Abstract
The time when an event occurs can become part of autobiographical memories. In brain structures that support such memories, a neural code should exist that represents when or how long ago events occurred. Here we describe a neuronal coding mechanism in hippocampus that can be used to represent the recency of an experience over intervals of hours to days. When the same event is repeated after such time periods, the activity patterns of hippocampal CA1 cell populations progressively differ with increasing temporal distances. Coding for space and context is nonetheless preserved. Compared with CA1, the firing patterns of hippocampal CA3 cell populations are highly reproducible, irrespective of the time interval, and thus provide a stable memory code over time. Therefore, the neuronal activity patterns in CA1 but not CA3 include a code that can be used to distinguish between time intervals on an extended scale, consistent with behavioral studies showing that the CA1 area is selectively required for temporal coding over such periods.
Keywords: episodic memory, pattern completion, place cells
Autobiographical memories include a detailed record about the place where events occurred and about when the events took place (1–4). The temporal aspect of these memories consists of detailed sequences of events as they occurred in real time, but many memories are also part of an extended timeline that relates the different episodes to each other, such as in a storyboard for filmmaking. The hippocampal system is required for remembering events over time intervals that exceed the capacity of short-term or immediate memories (5). Because these memories include temporal information over extended timescales, hippocampal neuronal activity should generate a code that can represent when or how long ago an event occurred within the remembered time range. However, hippocampal coding for time has only been described on a scale up to minutes. Temporal coding over these shorter intervals is thought to occur by the sequential or cumulative activation of cell populations or by the activation of delay-dependent cells (6–11). These mechanisms depend on ongoing activity patterns, which can be interrupted by unrelated neuronal firing patterns or by a change in brain state, and thus are not amenable to coding over longer timescales. It is known that humans and other animal species can remember temporal relations on the timescale of hours and days (12–20), and theoretical considerations have suggested that the coding of such extended temporal relations may emerge from fluctuations within the memory network (21–25). Nonetheless, a neural code for remembering when or how recently an episode occurred within the last hours or days has not yet been identified experimentally.
Beyond the timescale during which sustained firing patterns of hippocampal neurons can maintain a continuous representation of events, each new memory is thought to be stored as a change in synaptic strengths resulting from neuronal firing patterns at the time of encoding (26, 27). Later retrieval would be achieved by reinstating the corresponding neuronal activity from the stored pattern of synaptic strengths (28, 29). Under such a coding scheme, the activation of neuronal firing patterns for familiar, repeated events would be expected to be highly similar. If the reinstated firing patterns were exactly the same during each repetition, the event would be represented with high fidelity, but the neuronal code would not contain any information about when the events occurred or about how much time had passed between the events. Alternatively, random fluctuations or a continuous change in neuronal firing patterns could result in a somewhat distinct neuronal firing pattern for each occurrence of an otherwise highly similar event and thereby generate information about temporal distances or the temporal context of events (21–25). We therefore asked whether these proposed neural coding schemes could be observed in hippocampal neural networks for events that were identical except for the time when they occurred.
If differences in neuronal activation emerge over extended time periods, this might result in a loss of detailed information about past events. We therefore also asked how encoding of time might be complementary to retaining precise representations for other types of contextual information. To address these questions, we used a behavioral paradigm in which the firing patterns of the same hippocampal cell populations were repeatedly measured in the same behavioral apparatus over extended time intervals. To examine if coding differences over time might result in a loss of precise information about other aspects of the context, we tested, at each time point, to what extent the firing patterns were able to code for differences between enclosures that only differed in their shape.
Results
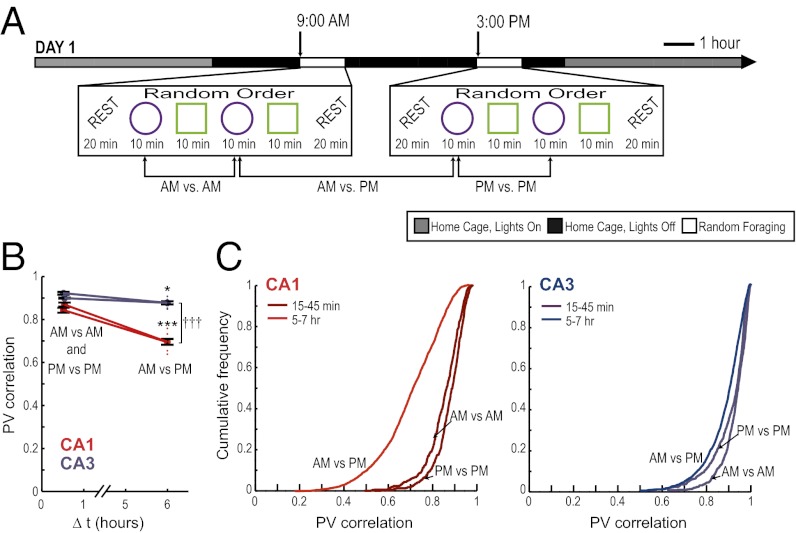
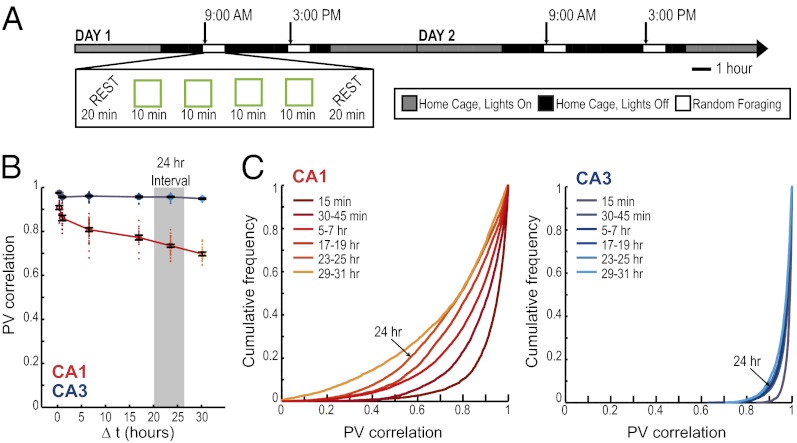
We trained six rats to randomly forage in a square and in a circular enclosure in the morning (AM) and, after an interval of 6 h, in the afternoon (PM) of each day. The training at each time of the day consisted of four 10-min sessions, two in each enclosure shape, presented in random order (Fig. 1A). The training schedule remained consistent over many days. Rats were therefore highly familiar with the behavioral paradigm when recordings began such that any observed differences in firing patterns could not be attributed to novelty-related effects.
