Abstract
Inflammatory autoimmune diseases such as systemic lupus erythematosus (SLE) and polyarthritis are characterized by chronic cytokine overproduction, suggesting that the stimulation of host innate immune responses, speculatively by persistent infection or self nucleic acids, plays a role in the manifestation of these disorders. Mice lacking DNase II die during embryonic development through comparable inflammatory disease because phagocytosed DNA from apoptotic cells cannot be adequately digested and intracellular host DNA sensor pathways are engaged, resulting in the production of a variety of cytokines including type I IFN. The cellular sensor pathway(s) responsible for triggering DNA-mediated inflammation aggravated autoimmune disease remains to be determined. However, we report here that Stimulator of IFN Genes (STING) is responsible for inflammation-related embryonic death in DNase II defective mice initiated by self DNA. DNase II-dependent embryonic lethality was rescued by loss of STING function, and polyarthritis completely prevented because cytosolic DNA failed to robustly trigger cytokine production through STING-controlled signaling pathways. Our data provides significant molecular insight into the causes of DNA-mediated inflammatory disorders and affords a target that could plausibly be therapeutically controlled to help prevent such diseases.
Keywords: apoptosis, necrosis, macrophage, dendritic cells
Autoimmune and inflammatory disease such as systemic lupus erythematosus (SLE) or chronic polyarthritis is characterized by multiple organ injury, high titers of autoantibodies, and abnormal cytokine production including type I IFN (1–3). The causes remain unclear but involve the overstimulation of innate immune signaling pathways speculatively through chronic infection or perhaps by inappropriately digested apoptotic DNA (1). Defects in lysosome function, for example, by loss of DNase II activity, results in inefficiently digested DNA that can escape from lysosomal regions and trigger host–defense mechanisms (4, 5). Mice lacking DNase II (DNase II−/−) die during development because of the overproduction of type I IFN. However, DNase II−/− mice lacking type I IFN function are viable but suffer from severe arthritis after 8 wk because of the production of other cytokines such as TNFα (4–7). The cellular sensors and mechanisms of DNA-mediated inflammation augmented autoimmune disease largely remain to be clarified although they are not considered to involve the Toll-like receptor (TLR) pathway (8). However, we have shown that Stimulator of IFN Genes (STING), an endoplasmic reticulum (ER)-associated transmembrane molecule and part of the translocon complex, is a key component in controlling cytoplasmic DNA innate immune signaling and is responsible for facilitating host–defense events that comprises the production of type I IFN (9, 10). Given that phagocytosed apoptotic cells can activate the production of type I IFN and inflammatory cytokines in macrophages defective in DNase II, it was plausible to examine whether STING, also referred to as MPYS and MITA (11, 12), possibly played a role in facilitating these events.
