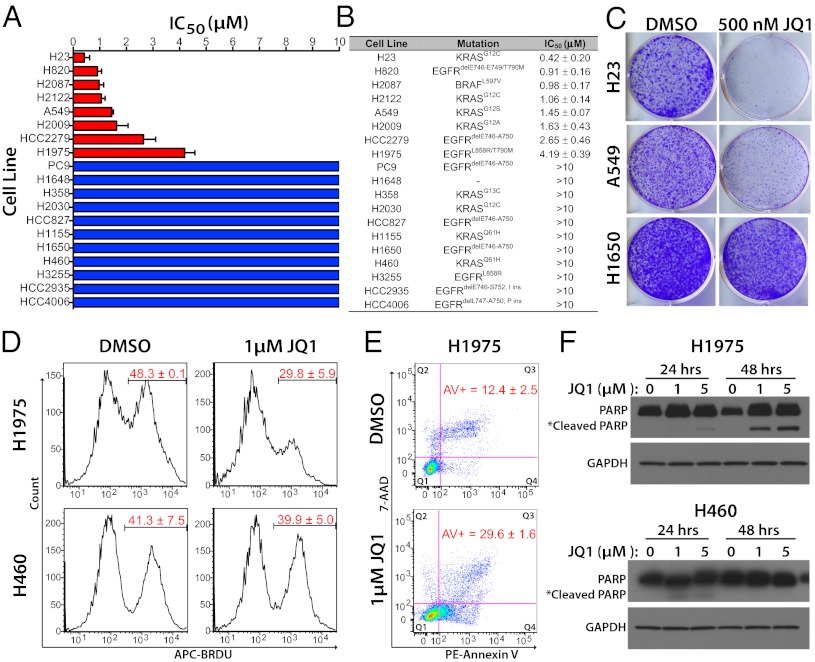
We treated a panel of 19 lung cancer cell lines for 72 h with a range of concentrations of the cell-permeable, small-molecule bromodomain inhibitor JQ1, which displays high potency and specificity toward the acetyl-binding cavity of BET proteins (10). [Two nonsmall cell lung cancer cell lines used in this study are not classified as adenocarcinomas. H1155 is a generic nonsmall cell carcinoma, and H460 (see Figs. 1 and 3) is a large cell carcinoma.] A subset of lung cancer cell lines (8/19–42%) demonstrated half maximal inhibitory concentration (IC50) values of less than 5 μM (range 0.42–4.19 μM, median = 1.26 μM), which we deemed sensitive to JQ1 treatment (Fig. 1A). All other cell lines had IC50 values >10 μM, the maximum dose used in the treatments. Although all sensitive cell lines were LACs, there was no apparent association between JQ1 sensitivity and known driver mutations in the cell lines (Fig. 1B). To complement the results from short-term treatments, we performed long-term colony-forming assays to determine if the inhibitory effects of JQ1 are sustained over time. Even at low JQ1 concentrations (500 nM), proliferation of sensitive cell lines was severely inhibited (>90% approximate reduction in cell number), whereas the insensitive line showed little effect (Fig. 1C), confirming that some lung cancer cell lines are inherently sensitive to BET protein inhibition.
Fig. 1.
JQ1 treatment inhibits the growth of a subset of lung cancer cell lines. (A) Average JQ1 IC50 values for the indicated 19 lung cancer cell lines. Cells were treated with increasing doses of JQ1 for 72 h and the number of viable cells was determined using Alamar Blue. Red bars signify sensitive cell lines (IC50 values <5 μM); blue bars mark the insensitive cell lines (IC50 values >10 μM). Error bars denote the SDs of independent experiments. (B) Putative driver mutations and JQ1 IC50 values for the 19 cell lines tested. Values are presented as averages ± SD of two to four experiments. (C) Long-term colony formation assay of cell lines deemed sensitive (H23 and A549) and insensitive (H1650) in the short-term viability assays. Cells (2,000–8,000 per six-well plate) were grown in the absence or presence of 500 nM of JQ1 for 10 d, stained with crystal violet, and photographed. (D) Cell cycle analysis of H23 (sensitive) and H460 (insensitive) cells treated with 1 μM JQ1 for 24 h. BrdU incorporation (represented by the proportion of cells stained with an anti-BrdU antibody labeled with APC fluorescent dye) indicates cells in S phase. Numbers in red represent the average percentage of cells in S phase ± SD (n = 2). (E) Induction of apoptosis by JQ1 in H1975 cells. Cells were treated with 1 μM JQ1 for 48 h and flow cytometry was performed using 7-aminoactinomycin D (7-AAD) and annexin V staining. Values represent the average percentage of annexin V+ cells (early (Q4) + late (Q3) apoptotic) ± SD (n = 2). (F) Induction of cleaved PARP1 in JQ1 sensitive (H1975) but not insensitive (H460) cell lines. Cells were treated with the indicated concentrations of JQ1 for 24 and 48 h and assessed for full-length and cleaved PARP1 protein levels by Western blot using an anti-PARP antibody. GAPDH serves as a loading control.