Fig. 1.
Decorrelated neuronal firing patterns between morning and afternoon sessions in hippocampal cell populations. (A) Behavioral design and experimental timeline. A series of four random-foraging sessions was conducted in the morning (AM), and a second series of four sessions was conducted in the afternoon (PM). Each series consisted of a random sequence of two sessions in a square and of two sessions in a circular enclosure. The enclosure was placed in the same location within the room, and extramaze cues remained constant. Recordings began after 9–26 d of pretraining in this paradigm. (B) The firing patterns of simultaneously recorded CA1 and CA3 neuronal populations were compared across repetitions of the same enclosure shape (as illustrated for circle comparisons in A). A PV represents the firing rates of all active cells within a 5 × 5 cm pixel in the spatial map. The activity patterns of an active cell population were compared between sessions by calculating the correlation coefficients of PVs from corresponding pixels between two sessions and by taking the average over all pixels. The values for pair-wise comparisons at short time intervals (within AM or within PM) and at long time intervals (AM vs. PM) were grouped. Each of the comparisons is shown as a red or blue dot, and black error bars report the mean ± SEM of between-session comparisons at each time interval. CA1 cell populations (red) showed a decreased correlation between sessions that occurred at 6-h intervals compared with sessions within <1 h. PVs of CA3 cells (blue) also showed a small degree of decorrelation between time intervals, but the degree of decorrelation was substantially lower in CA3 compared with CA1. (C) Cumulative distribution functions for PV correlations between pairs of recordings in the same enclosure shape at different time intervals. *P < 0.05; ***P < 0.001 for comparisons between time intervals; †††P < 0.001 for comparisons between CA1 and CA3; see text for statistics.
Because it was critical for all aspects of the data analysis that each recorded cell could be identified accurately throughout the entire sequence of sessions, we used a technique for electrode placement that was optimized for recording stability across time, and we applied stringent standards to the acceptance of cells for further analysis (Materials and Methods and Fig. S1). In particular, we flanked the recordings in behavior with recording periods during rest. Most hippocampal cells fire bursts of action potentials during rest periods (30, 31), and these recordings can thus be used to confirm that the signals of each cell could be reliably recorded before, after, and throughout the entire experimental paradigm, even if the cell was not active during all periods of random foraging.
In the dataset with confirmed cell identity within an entire recording day (n = 99 CA1 cells and n = 126 CA3 cells), we found in both the hippocampal CA1 and CA3 subregions that repetitions of the same enclosure shape resulted in highly reproducible firing of hippocampal cells at well-defined spatial locations, as reported consistently since hippocampal place fields were first described (32–35). However, when considering the network activity pattern of the CA1 cell population, we found that the similarity in neuronal activity decreased for the 6-h interval compared with intervals of less than 1 h (t = −7.71, P < 0.001), indicating that events that are farther separated in time have more distinct neuronal representations (Fig. 1 B and C). In contrast, the similarity of firing patterns in the CA3 cell population decreased to a much smaller extent between the <1-h interval and the 6-h interval (t = −2.95, P < 0.05 for the comparison between time intervals; t = −9.92, P < 0.001 for the comparison between CA1 and CA3). Differences between hippocampal subregions were also observed for the firing rates of single cells such that subsets of CA1 but not CA3 cells showed a high variability between repetitions of the same enclosure shape in the morning and afternoon. Thus, we find that CA3 maintains highly similar representations for repeated events, irrespective of the elapsed time between them, whereas CA1 representations vary to a larger extent between repeated events that are separated over intervals of several hours [F(103, 49) = 1.98, P < 0.01] (Fig. 2).