Results
STING Facilitates Apoptotic or Necrotic DNA Triggered Cytokine Production.
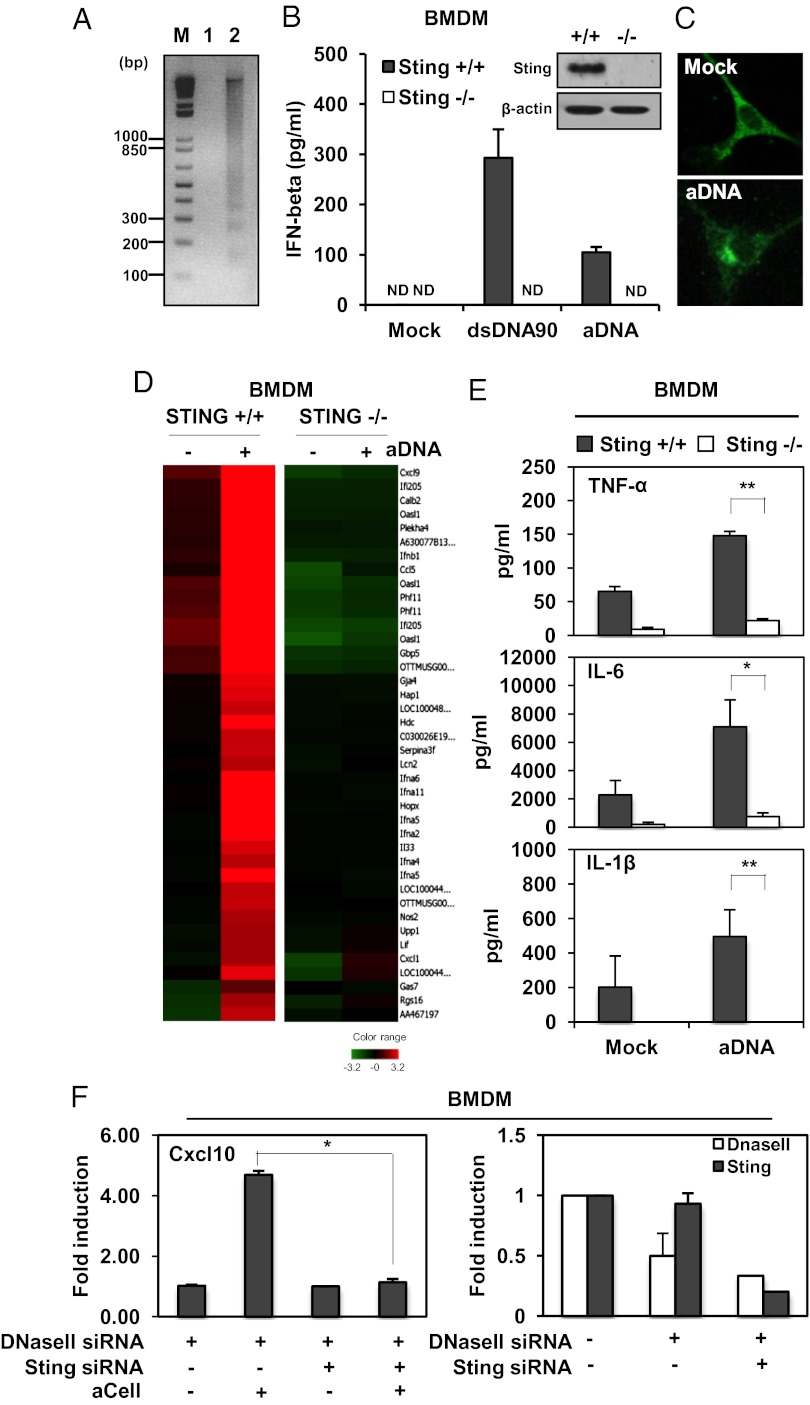
We obtained bone marrow-derived macrophages (BMDMs) from Sting+/+ and Sting−/− mice and transfected them with 90-bp dsDNA (dsDNA90) that is known to activate the STING pathway, or with apoptotic DNA (aDNA) derived from dexamethasone (Dex)-treated thymocytes. We observed that both types of DNA potently induced the production of IFNβ in BMDM and conventional dendritic cells (BMDCs) in a STING-dependent manner (Fig. 1 A and B, Fig. S1, and Table S1). This event caused STING to traffic to endosomal perinuclear regions in BMDMs, likely to interact with transcription factors required for the production of innate immune response genes (Fig. 1C) (10, 13). DNA microarray experiments confirmed that aDNA triggered STING-dependent production of an array of innate immune and inflammatory-related cytokines such as IFNβ and TNFα (Fig. 1D). We confirmed that cytokine production including TNF-α, IL-6, and IL-1β was highly induced in Sting+/+ BMDM but not in Sting−/− BMDM treated with aDNA (Fig. 1E). To determine whether STING played a role in DNase II-related inflammatory disease, we knocked down STING and/or DNase II expression in BMDM by using RNAi and exposed them to apoptotic thymocytes. We confirmed that loss of DNase II facilitated the up-regulation of select cytokines such as Cxcl10 in response to being exposed to apoptotic cells, as has been reported (8) (Fig. 1F). However, knockdown of STING was observed to largely prevent Cxcl10 induction generated through loss of DNase II (Fig. 1F). We similarly noted that necrotic cells were also able to induce the production of cytokines in BMDM’s in a STING-dependent manner (Fig. S2). Thus, STING facilitates apoptotic and necrotic DNA-mediated proinflammatory gene production in hematopoietic cells and may play a role in apoptotic cell manifested cytokine production.
Fig. 1.
Role of STING in facilitating cytokines production by apoptotic DNA. (A) DNA from thymocytes treated with or without Dex (10 μM, 5 h). (B) STING expression as analyzed by immunoblot and IFNβ ELISA of Sting+/+ and Sting−/− BMDM treated with dsDNA90 or aDNA. ND, nondetectable. (C) Confocal analysis of BMDMs transfected with aDNA and immunostained with anti-STING antibody. (Magnification: 1260×.) (D) Microarray of Sting+/+ or Sting−/− BMDM transfected with aDNA (6 h). (E) ELISA of IL-β, IL-6, and TNFα in BMDMs treated with or without aDNA. (F) Cxcl10 mRNA level levels in BMDMs treated with STING and/or DNase II siRNA after exposure for 6 h to apoptotic thymocytes (aCell). Cxcl10 mRNA level was normalized to the expression of GAPDH (Left). *P < 0.05, **P < 0.005 by Student’s t test.
Self DNA Activated Cytokine Production in DNase II−/− Embryos Is Abolished by Loss of STING.
