Abstract
No conventional therapy exists for salivary hypofunction in surviving head and neck cancer patients with Radiation Therapy Oncology Group late grade 2–3 toxicity. We conducted a phase I clinical trial to test the safety and biologic efficacy of serotype 5, adenoviral-mediated aquaporin-1 cDNA transfer to a single previously irradiated parotid gland in 11 subjects using an open label, single-dose, dose-escalation design (AdhAQP1 vector; four dose tiers from 4.8 × 107 to 5.8 × 109 vector particles per gland). Treated subjects were followed at scheduled intervals. Multiple safety parameters were measured and biologic efficacy was evaluated with measurements of parotid salivary flow rate. Symptoms were assessed with a visual analog scale. All subjects tolerated vector delivery and study procedures well over the 42-d study period reported. No deaths, serious adverse events, or dose-limiting toxicities occurred. Generally, few adverse events occurred, and all were considered mild or moderate. No consistent changes were found in any clinical chemistry and hematology parameters measured. Objective responses were seen in six subjects, all at doses <5.8 × 109 vector particles per gland. Five of these six subjects also experienced subjective improvement in xerostomia. AdhAQP1 vector delivery to a single parotid gland was safe and transfer of the hAQP1 cDNA increased parotid flow and relieved symptoms in a subset of subjects.
Keywords: gene therapy, radiation damage, salivary glands, dry mouth, water channel
Each year, ∼40,000 patients develop head and neck cancers in the United States, with ∼500,000 cases worldwide (1). Typical treatment for these patients includes irradiation (IR). About half of all surviving patients [Radiation Therapy Oncology Group (RTOG) late grade 2–4 toxicity (2–4)] experience irreversible damage to salivary glands in the IR field that dramatically reduces saliva output. Saliva is critical to the physiology and maintenance of upper gastrointestinal tract tissues, providing critical antimicrobial, lubricatory, remineralizing, and reparative functions (5). As a result, these patients suffer considerable morbidity, including oral infections (Candidiasis, rampant caries), mucositis, dysphagia, and frank discomfort, with a marked decline in quality of life (4, 6).
Salivary glands consist of two types of epithelia (7). Approximately 80% of the cells are acinar, secreting most exocrine proteins and being the only sites of water movement. Duct cells, the other major general cell type, are essentially water impermeable and NaCl absorbing. Following IR, patients with RTOG late-grade 2–3 toxicity lose most acinar cells, and surviving parenchymal cells are primarily ductal (8). Acinar cells are highly differentiated and postmitotic, but markedly radiosensitive by as yet unexplained mechanisms. Without sufficient acinar tissue, RTOG grades 2 and 3 patients do not respond well to the cholinergic agonist sialogogues. The only available treatments for their severe xerostomia are minimally effective wetting agents.
This absence of conventional therapy prompted our efforts to use gene transfer (8) to increase fluid secretion. Using evidence from rodent studies, we reasoned that without significant acinar cell function duct cells could generate an osmotic gradient (lumen > interstitium). If these cells expressed a facilitated water-permeability pathway, fluid secretion could occur (9). To test this hypothesis, we constructed a first-generation serotype 5, adenoviral (Ad5) vector encoding human aquaporin-1 (hAQP1) and used it to treat irradiated salivary glands of rats (9) and miniature pigs (10). In both species, salivary flow was reduced dramatically following IR. However, treatment of irradiated glands with AdhAQP1 led to near normal levels of salivary fluid secretion, albeit transiently, but a control Ad5 vector had no benefit.
Following a large safety study in rats (11), we received all required approvals to test AdhAQP1 in single irradiated parotid glands of subjects ≥ 5 y posttreatment for head and neck cancer (http://www.clinicaltrials.gov/ct/show/NCT00372320). Because AdhAQP1 displayed transient efficacy in preclinical studies (for ∼2 wk) (10), and Ad5 vectors can elicit significant early toxicity (e.g., ref. 11), this report focuses on the initial (through day 42) safety and biologic responses of the 11 subjects treated in this phase I trial (Fig. 1). This study is unique in representing direct gene therapy in the oral cavity for a nonmalignant condition, and is one of the few gene-therapy studies addressing a quality of life disorder.