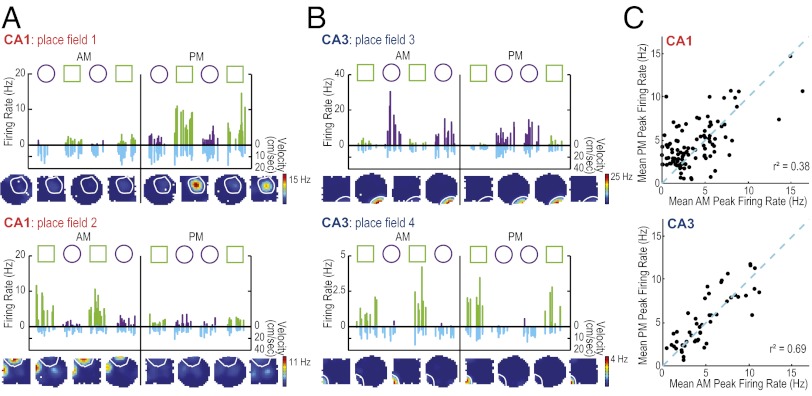
Fig. 2.
CA1 place fields show variability in firing rate between morning and afternoon sessions. (A) Firing rates for two representative CA1 place fields and (B) for two CA3 place fields. Fields 2, 3, and 4 were recorded simultaneously. Each 10-min recording session throughout the AM and PM is shown. Symbols above each graph indicate the order of enclosure shapes. Each bar (green for the square shape and purple for the circular shape) represents the firing rate of the cell during a pass of the animal through the place field (see Fig. S6 for methods and Fig. S7 for additional examples). For each pass, the corresponding running speed of the animal is plotted downward below the x-axis (cm/s, in blue). The variability in firing rates cannot be explained by movement velocity or by the proximity of the path to the field center (Fig. S8). For each firing field the corresponding color-coded rate maps (averaged across each 10-min recording session) are shown below the line graph. The color scale for rate maps is from 0 Hz (blue) to the peak rate of the day (red). Coding differences emerged, in part, from CA1 place fields that changed or became silent at a subset of time points. In all cases in which cells became silent, it was verified in preceding or subsequent rest sessions that spikes from these cells could be detected (Fig. S1). (C) For each place field, mean peak rates within each enclosure shape were calculated in the morning and in the afternoon. These rates were compared within each hippocampal subregion. Place fields that were not active (mean peak rate <2 Hz) at either time point were excluded. For active cells, the firing rates between AM and PM were more variable in CA1 compared with CA3 (see text for statistics; SI Materials and Methods).
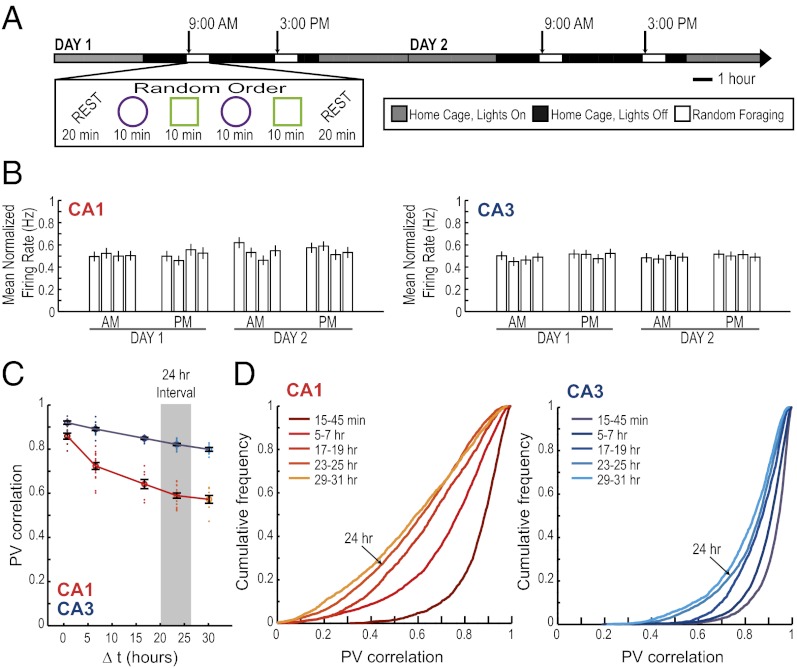
Next, we asked whether differences in hippocampal firing patterns were circadian. Pronounced fluctuations in hippocampal gene expression patterns, neurotransmitter release, and in hippocampal synaptic excitability occur with the circadian clock (36–40). Any differences in the population code for an identical environment after a time interval of 6 h might therefore reflect a circadian or ultradian modulation of activity patterns. If hippocampal neural network activity were controlled by internal states associated with circadian or other endogenous clocks, one would expect to find a cyclical pattern in neuronal firing such that the similarity at matching times of the day is higher than at nonmatching times within a day. To examine this possibility, we identified the hippocampal place cells that could be reliably tracked through the morning and afternoon of two consecutive recording days (n = 50 CA1 cells and n = 71 CA3 cells). We first looked for circadian fluctuations in normalized firing rates. Consistent with a previous study (41), we found no differences across time in the average normalized firing rate [F(15) = 0.90 for CA1 and 0.32 for CA3; not significant (n.s.)] (Fig. 3 A and B). We then asked whether the differences in neuronal activity patterns in the hippocampal CA1 area, when comparing sessions recorded 24 h apart and thus matched for time of day, showed any increase in similarity compared with those at 6-h time intervals, as would be expected for a circadian cycle. Instead, the recordings after 24 h exhibited a further decrease in similarity compared with the 6-h time point, such that the similarity in hippocampal firing in CA1 monotonically decreased for time intervals between 30 min up to at least 30 h [F(4) = 46.3, P < 0.001] (Fig. 3 C and D; Fig. S2). The network activity in CA3 also showed a gradual decrease in similarity with time [F(4) = 50.5, P < 0.001], although to a much smaller extent than the decrease in CA1. The correlation at 30 h was 0.80 ± 0.003 in CA3 compared with 0.57 ± 0.006 in CA1 (t = −11.5, P < 0.001). Therefore, we do not find a distinct coding scheme in the CA1 or CA3 network for representing the exact time of day at which an event occurred, but rather find that the degree of similarity between two representations differs depending on the temporal distance between them, in particular within the CA1 cell population.