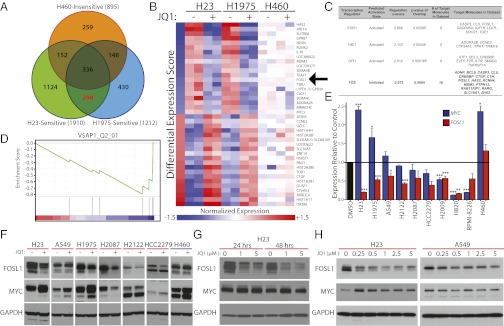
Fig. 3.
JQ1 represses expression of FOSL1 and produces a downstream gene expression signature in drug-sensitive lung cancer cell lines. (A) Venn diagram depicting the overlap of significantly differentially expressed genes (Benjamini–Hochberg corrected P ≤ 0.01) after exposure to 1 μM JQ1 for 6 h in two sensitive (H23 and H1975) and one insensitive (H460) lung cancer cell lines. The red font highlights the number of genes differentially expressed in both sensitive cell lines but not the insensitive cell line. (B) Heatmap representation of the top-20 down-regulated (blue) and top-20 up-regulated (red) genes following JQ1 treatment in sensitive and insensitive lung cancer cell lines. Genes are ranked by the differential expression score, which is shown in the Left column (details in SI Materials and Methods). Data presented are mean normalized by row for each cell line. FOSL1 (arrow) is down-regulated by JQ1 treatment. (C) Ingenuity Pathway Analysis of transcription factor programs significantly deregulated (z score ± 2.0, P < 0.05) by JQ1 treatment of drug-sensitive lung cancer cell lines. The 298 genes highlighted in A, along with their differential expression scores, revealed that a gene expression program consistent with inhibition of FOS genes is induced by JQ1 treatment. The number of genes predicted to be regulated by each transcription factor program that are also deregulated by JQ1 treatment are presented along with the corresponding gene symbols. (D) Gene set enrichment analysis plot displaying the down-regulation of genes with AP-1 DNA binding motifs after JQ1 treatment in drug-sensitive cell lines. The 298 genes from A are ranked according to their differential expression score from highest to lowest along the x axis. The overrepresentation of genes with AP-1 sites (represented by the black lines) at the bottom of the ranked gene list suggests that there is a correlation between genes with this binding motif and JQ1 down-regulated genes. The green line represents the running enrichment score. Additional details are provided in Fig. S4 and SI Materials and Methods. (E) Quantitative RT-PCR for FOSL1 (red) and c-MYC (blue) RNA levels in JQ1-treated cell lines. Data are presented as the average ratio of each gene’s expression for each cell line, relative to corresponding DMSO-treated controls (mean ± SEM). All adenocarcinoma cell lines displayed are sensitive to JQ1 except H460. The MM cell line RPMI-8226 is also depicted. Asterisks denote the level of statistical significance (*P < 0.05, **P < 0.01, ***P < 0.005; two-tailed t test). (F) Analysis of FOSL1 and c-MYC protein levels in JQ1-treated sensitive (red) and insensitive (blue) lung cancer cell lines. Cells were treated with DMSO (−) or 5 μM JQ1 (+) for 6 h before assay with anti-FOSL1 and anti–c-MYC antibodies as in Fig. 2. (G) FOSL1 protein levels diminish with the duration of JQ1 treatment. H23 cells were treated with the indicated doses of JQ1, and FOSL1 proteins were assessed at 24 and 48 h. c-MYC proteins are shown for comparison. (H) Dose-dependent effects of JQ1 treatment on FOSL1 protein levels in a drug-sensitive and a drug-resistant lung cancer cell line. c-MYC protein levels are shown for comparison. Cells were treated with the indicated doses for 6 h before analysis. GAPDH serves as a loading control for all Western blots.