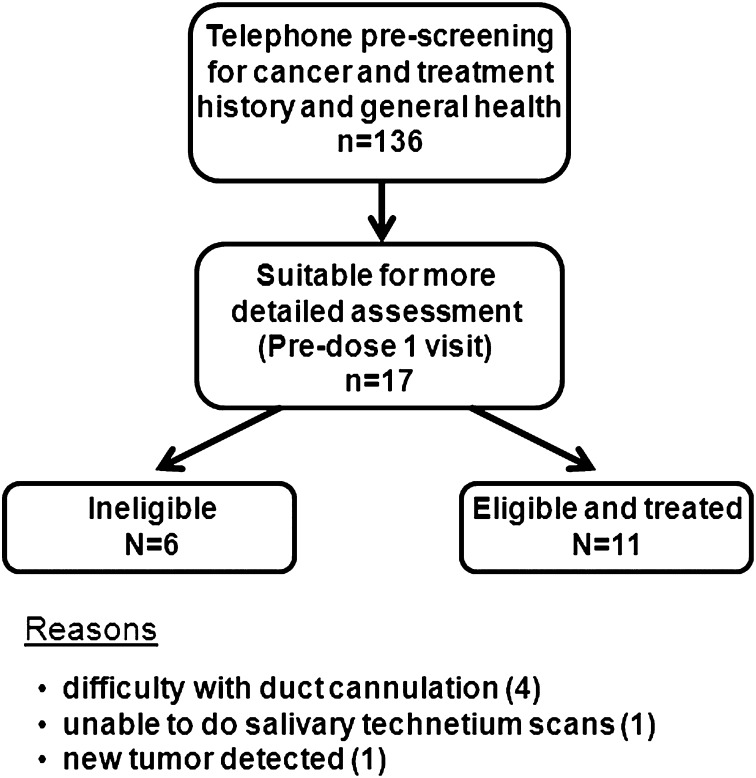
Fig. 1.
Screening and enrollment of study participants. Flowchart depicts the enrollment process for all individuals contacted for study participation. Of the 17 patients evaluated for enrollment at the predose 1 visit, 11 were enrolled (all subjects treated with AdhAQP1) and 6 were deemed ineligible, for the reasons indicated in the figure.
Results
All 11 subjects had been successfully treated for a squamous cell carcinoma (seven tonsil, three base of tongue, one hypopharynx) at least 5 y before enrollment. Key characteristics of these individuals are shown in Table 1.
Table 1.
Baseline characteristics of all treated subjects
| Dose group | Subject # | Age (y) | Sex | Ethnicity | Tumor locale | Tumor stage | Radiation Gy (maximum) | Ad5 NAb | Gland target | Baseline flow (mL/min) | Infusate vol (mL) | vp/μL infused |
| 4.8 × 107 | 40 | 68 | M | C | L tonsil | IVA | 66.6 | <1:4 | Left | tubing* | 0.7 | 6.86 × 104 |
| 25 | 60 | M | C | R tonsil | IVA | 68.4 | <1:1,024 | Right | 0.162 | 0.47 | 1.02 × 105 | |
| 19 | 58 | M | C | L tonsil | III | 50.4 | <1:256 | Right | 0.073 | 0.76 | 6.32 × 104 | |
| 2.9x × 108 | 50 | 58 | M | C | BOT | IVA | 59.4 | <1:8 | Left | 0.145 | 0.55 | 5.27 × 105 |
| 73 | 71 | M | C | R tonsil | IVA | 69.6 | <1:8 | Right | 0.142 | 0.7 | 4.14 × 105 | |
| 99 | 53 | M | C | R tonsil | IVA | 70 | <1:128 | Left | 0.092 | 0.81 | 3.58 × 105 | |
| 1.3 × 109 | 105 | 57 | F | C | hypopharynx | IVA | 73.8 | <1:16,384 | Left | 0.129 | 0.95 | 1.36 × 106 |
| 118* | 56 | M | C | BOT | IVB | 71.9 | <1:8,192 | Left | 0.044 | 0.7 | 3.19 × 106 | |
| 103 | 62 | M | C | BOT | IVA | 75.4 | <1:2,048 | Right | 0.136 | 1.8 | 7.22 × 105 | |
| 5.8 × 109 | 4 | 53 | M | H | L tonsil | IVB | 75.6 | <1:512 | Right | 0.085 | 0.67 | 8.66 × 106 |
| 116 | 62 | M | C | R tonsil | IVA | 66.6 | <1:8 | Left | 0.115 | 0.78 | 7.44 × 106 |
AdhAQP1 dose is given as vp/gland. For ethnicity, C indicates Caucasian and H indicates Hispanic. For tumor locale, BOT is base of tongue, L is left and R is right. The radiation dose given is the maximum dose to the targeted parotid gland. Ad5 NAb indicates neutralizing antibody titers at baseline (see SI Methods for details). Baseline flow from the targeted parotid gland is shown as milliliters per minute. “Tubing” means saliva was in the collection tubing, but was not able to be collected and quantified. For statistical analyses “tubing” was counted as 0.01 mL/min. All subjects experienced late grade 2 toxicity [RTOG classification (2)]. “vol” indicates the volume of the targeted parotid gland measured by contrast radiography (SI Methods). The last column to the right indicates the vector particles infused per microliter of infusate, as an in vivo measure of the multiplicity of infection (i.e., vp/tissue mass).