Fig. 3.
The decorrelation in CA1 network activity over extended time periods does not repeat cyclically across days. (A) To determine whether differences in CA1 network activity patterns can be explained as a circadian effect, we extended the hippocampal recordings across 2 d. (B) The mean ± SEM normalized firing rate for all active CA1 (Left) and CA3 (Right) principal neurons is shown for each 10-min recording session across the 2-d experiment. For each cell, the normalized firing rate was calculated by dividing the average firing rate for each session by that cell’s maximum average firing rate in any of the sessions. A circadian variation in firing rate was not observed (see text for statistics). (C) PV correlations between pairs of recordings in the same enclosure shape are shown as dots. Every pair-wise comparison is aligned to its time interval, so that, for example, the AM/PM comparisons on day 1 and the AM/PM comparisons on day 2 are all aligned to the 6-h interval (see Fig. S9 for the complete pair-wise correlation matrix). The black error bars report the mean ± SEM for pair-wise comparisons at each time interval. The correlation coefficients for the CA1 population activity (red) decreased monotonically as a function of elapsed time between recording sessions up to at least 30 h (see text for statistics and Fig. S2 for comparisons of up to 60 h). Repeated CA1 recordings at matching times of day on two consecutive days (24-h interval) show a smaller correlation than recordings at shorter intervals but at different times of the day (P < 0.001 for the post hoc comparison). Thus, the effect we observed is not due to circadian fluctuations. (D) Cumulative distribution functions for PV correlations between pairs of recordings in the same enclosure shape at different time intervals.
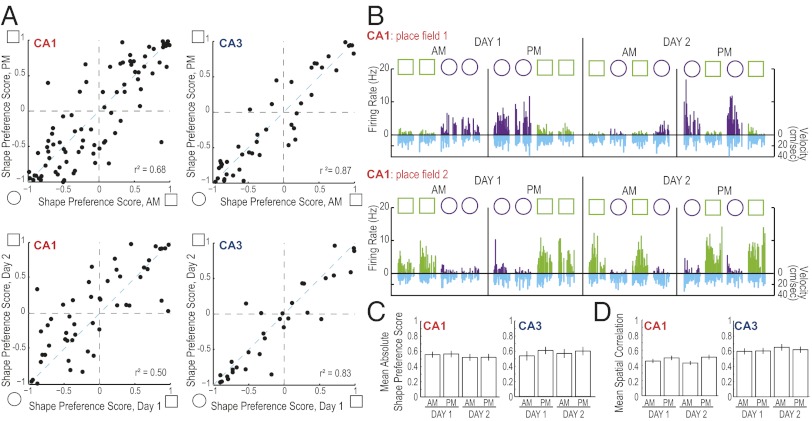
If the decrease in similarity over time reflected merely an unconstrained random drift in firing patterns, such continuous change could eventually result in a decrease in the accuracy with which hippocampal neuronal firing patterns code for a repetition of the same event after a long time interval and, consequently, in a change of the ability to represent the relative similarity between contexts (42, 43). We observed that, at any time point, there were many CA1 cells that discriminated between the two different enclosure shapes, but that individual CA1 cells showed inconsistency across extended time periods in their strength of preference for firing in one of the enclosure shapes (Fig. 4 A and B; see figure legend for statistics). In contrast, individual CA3 cells showed a striking consistency in enclosure shape coding by firing rate over extended time periods. Although individual CA1 cells showed high variability in preferentially firing in one of the enclosure shapes, the average degree of shape preference within the CA1 cell population did not change over time periods of 6 h or between days [absolute shape preference: CA1, F(3) = 0.28, n.s., CA3, F(3) = 0.33, n.s.; spatial correlation: CA1, F(3) = 0.30, n.s., CA3, F(3) = 2.59, n.s.] (Fig. 4 C and D). This pattern of coding in CA1 can result in consistent representations of contextual differences, even though the population vector correlations for repetitions of identical shapes were as low as 0.57 between days (see above and Fig. 3).
Fig. 4.