To characterize the physiological effects of JQ1, we performed flow cytometry of JQ1-exposed cells to determine the consequence on cell cycle progression and apoptosis. A549 and H1975 cells, which were sensitive to the drug in the proliferation assays, demonstrated a pronounced decrease in the proportion of cells in S phase, with a concurrent increase in cells in G0/G1 during treatment with 1 μM JQ1 for 24 h, whereas H460 cells showed no significant changes in cell cycle progression (Fig. 1D and Fig. S1). This pattern is consistent with previous studies that demonstrated a critical role for the BET member BRD4 in the transition from mitosis to G1 and is similar to the effects on cell cycle induced by JQ1 in MM and BL cell lines (4, 13). In addition to cell cycle arrest, treatment with modest levels (1 μM) of JQ1 also increased the number of cells undergoing apoptosis after 48 h, as measured by annexin V staining and PARP cleavage in sensitive cell lines (Fig. 1 E and F and Fig. S2). In contrast, no evidence of apoptosis was observed in H460 cells at 48 h even at high JQ1 doses (5 μM) (Fig. 1F). Together, these data suggest that JQ1 treatment decreases proliferation, blocks cell cycle progression, and induces apoptosis in a subset of lung adenocarcinoma cell lines that are sensitive to inhibition of BET proteins.
JQ1 Inhibition Does Not Prompt MYC Down-Regulation.
Based on the recent findings that JQ1 reduces levels of c-MYC in drug-sensitive cell lines derived from hematological malignancies, we next determined if JQ1 suppresses expression of c-MYC in drug-sensitive LAC cell lines. Comparison of basal c-MYC mRNA and protein levels in JQ1-sensitive and -insensitive cell lines revealed a significant association between high c-MYC expression and JQ1 sensitivity (Fig. S3 A and B). Furthermore, gene set enrichment analysis (GSEA) (14) of microarray data identified significant up-regulation of c-MYC transcriptional targets in the sensitive cell lines, suggesting these cancers may be driven by c-MYC activation (Fig. S3C). Surprisingly, however, c-MYC mRNA levels either significantly increased or remained unchanged after JQ1 treatment in the majority (6/8) of the sensitive lung cancer cell lines (Fig. 2A). Of note, c-MYC transcript levels increased more than twofold in H23 cells, although this cell line is the most sensitive to JQ1. In contrast, consistent with previous reports (8), c-MYC levels were dramatically suppressed by JQ1 in the MM cell line RPMI-8226 (Fig. 2A). c-MYC protein levels, like mRNA levels, were elevated or unaffected by JQ1 exposure in most lung cancer cell lines (Fig. 2B). In addition, c-MYC protein levels were stable after long-term treatment and did not decrease when cells underwent apoptosis as measured by cleaved poly (ADP-ribose) polymerase 1 (PARP1) (Fig. 2C). Lastly, there was a dose-dependent increase in c-MYC levels in H23 and H1975 cells, mirrored by increasing PARP1 cleavage; in contrast, c-MYC levels decreased with the appearance of cleaved PARP1 in RPMI-8226 cells, as anticipated. Collectively, these findings suggest that c-MYC down-regulation is not a requirement for JQ1-mediated inhibition in LAC.
Fig. 2.
Growth inhibition by JQ1 in lung adenocarcinoma cells is not dependent on c-MYC down-regulation. (A) Quantitative RT-PCR for c-MYC RNA levels in JQ1-treated cell lines. The MM control cell line RPMI-8226 (blue) and sensitive lung cancer cell lines (red) were treated with 1 μM JQ1 for 6 h before RNA extraction and analysis. Data are presented as the average ratio of MYC expression for each cell line relative to its corresponding DMSO-treated control (mean ± SEM). Asterisks denote the level of statistical significance (*P < 0.05, **P < 0.01, ***P < 0.005; two-tailed t test). (B) c-MYC protein levels in JQ1-treated sensitive lung cancer cell lines. Cells were treated with DMSO (−) or 5 μM JQ1 (+) for 6 h before lysis and Western blot analysis with an anti–c-MYC antibody. GAPDH serves as a loading control. (C) c-MYC protein levels are stable over time in response to JQ1 treatment. H1975 cells were treated with the indicated doses of JQ1, then c-MYC and PARP levels were evaluated at 24 and 48 h. Cleavage of PARP occurs in the absence of c-MYC down-regulation. (D) Dose-dependent effects of JQ1 treatment on c-MYC protein levels in sensitive lung cancer cell lines (red bars) and the control MM cell line (blue bar). Cells were treated with the indicated doses of JQ1 for 24 h before analysis. Again, cleavage of PARP occurs without c-MYC down-regulation.
BET Protein Inhibition Represses FOSL1 and Its Targets in LAC Cells.