*Subject #118 received a dose 71.5% higher than intended because of a pharmacy dilution error.
No deaths, dose-limiting toxicities, or serious adverse events occurred as a result of vector delivery. Indeed, there were no consistent or systematic changes seen in any serum chemistry or hematology parameters measured. Sixty-five adverse events occurred over the first 42 d postvector delivery (Table 2). All adverse events were mild (∼91%) or moderate (∼9%); most (>75%) were judged as unrelated or unlikely related to AdhAQP1 treatment. Of the remaining adverse events, 10 were considered possibly related, 4 probably related, and 1 definitely related to vector treatment. The latter five events all occurred with subject #105 (see below). Table S1 shows the distribution of all adverse events classified by organ system.
Table 2.
Summary of adverse events through day 42
| Dose tier (n) | Grade 1 (mild) | Grade 2 (moderate) | Grade 3 (severe) |
| 1 (3) | 18* | 2 | 0 |
| 2 (3) | 19† | 3 | 0 |
| 3 (3) | 19‡ | 1 | 0 |
| 4 (2) | 3 | 0 | 0 |
| Total (%) | 59 (90.8) | 6 (9.2) | 0 (0) |
Data shown are the number of adverse events (grades 1, 2, or 3) recorded in each dosing tier. The percentages shown are of the total number (i.e., 65). See footnotes below for specific adverse events; all other adverse events (50/65; 76.9%) were judged as unlikely related or unrelated to treatment.
*Five were judged possibly related to treatment (subject #s 19, 25, 40).
†Three were judged possibly related to treatment (subject #s 73, 99).
‡Two were judged possibly, four probably, and one definitely related to treatment (all with subject 105).
Four subjects had laboratory or clinical signs likely because of parotid gland inflammation resulting from AdhAQP1 delivery. One subject (#105, dose tier 3; the only female studied) developed a clinically visible, mild parotitis on days 2 and 3 following vector administration, associated with elevations in serum amylase and C-reactive protein, that resolved without intervention. This subject also exhibited the highest pretreatment serum-neutralizing antibody titer at baseline, <1:16384 (Table 1). Three other subjects exhibited laboratory or imaging changes, but not visible clinical changes, consistent with targeted gland inflammation. Subject #118 had an ∼100% increase, versus baseline, in absolute WBC level at 12 h, although this was not above the upper limit of normal. Subject #4 exhibited a >50% increase in serum amylase levels at 12 h, 2 and 3 d postvector delivery, although none were above the upper limit of normal. Subject #s 4, 105, and 116 showed a marked increase in 67Ga uptake in the AdhAQP1 targeted gland (see below and Table 3).
Table 3.
Summary of 67Ga citrate uptake results
| Dose tier | Subject # | Baseline | +24 h | +96 to 168 h |
| 1 | 40 | 1.205 | 1.168 (0.97) | ND |
| 25 | 0.905 | 0.886 (0.98) | ND | |
| 19 | 0.941 | 1.010 (1.07) | ND | |
| 2 | 50 | 1.250 | 1.234 (0.99) | ND |
| 73 | 0.846 | 0.884 (1.04) | ND | |
| 99 | 1.063 | 1.176 (1.11) | ND | |
| 3 | 105 | 1.115 | 1.434 (1.29) | 2.720 (2.44) |
| 118 | 1.025 | 0.979 (0.96) | 1.151 (1.12) | |
| 103 | 0.991 | 1.007 (1.02) | 1.124 (1.13) | |
| 4 | 4 | 0.995 | 1.483 (1.49) | 1.778 (1.79) |
| 116 | 1.133 | 1.381 (1.22) | 1.578 (1.39) |
Data shown are the results of 67Ga citrate scans, to assess inflammation in parotid glands, performed at baseline, 24 h or 96–168 h following AdhAQP1 administration to the targeted parotid gland. A region of interest, defining either the targeted or nontargeted contralateral gland, was identified and applied to both glands; the number of counts in each region was quantified, and then the ratio of counts in the targeted/nontargeted gland was calculated (see SI Methods for additional details). Next, a ratio of these quotients, at each time-point (+24 h or +96 to 168 h) to that at baseline, was determined. These ratios are in parentheses within the table. ND, not done.