Differences in firing patterns over extended time periods did not preclude the encoding of spatial information or of contextual differences. (A) For each place field a shape preference score was calculated as a measure of the difference in firing rates between the circular and the square enclosure (scores of −1 or +1 indicate that the cell fired only in the circle or only in the square, respectively). This score was compared between recording blocks (Left, CA1; Right, CA3) in the AM and PM (Upper) and between days (Lower). For calculating each day’s score, all AM and PM recording sessions within a day are used. Individual CA1 fields show more variable shape coding over 6-h intervals and over 1-d intervals than individual CA3 fields [F(82, 43) = 2.51 and F(50, 31) = 2.55, P < 0.01 for comparisons of shape preference scores at both time intervals]. (B) Firing rates within two representative CA1 place fields from the same cell over 2 d (data presented as described in Fig. 2; see Fig. S10 for additional examples). The CA1 place fields showed a change in the degree of discrimination between the square and circular enclosure between time points (Fig. 2A). Field 1 normally fired at its highest rate in the circular shape, but became silent in the AM session on day 2. The field resumed its firing in the circular enclosure during the PM session on day 2. It was therefore observed that shape preferences could be lost and regained between blocks of sessions at different time points. (C and D) Even though individual CA1 cells show coding differences between time points, the average degree of context and place coding is consistent within CA1 and within CA3 cell populations (see text for statistics).
Did the dissimilarity in the CA1 hippocampal population code emerge as a function of elapsed time, or could differences in neuronal firing patterns be the outcome of cumulative rate changes during the behavioral sessions such that larger differences were reached with increased random foraging experience? If experience in the recording enclosure were primarily responsible for the reorganization of the network, then the changes in network activity would be expected to occur predominantly during running in the enclosure, the period during which place cells are active and when activity-dependent network plasticity occurs (44). To examine this possibility, we established several control conditions. First, we tested whether the amount of random foraging experience between two time points had an effect on the similarity of CA1 population coding. At the 24-h and at the 30-h interval, we found the same degree of decorrelation irrespective of the number of intervening four-session blocks (t = −0.64, n.s. for 24-h intervals; t = −1.82, n.s. for 30-h intervals; Fig. S3A). Second, we observed decorrelation with time even when limiting our analysis to time intervals without any intervening AM or PM blocks [F(3) = 41.6, P values for all post hoc comparisons <0.001 except that the comparisons between the 18- and 24-h interval is n.s.] (Fig. S3B). Third, we asked whether the continued switching between two different enclosure shapes might explain why CA1 firing patterns accumulated firing differences across a series of recording sessions. To examine this possibility, we performed a 2-d series of AM and PM recordings in only one enclosure shape (Fig. 5A). We found that this paradigm abolished the already small degree of decorrelation that we had observed over long time periods in CA3 [all mean population vector (PV) correlations >0.96; all post hoc comparisons between time intervals ≥0.5 h, n.s.] (Fig. 5 B and C). At the same time, a monotonic decrease in population similarity with extended time was still observed in hippocampal CA1 cell populations [F(5) = 54.0, P < 0.001; P < 0.001 for all post hoc comparisons, except P < 0.01 for <1 h compared with 6 h, and n.s. for 6 h compared with 18 h, 18 h compared with 24 h, and 24 h compared with 30 h]. Finally, we examined whether a repetitive increase or a repetitive decrease in the firing rate of place fields within each 10-min session might contribute to progressive changes over long time periods. We observed that a large fraction of CA3 place fields (57.9%) exhibited either a consistent decrease or a consistent increase in firing rate within each 10-min behavioral session. However, consistent rate changes were uncommon in CA1 cells (11.1%; Fig. S4). Systematic changes in firing patterns within recording sessions—as expected for activity-dependent plasticity mechanisms—were thus primarily observed in the CA3 hippocampal subregion, which showed only minor differences in activity patterns over long time periods, and not in the CA1 subregion, where marked changes occurred across longer timescales.
Fig. 5.
When testing with a single enclosure shape, firing patterns of the CA3 network remained highly consistent for repetitions of the same environment over extended time intervals, whereas activity patterns in the CA1 network changed. (A) An experimental design with a single enclosure shape was used to test whether the decorrelation of hippocampal activity patterns could have been an effect of intervening experiences in a different context (Fig. 3). The mean PV correlation between pairs of recordings in the same enclosure shape (B) and the corresponding cumulative distribution function for the PVs (C) are shown as described in Fig. 3. Highly consistent firing patterns in the CA3 population were observed over time intervals of 30 min to 30 h. In contrast, the CA1 network continued to show a pronounced monotonic decrease in firing similarity with time (see text for statistics).
Discussion
Theories of long-term memory coding require that stored firing patterns are accurately reinstated during later retrieval (26–29). In contrast, theoretical considerations for representing temporal aspects in long-term memory require fluctuations of activity patterns in neuronal networks such that relative temporal distances or temporal order can be represented even when the events are otherwise identical (21–25). We provide evidence for both of these neuronal coding schemes within distinct subregions of hippocampus on a timescale of hours and days. In the CA1 cell population, the degree of similarity of neuronal responses for identical locations in the same context decreases monotonically as a function of the time between experiences for at least 30 h. These changes in neuronal network activity were measured in highly familiar environments and thus appear unrelated to changes that have been reported during new learning (41, 45–47). The fluctuations also seem not to correspond merely to noise, as it would be unlikely for a cell to stop firing for a time and then resume its prior firing field, firing rate, and shape preference by chance. Finally, we could not detect a circadian component in the coding difference within the hippocampal CA1 cell populations. The increasing decorrelation with longer time intervals in CA1, but not in CA3, is consistent with behavioral studies showing that the hippocampal CA1 area is selectively required for temporal coding over extended time periods (20), and that rats are able to use “how long ago” but not “when in a day” as a cue for locating a food reward (15, 19).