Because c-MYC was not suppressed by JQ1 in drug-sensitive LAC cells, the phenotypic effects of BET protein inhibition are likely to be mediated by different transcriptional targets in this type of tumor. To seek the relevant targets, we performed gene expression profiling of two sensitive (H23 and H1975) and one insensitive (H460) cell line treated with JQ1. To increase the likelihood of identifying direct effects of JQ1, we collected samples after just 6 h of treatment. The insensitive cell line was used to identify changes in gene expression that did not inhibit growth. Comparison of significantly differentially expressed genes identified a set of 298 annotated transcripts that are regulated by BET inhibition exclusively in sensitive cells (Fig. 3A). Ranking these transcripts by their differential expression score (defined in Materials and Methods) revealed numerous genes previously implicated in tumorigenesis that were strongly down-regulated by JQ1 treatment; these included HAS2, ILR7, MDM2, RUNX2, TRAF1, and FOSL1 (Fig. 3B and Dataset S1).
To determine if the deregulation of a specific transcription factor could potentially explain the changes in gene expression induced by BET inhibition, we performed ingenuity pathway analysis (IPA) using the JQ1-affected genes. Four transcription regulators were found to be significantly associated with the JQ1 gene signature, with three predicted to be activated (EGR1, HIC1, and GFI1) and one inhibited (FOS), based on whether their target genes were up- or down-regulated (Fig. 3C). To complement the IPA analysis, we used GSEA to identify common transcription-factor binding sites associated with genes affected by BET inhibition. Genes repressed by JQ1 treatment were significantly enriched for activator protein 1 (AP-1) DNA binding motifs; these serve as targets for the heterodimeric AP-1 transcription factor complex formed by FOS and JUN protein family members (Fig. 3D and Fig. S4) (15). Thus, both IPA and GSEA demonstrated a significant enrichment for FOS targets within the JQ1-regulated gene set. In contrast, binding motifs for the other transcription factors predicted by IPA to be associated with JQ1 response (EGR1, HIC1, and GFI1) did not significantly overlap with the gene signature determined by GSEA (Fig. S4). Lastly, IPA or GSEA did not identify c-MYC targets as being significantly repressed upon JQ1 treatment, suggesting that BET inhibition is not interfering with c-MYC transcription function in a manner independent of c-MYC down-regulation (Fig. 3C and Fig. S4).
Gene expression analyses highlighted a potential role for the transcription factor FOS in mediating the response to JQ1 in LAC cell lines. Although FOS itself was not differentially expressed upon JQ1 treatment, its closely related family member, FOSL1, was one of the most significantly down-regulated genes (Fig. 3B). Like FOS, FOSL1 forms dimers with JUN family members to regulate AP-1 target genes (16), and FOS and FOSL1 are thought to control similar sets of genes, because FOSL1 is able to replace FOS in genetically altered animal models (16). Although both proteins have previously been implicated in tumorigenesis, FOSL1 is the main FOS family member linked to lung cancer (17). Analysis of RNA-Seq data from The Cancer Genome Atlas revealed that it is the only FOS member commonly overexpressed in LAC (Fig. S5). Furthermore, the BET protein BRD4 is known to localize to the FOSL1 enhancer where it initiates transcriptional initiation and elongation (18). Thus, we predict that deregulation of FOSL1 might be responsible, at least in part, for the FOS gene expression signature induced by JQ1 treatment and may represent a key target of BET inhibition in sensitive cell lines.
Consistent with this hypothesis, FOSL1 mRNA was repressed upon treatment of all JQ1-sensitive cell lines, with 6/8 reaching statistical significance (Fig. 3E). In addition, FOSL1 protein levels decreased following BET inhibition in most drug-sensitive cell lines (Fig. 3F), whereas the insensitive cell line H460 showed no appreciable effects on FOSL1 expression or protein levels after JQ1 treatment (Fig. 3 E and F, respectively), consistent with the findings from the gene expression profiling analysis (Fig. 3 A and B). The MM cell line RPMI-8226 also showed decreased FOSL1 mRNA after treatment, although this did not reach significance. This contrasts with c-MYC expression levels (Fig. 3 E and F). FOSL1 protein also diminished with increased duration of JQ1 treatment in H23 cells (Fig. 3G); unlike c-MYC FOSL1 protein appeared to decline in association with cleaved PARP1 (Fig. 3G and Fig. S2), suggesting a functional link between FOSL1 suppression and the induction of apoptosis in this cell line. Lastly, there was a dose-dependent decrease in FOSL1 protein in H23 and A549 cells, demonstrating the dependency of FOSL1 levels on BET activity. Together, these data suggest that FOSL1 is a direct target of BET inhibition in LAC cell lines.