During the study, one stopping rule was met, resulting in a Food and Drug Administration (FDA)-ordered, ∼3 mo clinical hold. This hold involved subject #25 (dose tier 1). On day 7 after vector administration, his saliva, but not serum, was positive for both replication competent adenovirus (RCA; per protocol, required stopping rule) and AdhAQP1. As reported (12), this resulted from activation of a latent Ad5 infection in the targeted gland, was without clinical consequence, and was judged a mild adverse event. No other subject had saliva or serum samples positive for RCA or AdhAQP1, and none developed serum antibodies to hAQP1.
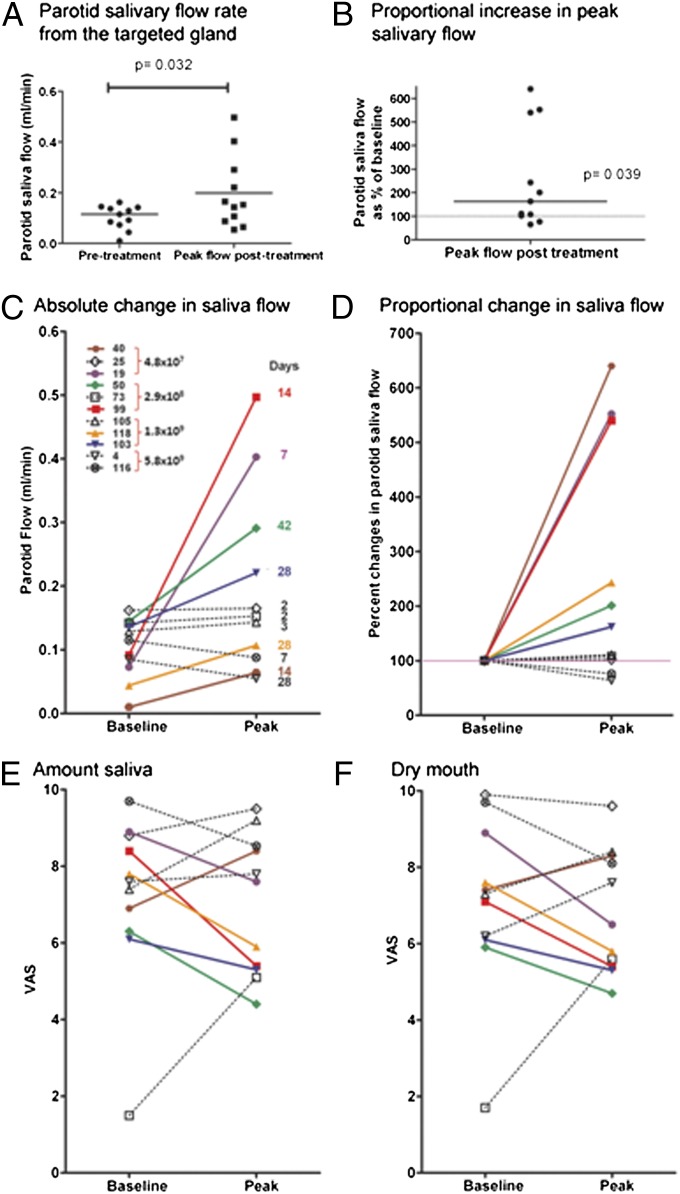
The hypothesis underlying this intervention was that hAQP1 cDNA delivery to IR-damaged parotid glands would increase salivary secretion. To test this theory we compared saliva flow rates in targeted glands before treatment with peak values obtained during the first 42 d posttreatment. We found significant improvement in both absolute volume (Fig. 2A) (baseline: median 0.115 mL/min; peak: median 0.153 mL/min, P = 0.032; Wilcoxon matched-pairs signed rank test) and proportional change (Fig. 2B) (median 164.5%, 25% percentile: 101.9, 75% percentile: 540.2%; P = 0.039; Wilcoxon signed rank test) in parotid flow rates following treatment. Closer examination of the data revealed a heterogeneous response, with six subjects showing a 60–540% increase in parotid flow rates at different times between days 7–42 (Fig. 2 C and D), whereas during this period for the other five subjects parotid saliva flow rates showed no improvement or worsened (min −35%, max +10%). Based on these observations, we considered the six subjects with at least 50% improvement as responders and the remaining subjects as nonresponders. An increase in parotid saliva flow rate was observed in 66% (two of three) of participants in each of the first three dose cohorts; both subjects in the fourth dose cohort experienced a decrease in their parotid saliva flow rate.