In contrast to CA1, CA3 cell populations showed highly reproducible firing patterns over extended time periods between repeated recordings. We therefore find a complementary neural code in CA3, which provides a highly stable representation of space and context without the possibility of contributing information about extended time. The striking stability of firing patterns in CA3 compared with the pronounced decorrelation in CA1 suggests that differences in neuronal activity over time are not an inevitable consequence within highly plastic hippocampal networks. To the contrary, CA3 generates nearly identical firing patterns for repeated events over time intervals when changes in synaptic strengths are expected (36–39). This function is consistent with the proposed role of the recurrent network architecture in CA3 for pattern completion (27, 48–50). Although the typical definition of pattern completion proposes that a partial sensory input pattern is expanded to a pattern that is stored in memory, our finding of network stability for exact repetitions across long time intervals suggests that accurate neuronal firing patterns can be generated not only from degraded sensory inputs, but also when fluctuation or degradation in synaptic strength may emerge within neural circuits over extended time intervals. Ongoing changes in synaptic strength could emerge not only from random variability, but also by network reorganization over long time periods, as predicted by theories of consolidation and reconsolidation (51). Our results indicate that hippocampal memory circuitry includes network mechanisms within CA3 that provide consistency of neuronal representations despite fluctuations or circuit reorganization over extended time intervals within CA1 and, possibly, within a wider cortical network.
Even though neuronal firing patterns in CA3 remained remarkably consistent, the input from CA3 to CA1 did not result in equally consistent firing patterns in CA1. This finding is perhaps not unexpected because CA1 also receives major direct input from entorhinal cortex (52) and is thus the site for convergence of firing patterns from layer III of entorhinal cortex—which may include temporal information (53, 54) and be critical for memory consolidation (55)—with the highly consistent firing patterns it receives from CA3. Even though the CA3 firing patterns are not completely transferred to CA1, they may nonetheless provide substantial firing stability to the CA1 cell population. This notion is supported by the finding that although each individual subregion became more decorrelated in the two-shape compared with the single shape condition, the difference in decorrelation over time between CA3 and CA1 remained consistent irrespective of the experimental condition (Figs. 3 and 5; Fig. S5). This consistency further supports the idea that a neuronal code for temporal distances might emerge in CA1 by adding fluctuations to a more stable representation of other aspects of context it receives from CA3.
Though temporal coding over periods of seconds and minutes can use delay-dependent and sequential coding (7–10), and though time-stamps over weeks might be explained by long-term structural reorganization (56), the mechanism that we describe can encompass temporal coding in the behaviorally relevant domain of hours and days. The observed pattern of neural activity rules out the possibility that the differences across time are due to a small subpopulation of hippocampal cells that is dedicated to temporal coding. Rather, differences in neuronal coding with extended time, along with sustained precision for other aspects of the context unrelated to time, are achieved by a high but balanced variability in the firing patterns of individual CA1 cells such that the average context discrimination remains consistent. Even though such a network pattern may, at least in part, be generated by random variability and decay in synaptic strength, by intervening modifications of synaptic strength, or by random fluctuations in network patterns, this would nonetheless correspond to theoretical considerations (21–25) that predict that temporal information can be retrieved from the resulting variability in the population code over time. Our findings provide experimental evidence of a neuronal code that can be used for encoding temporal distances on a timescale of hours and days and that can co-occur with a precise neuronal code for other aspects of memory such that the “when, what, and where” aspects of memories can be simultaneously represented within the hippocampal CA1 cell population.
Materials and Methods
Subjects.
Six male Long Evans rats with a preoperative weight of 400–485 g were housed individually and maintained on a 12-h light/12-h dark schedule with lights off at 6:00 AM. All behavioral testing occurred in the dark phase with one exception, as described below. All experimental procedures were performed as approved by the Institutional Animal Care and Use Committee at the University of California at San Diego and according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (57).
Surgical Procedures.
At the time of surgery, the rats were anesthetized with isoflurane gas [2–2.5% in O2 (20 mL /1 L per minute)] and an electrode assembly that consisted of 14 independently movable tetrodes was implanted above the right hippocampus (3.8–4.0 mm posterior and 3.0 mm lateral to bregma).
Behavioral Procedures.
After 1 wk of recovery from surgery, rats were partially food-deprived and trained to forage for randomly scattered cereal crumbs in an enclosure with walls that could be shaped either as a square (80 × 80 cm) or as a 16-sided polygon (50-cm radius; referred to as a circle) (43). A polarizing white cue card (20 cm wide) was placed on an inside wall of the enclosure. The center of the enclosure was always located at the same place in the room, and the angle of the cue card compared with external room cues was kept constant. Training was performed in two daily blocks. For all rats, the first block started between 8:30 and 9:30 AM, and the second block between 2:30 and 3:30 PM. For each individual rat, the daily start time of each block varied by less than 30 min. Rats were returned to the animal housing room between the morning and afternoon training blocks.