FOSL1 Is a Transcriptional Target of BRD4 in LAC Cell Lines.
BRD4 is known to localize to the FOSL1 enhancer where it recruits P-TEFb, leading to the activation of RNA Pol II and subsequent transcriptional elongation (18). To confirm the requirement of BRD4 for FOSL1 transcription, and thus, demonstrate its viability as a target of BET inhibition in LAC, we reduced expression of BRD4 with siRNA in JQ1-sensitive cell lines and assayed for FOSL1 mRNA and protein levels. H23, A549, and H1975 cells were treated with BRD4 siRNAs and reduced levels of BRD4 were confirmed by quantitative RT-PCR (Fig. 4A). BRD4 knockdown significantly reduced levels of FOSL1 transcripts in all JQ1-sensitive cell lines analyzed, mimicking the effects of JQ1 treatment (Fig. 4A). In addition, BRD4 siRNA suppressed FOSL1 protein in these lines, whereas it had no effect on c-MYC protein levels, similar to the results produced by JQ1 treatment in these cell lines (Fig. 4B and Fig. S6). Knockdown of the BET family members BRD2 and BRD3 had no apparent effects on FOSL1 mRNA or protein levels, implying that the decrease of FOSL1 upon JQ1 treatment is specifically due to inhibition of BRD4 (Fig. S6).
Fig. 4.
FOSL1 knockdown phenocopies the effects of JQ1 treatment and BRD4 knockdown in lung cancer cell lines. (A) Knockdown of BRD4 decreases the expression of FOSL1 in JQ1-sensitive cell lines. FOSL1 (blue) and BRD4 (red) mRNA levels were assessed by quantitative RT-PCR 72 h after transfection of the indicated siRNAs. Data presented are the average ratio of each gene’s expression relative to the levels observed in cells transfected with nontargeting (NonT) siRNA (mean ± SEM, n = 3). (B) Knockdown of BRD4 decreases FOSL1 protein levels. H1975 and A549 were transfected with the indicated siRNAs and FOSL1 and c-MYC levels were assessed by Western blot after 72 h as described in Fig. 3. GAPDH serves as a loading control. BRD4 knockdown decreases FOSL1 but not c-MYC levels, mimicking JQ1 treatment. (C) Knockdown of FOSL1 and BRD4 decreases viability of JQ1-sensitive lung cancer cell lines. Cell lines were transfected with the indicated siRNAs and viability was assessed by Alamar Blue after 72 h. Data are presented as the average viability of cells treated with each siRNA or JQ1, relative to cells transfected with the nontargeting (NonT) siRNA or DMSO (mean ± SEM, n = 3). KIF11 and KRAS siRNAs serve as positive controls. Asterisks denote the level of statistical significance (*P < 0.05, **P < 0.01, ***P < 0.005; one-tailed, one-sample t test).
JQ1-Sensitive LAC Cells Are Inhibited by FOSL1 Knockdown.
The sensitivity of a subset of LAC cell lines to BET inhibition, coincident with down-regulation of FOSL1 in these cell lines, suggested that FOSL1 might mediate the response to JQ1. Correspondingly, this would also mean that sensitive cell lines are dependent on sustained FOSL1 expression for survival. The finding that FOSL1 mRNA and protein levels were significantly higher in sensitive compared with insensitive cell lines could be interpreted to support this assumption (Fig. S7). Therefore, we used RNAi to inhibit FOSL1 in sensitive cell lines, because this should phenocopy the effects of BET protein knockdown and JQ1 treatment. H23, A549, and H1975 cells were treated with siRNAs for FOSL1 or BRD4, and knockdown was confirmed by quantitative RT-PCR and Western blot (Fig. 4 A and B and Fig. S6). Cell viability was assessed 72 h after knockdown, with nontargeting (NonT) and KRAS or KIF11 siRNAs serving as negative and positive controls, respectively. Knockdown of FOSL1 significantly reduced the viability of all three sensitive cell lines (average reduction in viability = 35%), in agreement with a role for FOSL1 in mediating survival (Fig. 4C). Although FOSL1 siRNA inhibited the cells to a lesser degree than JQ1 treatment (average reduction in viability = 64%), the effects were similar to those observed by BRD4 knockdown (average reduction in viability = 40%) (Fig. 4C). Importantly, knockdown of BRD2 and BRD3 also inhibited the viability of the sensitive cell lines, by 37 and 27%, respectively (Fig. S6). Although JQ1 is selective toward binding of BET, and not other, bromodomains, it has nearly equal activity against BRD2, BRD3, and BRD4. Thus, the effects of JQ1 treatment on LAC cells may be due to the cumulative inhibition of all BET family members and not a single protein alone. Coupled with the finding that BRD4 is the only BET family member that appeared to control FOSL1 levels (Fig. S6), these data suggest that FOSL1 may serve as a downstream factor mediating the BRD4-specific effects of JQ1 inhibition and support the conclusion that down-regulation of FOSL1 is at least a partial cause of growth inhibition induced by BET inhibitors in LAC cells.