Fig. 2.
Summary of clinical response data. Clinical responses following vector delivery as measured by (A) absolute parotid salivary flow rate from the targeted gland and (B) the proportional increase in peak parotid salivary flow shown as the percent of baseline. Statistical significance was determined using the Wilcoxon matched-pair rank test for the change in absolute values. The Wilcoxon signed rank test was used to test if the peak proportional increase in parotid salivary flow was significantly different from the baseline (100%). Individual changes in parotid salivary flow are shown (C) for absolute salivary flow rates and (D) for proportional changes compared with baseline. Coding for individual subjects is shown as indicated in the Inset in C. All subjects shown in black were considered nonresponders (< 50% increase in salivary flow rate; see text). All subjects shown in colors were considered responders (at least 50% increase in parotid salivary flow rate following AdhAQP1 administration). The days indicated to the right of each peak data point correspond to the days on which that peak parotid flow rate was observed. Visual analog scale (VAS) results from all subjects, at baseline and peak time of parotid salivary flow, are shown for both the amount of saliva perceived (E) (rate how much saliva is in your mouth) and dryness of their mouth (F) (rate the dryness in your mouth). Note that lower VAS results indicate an improvement in symptoms. The colors and symbols used to identify individual subjects are identical to those shown in C. See text for additional details.
Importantly, at the time of peak increase in parotid flow rate, five of six responders showed improvement in subjective perception of oral dryness and amount of saliva present in their mouth by visual analog scale assessments (Fig. 2 E and F; lower score represents improvement). These same five responders also showed improvements in at least five of the eight visual analog scale measurements at this time, which is notable considering only one major salivary gland was treated. Conversely, four of the five nonresponders exhibited either no change in their subjective assessments of saliva present or xerostomia, or perceived worsening in their symptoms (Fig. 2 E and F). The fifth individual experienced improvement in these two assessments, without increased parotid flow (Fig. 2 E and F). Thus, if both enhanced parotid flow and improved subjective responses are considered to indicate efficacy, then AdhAQP1 treatment was dose-dependent, with peak responses occurring at 2.9 × 108 and 1.3 × 109 vector particles (vp) per gland. There was no difference between responders and nonresponders in age (responders: 59.2 ± 2.1; nonresponders: 60.6 ± 3.0; P = 0.699), IR dose to the salivary glands (responders: 62 ± 3.8; nonresponders: 70.8 ± 1.7; P = 0.2724), or average baseline parotid saliva flow rates (responders: 0.083 ± 0.021; nonresponders: 0.127 ± 0.013; P = 0.135). Median serum-neutralizing antibody levels at baseline for responders and nonresponders were <1:192 and <1:512, respectively. (Fig. S1).
Generally, salivary 99mTcO4 scans were unhelpful for predicting subject responders and demonstrating positive responses; that is, for 10 subjects 99mTcO4 scan results and kinetic analyses were not distinguishing. However, subject #73 showed a widely scattered pattern of 99mTcO4 uptake, both before and following AdhAQP1 treatment, suggesting individuals with such a pattern initially would be poor candidates for hAQP1 gene transfer. Similarly, magnetic resonance imaging proved unhelpful for predicting patient responders and demonstrating positive responses to vector treatment. We compared the percent change from baseline of average signal intensities in all sequences, between treated and not treated glands and between responders and nonresponders. Signal intensities varied unpredictably in each scan and did not show any correlation with response to treatment. Conversely, 67Ga uptake scans were useful in demonstrating local inflammation in the targeted glands. As shown in Table 3, subjects in the first two dose tiers showed little difference in 67Ga uptake in targeted glands following AdhAQP1 delivery. However, three of five subjects treated in dose tiers 3 and 4 exhibited substantially increased 67Ga uptake in targeted glands (subject #s 4, 105, and 116; >20% increase in 24 h/baseline ratio), consistent with localized inflammation.
Discussion
There are three major findings through day 42 of this phase I study. First, delivery of an Ad5 vector to a single parotid gland is safe. No consistent or systematic changes were seen in any parameter of clinical safety measured. Furthermore, relatively few adverse events were recorded during this observation time, all being mild or moderate. Of adverse effects seen, two types were notable. One, RCA in one subject’s saliva, required us to invoke a stopping rule temporarily. This situation was extensively described earlier (12), occurred because of latent Ad5 infection in the targeted gland, and is likely an uncommon occurrence, difficult to predict prospectively. However, it was reassuring that the event was temporary, resulted in no viremia, and resolved without sequalae or need for intervention. The other situation, occurrence of inflammatory changes (clinical chemistry; 67Ga uptake) associated with vector delivery in targeted glands, was dose-dependent, occurring to some extent in all four subjects receiving an AdhAQP1 dose > 1 × 106 vp/μL infusate (Table 1) (Subject #s 105, 116, 118, and 4). Overall, the notion that at the doses administered, Ad5 vector delivery to parotid glands is essentially safe is consistent with earlier reports targeting several tissues (e.g., refs. 13 and 14).