Rats were trained to run for four 10-min sessions during each block, with two sessions in the square enclosure and two sessions in the circle enclosure. The order of the shapes varied randomly within each training block. The rats were allowed to rest for 5 min between sessions, and training blocks were flanked by sleep sessions (10–20 min before and after each block). The floor of the enclosure was cleaned with water between each session. Following the sleep session at the end of the afternoon training block, rats were screened for single-unit activity. Electrophysiological recordings throughout the morning and afternoon sessions were initiated when multiple well-isolated cells (>300 µV) were observed on most tetrodes. The recording phase of the experiment began after 14–26 d of behavioral training, except in one rat in which the recordings commenced after 9 d.
Recordings were first conducted for 2 d in the training paradigm (referred to as day 1 and day 2 in the text). In three animals, recordings were performed on a third day. The third day was identical to the first two recording days, except that the start times of the blocks were shifted by 6 h, so that the first block occurred at 3:00 PM and the second at 9:00 PM. The second block was thus during the light phase of the light/dark cycle. Two animals returned for 1 d to the standard training conditions before two additional days of recordings were performed in which all four random foraging sessions in the morning and afternoon block were conducted in one enclosure shape (single shape, day 1; single shape, day 2). This paradigm was identical to the standard paradigm, except that only one of the two enclosure shapes was used in all behavioral sessions throughout both days. One rat was tested in the square and the other in the circle enclosure. For each animal, we selected the shape in which we identified the larger number of active cells during the recording on the preceding day.
Cell Tracking.
Because our study depended on being able to follow the same set of principal cells over an extended time period, we developed a customized version of MClust (58) with added functions that allowed for the comparison of the cluster boundaries of each cell throughout a series of 10-min recording sessions. Clusters that persisted in the same region of parameter space throughout a day (or multiple days) were accepted for further analysis. Care was taken to accept only cells that could be precisely followed from the beginning to the end of the data analysis (Fig. S1), and for which all spikes were included in the cluster boundary such that observed rate changes could not be attributed to the definition of the cluster boundaries.
Data Analysis.
For tracked cells, we calculated the spatial map of each 10-min session and the spatial correlations between maps. For a more detailed analysis of firing rates within the place field, we also analyzed the firing during individual passes through the place field (Figs. S6 and S11). From firing-rate measurements within the place field, we derived scores for differences between square and circular enclosures for different times throughout the experiment. For the entire population of CA1 cells and for the population of CA3 cells, we calculated PV correlations between 10-min sessions that were recorded at different times, but within the same enclosure shape.
Histology.
Final tetrode locations were confirmed histologically. Tetrodes with tips in or near the border of the CA2 region were excluded from the analysis.
Additional details on the cell tracking, data analysis, and histology can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the Ray Thomas Edwards Foundation, a Walter F. Heiligenberg Professorship, National Science Foundation/National Institutes of Health/Bundesministerium für Bildung and Forschung Grant 101046, and Alberta Innovates–Health Solutions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214107109/-/DCSupplemental.
References
- 1.Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Academic; 1972. pp. 381–403. [Google Scholar]
- 2.Tulving E. Elements of Episodic Memory. Oxford: Clarendon; 1983. [Google Scholar]
- 3.Friedman WJ. Memory for the time of past events. Psychol Bull. 1993;113(1):44–66. [Google Scholar]
- 4.O’Keefe J, Burgess N, Donnett JG, Jeffery KJ, Maguire EA. Place cells, navigational accuracy, and the human hippocampus. Philos Trans R Soc Lond B Biol Sci. 1998;353(1373):1333–1340. doi: 10.1098/rstb.1998.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zola-Morgan S, Squire LR. Neuroanatomy of memory. Annu Rev Neurosci. 1993;16:547–563. doi: 10.1146/annurev.ne.16.030193.002555. [DOI] [PubMed] [Google Scholar]
- 6.Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56(3):530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321(5894):1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill PR, Mizumori SJ, Smith DM. Hippocampal episode fields develop with learning. Hippocampus. 2011;21(11):1240–1249. doi: 10.1002/hipo.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333(6043):773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 11.Howard MW, Viskontas IV, Shankar KH, Fried I. Ensembles of human MTL neurons “jump back in time” in response to a repeated stimulus. Hippocampus. 2012;22(9):1833–1847. doi: 10.1002/hipo.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395(6699):272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- 13.Clayton NS, Yu KS, Dickinson A. Scrub jays (Aphelocoma coerulescens) form integrated memories of the multiple features of caching episodes. J Exp Psychol Anim Behav Process. 2001;27(1):17–29. [PubMed] [Google Scholar]
- 14.Babb SJ, Crystal JD. Discrimination of what, when, and where: Implications for episodic-like memory in rats. Learn Motiv. 2005;36(2):177–189. [Google Scholar]
- 15.Babb SJ, Crystal JD. Discrimination of what, when, and where is not based on time of day. Learn Behav. 2006;34(2):124–130. doi: 10.3758/bf03193188. [DOI] [PubMed] [Google Scholar]
- 16.Babb SJ, Crystal JD. Episodic-like memory in the rat. Curr Biol. 2006;16(13):1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien J, Sutherland RJ. Evidence for episodic memory in a pavlovian conditioning procedure in rats. Hippocampus. 2007;17(12):1149–1152. doi: 10.1002/hipo.20346. [DOI] [PubMed] [Google Scholar]