Exogenous Expression of FOSL1 Is Not Sufficient to Rescue Cells from JQ1 Treatment.
Next, we asked whether exogenous expression of FOSL1 could rescue sensitive cell lines from the deleterious effects of BET inhibition. Two sensitive cell lines (H23 and H1975) were stably transduced with retroviral vectors that express FOSL1 cDNA and expression was confirmed by Western blots (Fig. S8). Exogenous expression of FOSL1 led to stable protein levels in these cell lines even after JQ1 treatment, consistent with the expectation that BRD4 is not required for transcription from the retroviral construct (Fig. S8). Cells were then treated with JQ1 and assayed as in the initial screens described in Fig. 1 to determine sensitivity to BET protein inhibition. Surprisingly, cells overexpressing FOSL1 and control cells were equally sensitive to JQ1 (Fig. S8), indicating that exogenous FOSL1 was unable to protect cells from growth inhibition mediated by JQ1. Thus, although FOSL1 down-regulation likely plays a role in response to the BET inhibitor, it is probable that additional factors also render LAC cells sensitive to inhibition of BET proteins.
Discussion
In this study, we show that a subset of LAC cell lines is highly sensitive to the BET inhibitor JQ1, suggesting that such inhibitors may provide a viable therapeutic strategy for treatment of solid as well as hematological tumors. Down-regulation of c-MYC and its target genes was identified as the main mechanism mediating the antiproliferative effects of BET inhibition in leukemia and lymphoma (8, 11, 13). In these cancer types, BRD4 actively binds to the c-MYC promoter and regulates signaling events leading to transcript elongation (13). In response to BET inhibitors, BRD4 is released from acetylated histones at the c-MYC locus, reducing gene expression (13). We found here that BET inhibition does not down-regulate c-MYC in sensitive lung cancer cells but instead affects the expression of several other genes potentially involved in the drug response. This was surprising, especially considering the fact that sensitive LAC cell lines demonstrated many aspects of c-MYC dependency, including high c-MYC mRNA and protein levels and up-regulation of c-MYC target genes (Fig. S3). However, as the epigenetic state of a cell is dramatically influenced by cell lineage, it is likely that BET and other chromatin binding proteins have different transcriptional targets in different cancer types. Indeed, our results imply that c-MYC, although dependent on BET proteins for transcription in the hematological cancer cell lines, is regulated by BET-independent mechanisms in LAC cells. These findings have important implications, as they suggest that not all cancers dependent on c-MYC for survival will respond to BET inhibition. Instead, sensitive cancers from different tissue types will likely respond to BET inhibition through different pathways. As a result, it may be difficult to predict which patients will benefit from therapy with BET inhibitors. Thus, a strategy that considers cell lineage, genetic background, and epigenetic status will likely be needed to achieve optimal treatment response. Furthermore, it should also be noted that reduction of c-MYC is not solely responsible for the antiproliferative effects of BET inhibition in hematological cancers, as exogenous expression of c-MYC from a retroviral vector cannot fully rescue cells from JQ1 treatment (8, 13). Also, in addition to potentially maintaining the expression of driver oncogenes, BET proteins serve many general functions involved in regulating cell cycle progression (9), such as bookmarking genes for postmitotic reactivation (19). As a result, the action of additional genes is likely, and the effects of BET inhibition may be mediated through multiple factors, even in a single cancer type.