Our second major finding was that hAQP1 gene transfer enhanced parotid flow rate in 6 of 11 treated subjects, suggesting our original hypothesis was achievable in humans. Thus, this study represents a potential advance for patients with RTOG late-grade 2–3 toxicity currently lacking suitable conventional therapy. The responder percentage in the first three dose tiers was identical, based on enhanced parotid saliva flow, with two of three subjects responding per group. Importantly, five of these six responders also had improvement in subjective symptoms (versus one of five among nonresponders), further supporting the notion that observed changes in parotid flow rates are clinically significant. In contrast to these findings, neither of the two subjects in the highest dose group had an objective response; in fact, both experienced a decrease in parotid flow rate following AdhAQP1 delivery. Based on the clinical outcomes and the presence of inflammation in subjects receiving an AdhAQP1 dose > 1 × 106 vp/μL infusate (subject #s 105, 116, 118, and 4), only one (#118) was considered to be a responder with an ∼threefold increase in salivary flow (Fig. 2); the other subjects (#s 105, 116, and 4) had no benefits from treatment and were considered nonresponders. These data suggest that Ad5 vector doses > 1 × 106 vp/μL infusate [vector dose/gland infusate volume; equivalent to >1.3 × 107 vp/kg body weight] are associated with an increased inflammatory response in parotid glands and less likely to yield clinical benefits when an Ad5 vector is used for mediating gene transfer. Of both remaining nonresponders, one had the RCA event and consequent target cell lysis (subject #25), and the other (subject #73) showed highly irregular 99mTcO4 uptake before and following AdhAQP1 treatment. We do not yet understand why subject #40 failed to experience subjective benefit from AdhAQP1 treatment, despite a measurable biologic effect. The lack of response caused by inflammation or Ad5 reactivation and RCA formation could be circumvented by using another vector-delivery system (e.g., based on a serotype 2, adeno-associated virus) (15). Overall, the proportion of responders seen herein is not surprising, given findings from previous phase I gene-therapy studies using Ad5 vectors in diverse tissues, such as the eye, heart, peripheral vasculature, and lung (e.g., refs. 16–19).
Our last major finding was that positive responses seen in the responders did not follow a time course predicted from previous Ad5 vector studies with mice, rats, miniature pigs, and macaques. Generally, those studies showed peak transgene expression times 24–72 h after vector delivery, with biological responses occurring shortly thereafter (e.g., refs. 20–23). Specifically, when AdhAQP1 was studied in irradiated miniature pigs, a peak response of increased salivary flow was seen on day 3 (first time-point measured), which then decreased on days 7 and 14 (10). However, among the six responders, the time at which peak elevations in parotid salivary flow occurred was quite variable and much later than in animal studies [i.e., days 7, 14 (twice), 28 (twice), and 42]. In part the reason may reflect use of inbred rodents for many preclinical studies, and controlled environments and nutritional intake for all animal model studies. Another consideration is that human species C adenoviruses, to which Ad5 belongs, function less efficiently in cells from nonhuman mammals, including monkeys (e.g., ref. 24). Furthermore, the kinetics of transgene expression or physiological responsiveness following Ad5 transduction in several clinical studies directed at other tissues, not involving treatment of a malignancy, have also shown considerable variability (e.g., refs. 16, 19, 25–27).
Interestingly, we found no relationship between the preexisting Ad5 neutralizing antibody titer and positive responses to AdhAQP1 (Table 1). In other studies examining neutralizing antibodies following Ad5 vector administration to multiple tissues, healthy and diseased individuals show great variability, ranging from little to no response to extremely high levels (e.g., refs. 14 and 16). Such behavior was unrelated to vector dose, but highly correlated with preexisting anti-Ad5 antibody levels (i.e., unlike the situation with naïve experimental animals) (28). It is likely that the inflammatory changes seen herein were because of cell-mediated immune reactivity, which we plan to assess using peripheral blood lymphocytes obtained from all subjects during the study.