- 18.Iordanova MD, Good MA, Honey RC. Configural learning without reinforcement: Integrated memories for correlates of what, where, and when. Q J Exp Psychol (Hove) 2008;61(12):1785–1792. doi: 10.1080/17470210802194324. [DOI] [PubMed] [Google Scholar]
- 19.Roberts WA, et al. Episodic-like memory in rats: Is it based on when or how long ago? Science. 2008;320(5872):113–115. doi: 10.1126/science.1152709. [DOI] [PubMed] [Google Scholar]
- 20.Kesner RP, Hunsaker MR. The temporal attributes of episodic memory. Behav Brain Res. 2010;215(2):299–309. doi: 10.1016/j.bbr.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 21.Estes WK. Statistical theory of spontaneous recovery and regression. Psychol Rev. 1955;62(3):145–154. doi: 10.1037/h0048509. [DOI] [PubMed] [Google Scholar]
- 22.Mensink GJ, Raaijmakers JGW. A model for interference and forgetting. Psychol Rev. 1988;95(4):434–455. [Google Scholar]
- 23.Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- 24.Murdock BB. Context and mediators in a theory of distributed associative memory (TODAM2) Psychol Rev. 1997;104(4):839–862. [Google Scholar]
- 25.Howard MW, Kahana MJ. A distributed representation of temporal context. J Math Psychol. 2002;46(3):269–299. [Google Scholar]
- 26.Hebb D. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- 27.Mcnaughton BL, Morris RGM. Hippocampal synaptic enhancement and information-storage within a distributed memory system. Trends Neurosci. 1987;10(10):408–415. [Google Scholar]
- 28.Reijmers LG, Perkins BL, Matsuo N, Mayford M. Localization of a stable neural correlate of associative memory. Science. 2007;317(5842):1230–1233. doi: 10.1126/science.1143839. [DOI] [PubMed] [Google Scholar]
- 29.Liu X, et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484(7394):381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olmstead CE, Best PJ, Mays LE. Neural activity in the dorsal hippocampus during paradoxical sleep, slow wave sleep and waking. Brain Res. 1973;60(2):381–391. doi: 10.1016/0006-8993(73)90797-x. [DOI] [PubMed] [Google Scholar]
- 31.Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41(2):461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 32.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 33.O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51(1):78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 34.Muller RU, Kubie JL, Ranck JB., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7(7):1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson LT, Best PJ. Long-term stability of the place-field activity of single units recorded from the dorsal hippocampus of freely behaving rats. Brain Res. 1990;509(2):299–308. doi: 10.1016/0006-8993(90)90555-p. [DOI] [PubMed] [Google Scholar]
- 36.Barnes CA, McNaughton BL, Goddard GV, Douglas RM, Adamec R. Circadian rhythm of synaptic excitability in rat and monkey central nervous system. Science. 1977;197(4298):91–92. doi: 10.1126/science.194313. [DOI] [PubMed] [Google Scholar]
- 37.Tononi G, Cirelli C. Modulation of brain gene expression during sleep and wakefulness: A review of recent findings. Neuropsychopharmacology. 2001;25(5)(Suppl):S28–S35. doi: 10.1016/S0893-133X(01)00322-0. [DOI] [PubMed] [Google Scholar]
- 38.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Hut RA, Van der Zee EA. The cholinergic system, circadian rhythmicity, and time memory. Behav Brain Res. 2011;221(2):466–480. doi: 10.1016/j.bbr.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 40.Valnegri P, et al. A circadian clock in hippocampus is regulated by interaction between oligophrenin-1 and Rev-erbα. Nat Neurosci. 2011;14(10):1293–1301. doi: 10.1038/nn.2911. [DOI] [PubMed] [Google Scholar]
- 41.Munn RG, Bilkey DK. The firing rate of hippocampal CA1 place cells is modulated with a circadian period. Hippocampus. 2012;22(6):1325–1337. doi: 10.1002/hipo.20969. [DOI] [PubMed] [Google Scholar]
- 42.Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7(7):1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 44.Mehta MR, Barnes CA, McNaughton BL. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc Natl Acad Sci USA. 1997;94(16):8918–8921. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416(6876):90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- 46.Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci. 2004;24(35):7681–7689. doi: 10.1523/JNEUROSCI.1958-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14(11):745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- 48.Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262(841):23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 49.Rolls ET. Functions of neuronal networks in the hippocampus and cerebral cortex in memory. In: Cotterill RMJ, editor. Models of Brain Function. Cambridge, UK: Cambridge Univ Press; 1989. pp. 15–33. [Google Scholar]
- 50.Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2(2):189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 51.Wang SH, Morris RG. 2010. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annu Rev Psychol 61:49–79, C41–C44.
- 52.Witter MP, Moser EI. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 2006;29(12):671–678. doi: 10.1016/j.tins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Howard MW, Natu VS. Place from time: Reconstructing position from a distributed representation of temporal context. Neural Netw. 2005;18(9):1150–1162. doi: 10.1016/j.neunet.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334(6061):1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- 55.Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431(7009):699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- 56.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 57. Committee on Care and Use of Laboratory Animals (1985) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 58.Redish AD. University of Minnesota, Minneapolis; 2009. MClust 3.5, Free-Ware Spike Sorting. Available at http://redishlab.neuroscience.umn.edu/MClust/MClust.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.