Down-regulation of the oncogenic transcription factor FOSL1 was identified as a potential cause of the antiproliferative effects of BET protein inhibition in LAC cells. FOSL1 mRNA and protein levels were reduced by JQ1, and genome-wide expression profiling identified a significant enrichment of putative FOSL1 targets within the set of JQ1-repressed genes. FOSL1 poses a reasonable target for mediating the effects of BET protein inhibition in LAC: numerous lines of evidence point to a key role for this transcription factor in lung tumorigenesis. For example, FOSL1 is persistently activated by toxicants in cigarette smoke, acts as a downstream transcription effector of signaling pathways commonly mutated in lung cancer, and induces lung epithelial cell invasion and anchorage-independent growth (17, 20). Furthermore, overexpression of FOSL1 but not other AP-1 components, in transgenic mouse models leads to the development of lung bronchoalveolar tumors (21). Our finding that FOSL1 is overexpressed in a large proportion of human LAC tumors further supports this conclusion. Lastly, FOSL1 is necessary for KRAS- and EGFR-induced neoplastic transformation in different model systems (22). Because many of the JQ1-sensitive LAC lines have mutations in KRAS or EGFR, FOSL1 may play a vital role in maintaining the malignant phenotype of these cells. Indeed, we found that many cell lines that are sensitive to JQ1 treatment have elevated FOSL1 levels and that FOSL1 knockdown inhibits their growth, suggesting that these cells are dependent on sustained FOSL1 for survival. However, as some of the LAC cell lines most sensitive to JQ1, notably H23, express very low levels of FOSL1 whereas some that are insensitive to JQ1 have high FOSL1 mRNA and protein—as well as mutations in EGFR and KRAS—the predictive value of these characteristics are clearly limited. Low or high levels of FOSL1 may sensitize LAC cell lines to JQ1, depending on the specific cellular context, such as the status of other FOS family members. Furthermore, JQ1-induced down-regulation of FOSL1 was less common in insensitive lung cancer cell lines and, unlike sensitive cell lines, their growth was not significantly inhibited by FOSL1 knockdown (Fig. S9), implying a possible correlation between FOSL1 dependency and JQ1 sensitivity in lung adenocarcinoma. Collectively, our data show that FOSL1 is down-regulated by BET inhibition and that this event is associated with the decreased viability of drug-sensitive LAC cells. Because FOSL1 has a critical role in lung cancer development, BET inhibitors may provide a therapeutic strategy for targeting tumors dependent on this gene.
Further support for FOSL1 as a target of BET inhibition is provided by the observation that BRD4 is required for expression of the FOSL1 gene (18). The mechanism by which this occurs is well established and is stimulated by the phosphorylation of serine 10 in histone H3 (H3S10ph) at the FOSL1 enhancer by PIM1 kinase. This phosphorylation triggers a cascade of events leading to histone acetylation, BRD4 binding, and subsequent enhancement of transcriptional elongation (18). Thus, it is assumed that treatment with JQ1 would directly interfere with localization of BRD4 to the FOSL1 enhancer by disrupting the ability of its bromodomain to bind to the acetylated histone H3 induced by this signaling cascade. Consistent with this notion, we found that knockdown of BRD4 mimicked the effect of JQ1 treatment, leading to decreased levels of FOSL1 transcripts and proteins in sensitive lung cancer cell lines. Interestingly, c-MYC is also known to localize to the FOSL1 enhancer where it is involved in recruiting PIM1 kinase and thus initiating the chain of events described above (23). Because JQ1-sensitive LAC cells express high levels of c-MYC RNA and protein, in addition to FOSL1 gene products, and c-MYC and FOSL1 transcript levels are highly correlated across lung cancer cell lines (Fig. S7), it will be interesting to explore the relationship between c-MYC, FOSL1, and BRD4 in this cancer type and investigate how the dynamics among these proteins are disrupted by exposure to BET inhibitors. Together, our findings, combined with those from previous studies, highlight the requirement for BRD4 in regulating FOSL1 gene transcription.
The finding that exogenous FOSL1 did not rescue sensitive LAC cell lines from the effects of JQ1 treatment might seem to contradict our conclusion that this gene is involved in mediating the drug response. We argue that this finding does not preclude a role for FOSL1. Because the knockdown of BRD2 and BRD3, in addition to the knockdown of BRD4, inhibited the growth of drug-sensitive lung cancer cells, it is likely that the cumulative inhibition of all BET proteins is responsible for the antiproliferative effects of JQ1 in this tumor type. However, because down-regulation of FOSL1 is attributed specifically to BRD4 inhibition, it is likely that exogenous FOSL1 cannot protect sensitive cells from the deleterious effects of BRD2 and BRD3 inactivation elicited by JQ1. In addition, because FOSL1 needs to form a hetrodimer to regulate transcription from AP-1 DNA binding motifs (16), it is possible that overexpressing FOSL1 alone may not be sufficient to rescue target genes without coexpression of an appropriate binding partner. Moreover, as JQ1 leads to the down-regulation of several genes with potential roles in tumorigenesis, such as HAS2, MDM2, ILR7, and TRAF1, in addition to FOSL1, we cannot rule out the possibility that other factors may be responsible for mediating the effects of BET inhibition in LAC. Indeed, a recent study on the effects of JQ1 in acute lymphoblastic leukemia identified IL7R as a critical target (24). Even so, our cumulative results support the conclusion that loss of FOSL1 is at least partly responsible for the cytotoxic effects of BET inhibition in LAC cells.