Clinical gene therapy, like clinical use of stem cells and novel biologicals, is being developed for conditions lacking adequate conventional therapies. The overwhelming majority of clinical gene-therapy applications have focused on cancer (70.3%), with monogenic (9.05%), cardiovascular (7.1%), and infectious diseases (6.15%) forming the next most frequent applications (http://oba.od.nih.gov/rdna/adverse_event_oba.html; accessed May 20, 2012). This study represents a unique direct gene-therapy clinical trial in the oral cavity for a nonmalignant condition and one of the few times that gene transfer has been used to treat a quality of life disorder. We chose an Ad5 vector over an adeno-associated virus vector to limit any concerns related to the long-term presence of a viral vector, in case the study did not show any benefit. The results are cautiously encouraging for the treatment of IR-induced xerostomia with patients in RTOG grades 2 and 3. Because Ad5 vectors do not result in permanent gene transfer, our findings represent an important proof-of-concept and suggest further study of hAQP1 gene transfer with less immunogenic vectors; for example, serotype 2 adeno-associated virus, capable of longer-lived expression in salivary glands (15), are warranted.
Methods
Subjects.
Subjects were assigned to a dose tier in the order in which it was determined that they met all eligibility criteria for enrollment (see Table S2 for all inclusion and exclusion criteria). Fig. 1 depicts the screening and enrollment process for subjects. A total of 136 individuals were screened by telephone for general eligibility. Seventeen individuals were deemed suitable for a detailed clinical assessment (predose 1 visit) (Fig. S2). Of these 17 candidates, 11 were eligible for enrollment and received AdhAQP1 vector treatment (below). This phase I clinical trial [National Institutes of Health (NIH) protocol 06-D-0206] was approved by the National Institute of Dental and Craniofacial Research Institutional Review Board, the NIH Biosafety Committee, the Recombinant DNA Advisory Committee, the FDA (IND BB-13,102), and an independent Data and Safety Monitoring Board (DSMB). The study was an open-label, single-dose, dose-escalation design, with all subjects treated and followed at the NIH Clinical Center. Detailed descriptions of this clinical protocol have been previously reported (8, 29). Dose escalation was done after DSMB review of all accumulated data following completion of the day 28 visit for all subjects in each dose tier. Subjects were seen twice before vector administration and 13 times following treatment [on days 1 (twice, at 6 and 12 h), 2, 3, 7, 14, 28, 42, 90, 120, 150, 180. and 360] (Fig. S2). As noted above, only results through day 42 are reported herein; longer follow-up will be reported separately. Subjects were in-patients during days 1–3 and outpatients for other visits. Each visit included a physical examination, oral/head and neck examination, saliva collection, detailed clinical chemistry, and hematologic and urine analyses.
Treatment.
Approximately 30 min before administration of AdhAQP1, subjects received 4 μg/kg of glycopyrrolate intravenously to stop existing salivary flow from interfering with vector transduction. The targeted parotid gland was determined during predose 1, based on access to Stensen’s duct for cannulation, salivary flow rate (per protocol <0.2 mL/min per gland) and a sialographic appearance consistent with IR damage. Sialograms were also used to determine the volume of vehicle in which vector dose was to be suspended for administration. Thereafter, following dilation of Stensen’s duct using graded lacrimal probes, cannulation was performed with a ProtectIV Plus Safety IV Catheter-Radiopaque, (Ethicon Endo-Surgery; catalog #3060; 22 gauge by 1 in). AdhAQP1 at the indicated dose and suspended in vehicle was then infused into the targeted parotid gland in a retrograde direction using the predetermined gland infusate volume. The cannula was held in place for 10 min to ensure a reasonable contact time between the vector and gland parenchyma, and to help prevent anterior loss of infusate. Following removal of the cannula, the “draining” AdhAQP1 suspension was suctioned along the buccal mucosa and floor of the mouth for 5 min. There was no manipulation of the contralateral parotid gland.