Materials and Methods
Cell lines were cultured under standard conditions in 96-well plates and treated with JQ1 for 72 h followed by the addition of Alamar Blue cell viability reagent for dose–response analysis. Cell cycle analysis, Annexin V staining, protein extraction, western blots, quantitative RT-PCR, gene expression profiling, and siRNA transfections were performed using standard methods. A detailed description of the reagents, protocols, and computational analyses used in this study can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Nancy Colburn and Matthew Young for providing the pFB-FRA1 construct. This work was funded by the National Institutes of Health Intramural Research Program. W.W.L. is supported by the Canadian Institutes of Health Research Jean-Francois Saint Denis Fellowship in Cancer Research.
Footnotes
Conflict of interest statement: Dana Farber Cancer Institute has licensed drug-like derivatives of JQ1 from the J.E.B. laboratory to Tensha Therapeutics for clinical development as anticancer agents.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216363109/-/DCSupplemental.
References
- 1.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: A new frontier for drug discovery. Nat Rev Drug Discov. 2012;11(5):384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- 3.Filippakopoulos P, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149(1):214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z, He N, Zhou Q. Brd4 recruits P-TEFb to chromosomes at late mitosis to promote G1 gene expression and cell cycle progression. Mol Cell Biol. 2008;28(3):967–976. doi: 10.1128/MCB.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell. 2009;20(23):4899–4909. doi: 10.1091/mbc.E09-05-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeRoy G, Rickards B, Flint SJ. The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol Cell. 2008;30(1):51–60. doi: 10.1016/j.molcel.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicodeme E, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468(7327):1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delmore JE, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S-Y, Chiang C-M. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282(18):13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- 10.Filippakopoulos P, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuber J, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson MA, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478(7370):529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertz JA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA. 2011;108(40):16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eferl R, Wagner EF. AP-1: A double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 16.Verde P, Casalino L, Talotta F, Yaniv M, Weitzman JB. Deciphering AP-1 function in tumorigenesis: Fra-ternizing on target promoters. Cell Cycle. 2007;6(21):2633–2639. doi: 10.4161/cc.6.21.4850. [DOI] [PubMed] [Google Scholar]
- 17.Reddy SPM, Mossman BT. Role and regulation of activator protein-1 in toxicant-induced responses of the lung. Am J Physiol Lung Cell Mol Physiol. 2002;283(6):L1161–L1178. doi: 10.1152/ajplung.00140.2002. [DOI] [PubMed] [Google Scholar]
- 18.Zippo A, et al. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138(6):1122–1136. doi: 10.1016/j.cell.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 19.Zhao R, Nakamura T, Fu Y, Lazar Z, Spector DL. Gene bookmarking accelerates the kinetics of post-mitotic transcriptional re-activation. Nat Cell Biol. 2011;13(11):1295–1304. doi: 10.1038/ncb2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adiseshaiah P, Lindner DJ, Kalvakolanu DV, Reddy SP. FRA-1 proto-oncogene induces lung epithelial cell invasion and anchorage-independent growth in vitro, but is insufficient to promote tumor growth in vivo. Cancer Res. 2007;67(13):6204–6211. doi: 10.1158/0008-5472.CAN-06-4687. [DOI] [PubMed] [Google Scholar]
- 21.Jochum W, et al. Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nat Med. 2000;6(9):980–984. doi: 10.1038/79676. [DOI] [PubMed] [Google Scholar]
- 22.Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41(16):2449–2461. doi: 10.1016/j.ejca.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Zippo A, De Robertis A, Serafini R, Oliviero S. PIM1-dependent phosphorylation of histone H3 at serine 10 is required for MYC-dependent transcriptional activation and oncogenic transformation. Nat Cell Biol. 2007;9(8):932–944. doi: 10.1038/ncb1618. [DOI] [PubMed] [Google Scholar]
- 24.Ott CJ, et al. BET bromodomain inhibition targets both c-MYC and IL7R in high-risk acute lymphoblastic leukemia. Blood. 2012;120:2843–2852. doi: 10.1182/blood-2012-02-413021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.