Supplementary Material
Acknowledgments
We thank Dr. Jane Atkinson for many helpful suggestions during this study. We thank Dr. Sarfaraz Hasni for help in the medical evaluation of several subjects; Janet Rowan, RN for assistance with many subjects; multiple physicians, for referring their patients to us; and the 11 study subjects, who gave so generously of their time and themselves, enabling this trial to be conducted. The Intramural Research Program of the National Institute of Dental and Craniofacial Research supported this study.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210662109/-/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 3.Jensen SB, et al. Salivary Gland Hypofunction/Xerostomia Section. Oral Care Study Group. Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Management strategies and economic impact. Support Care Cancer. 2010;18(8):1061–1079. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 4.Vissink A, et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: Successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78(4):983–991. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amerongen AV, Veerman EC. Saliva—The defender of the oral cavity. Oral Dis. 2002;8(1):12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- 6.Jensen SB, et al. Salivary Gland Hypofunction/Xerostomia Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO) A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18(8):1039–1060. doi: 10.1007/s00520-010-0827-8. [DOI] [PubMed] [Google Scholar]
- 7.Baum BJ. Principles of saliva secretion. Ann N Y Acad Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- 8.Baum BJ, et al. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncol. 2010;46(1):4–8. doi: 10.1016/j.oraloncology.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delporte C, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94(7):3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan Z, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11(3):444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Zheng C, et al. Toxicity and biodistribution of a first-generation recombinant adenoviral vector, encoding aquaporin-1, after retroductal delivery to a single rat submandibular gland. Hum Gene Ther. 2006;17(11):1122–1133. doi: 10.1089/hum.2006.17.1122. [DOI] [PubMed] [Google Scholar]
- 12.Zheng C, et al. Transient detection of E1-containing adenovirus in saliva after the delivery of a first-generation adenoviral vector to human parotid gland. J Gene Med. 2010;12(1):3–10. doi: 10.1002/jgm.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crystal RG, et al. Analysis of risk factors for local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of comorbid conditions. Hum Gene Ther. 2002;13(1):65–100. doi: 10.1089/10430340152712647. [DOI] [PubMed] [Google Scholar]
- 14.Harvey BG, et al. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002;13(1):15–63. doi: 10.1089/10430340152712638. [DOI] [PubMed] [Google Scholar]
- 15.Gao R, et al. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther. 2011;18(1):38–42. doi: 10.1038/gt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campochiaro PA, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: Results of a phase I clinical trial. Hum Gene Ther. 2006;17(2):167–176. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 17.Harvey BG, et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J Clin Invest. 1999;104(9):1245–1255. doi: 10.1172/JCI7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäkinen K, et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery: A randomized, placebo-controlled, double-blinded phase II study. Mol Ther. 2002;6(1):127–133. doi: 10.1006/mthe.2002.0638. [DOI] [PubMed] [Google Scholar]
- 19.Rosengart TK, et al. Angiogenesis gene therapy: Phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation. 1999;100(5):468–474. doi: 10.1161/01.cir.100.5.468. [DOI] [PubMed] [Google Scholar]
- 20.Kagami H, O’Connell BC, Baum BJ. Evidence for the systemic delivery of a transgene product from salivary glands. Hum Gene Ther. 1996;7(17):2177–2184. doi: 10.1089/hum.1996.7.17-2177. [DOI] [PubMed] [Google Scholar]
- 21.Li J, et al. Developing a convenient large animal model for gene transfer to salivary glands in vivo. J Gene Med. 2004;6(1):55–63. doi: 10.1002/jgm.476. [DOI] [PubMed] [Google Scholar]
- 22.Voutetakis A, et al. Sorting of transgenic secretory proteins in rhesus macaque parotid glands after adenovirus-mediated gene transfer. Hum Gene Ther. 2008;19(12):1401–1405. doi: 10.1089/hum.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, et al. Adenoviral-mediated gene transfer to mouse salivary glands. J Dent Res. 2000;79(2):701–708. doi: 10.1177/00220345000790020201. [DOI] [PubMed] [Google Scholar]
- 24.Wold WSM, Horwitz MS. Adenoviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields’ Virology. 5th ed. Philadelphia PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 25.Bellon G, et al. Aerosol administration of a recombinant adenovirus expressing CFTR to cystic fibrosis patients: A phase I clinical trial. Hum Gene Ther. 1997;8(1):15–25. doi: 10.1089/hum.1997.8.1-15. [DOI] [PubMed] [Google Scholar]
- 26.Matyas L, et al. Arteriogenic gene therapy in patients with unreconstructable critical limb ischemia: A randomized, placebo-controlled clinical trial of adenovirus 5-delivered fibroblast growth factor-4. Hum Gene Ther. 2005;16(10):1202–1211. doi: 10.1089/hum.2005.16.1202. [DOI] [PubMed] [Google Scholar]
- 27.Zuckerman JB, et al. A phase I study of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator gene to a lung segment of individuals with cystic fibrosis. Hum Gene Ther. 1999;10(18):2973–2985. doi: 10.1089/10430349950016384. [DOI] [PubMed] [Google Scholar]
- 28.Harvey BG, et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J Virol. 1999;73(8):6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samuni Y, Baum BJ. Gene delivery in salivary glands: From the bench to the clinic. Biochim Biophys Acta. 2011;1812(11):1515–1521. doi: 10.1016/j.bbadis.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.