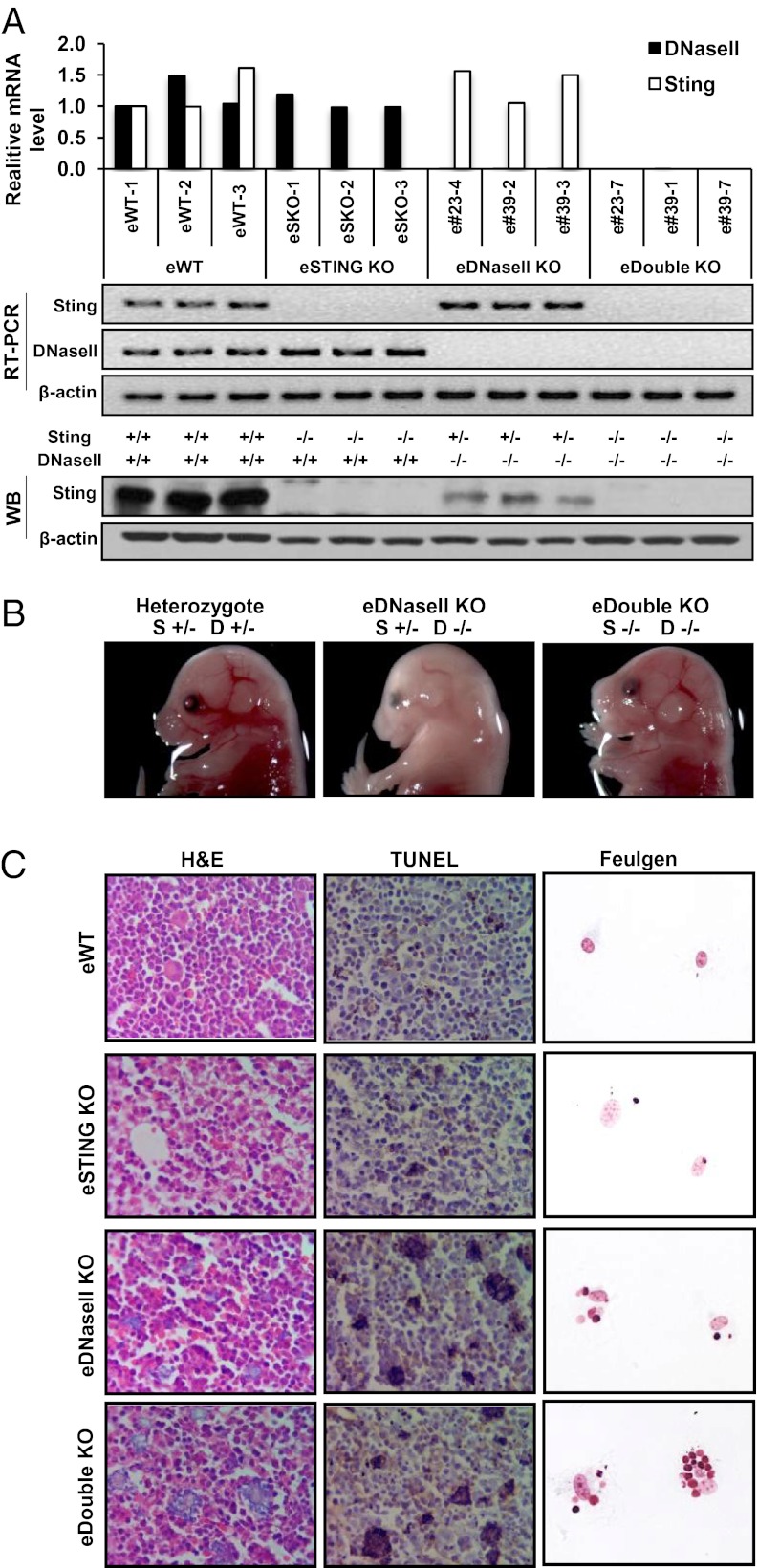
DNase II−/− mice die before birth because of high levels of type I IFN being produced by macrophages engulfing apoptotic cells that harbor undigested self DNA. Thus, to evaluate STING’s role in DNaseII lethality, we next crossed DNase II−/+mice with Sting−/− mice and analyzed DNase II−/−, Sting−/−, or Sting−/− DNase II−/− double-knock out (DKO) from embryonic day 17 (E17). Genotyping analysis, including RT-PCR and immunoblot, confirmed that the embryos lacked Sting, DNase II, or both functional genes (Fig. 2A). We observed that DNase II−/− embryos exhibited anemia, as previously described, which was in significant contrast to Sting−/− DNase II−/− DKO embryos or controls that noticeably lacked this phenotype (Fig. 2B) (1). Lethal anemia has been reported to be due to type I IFN inhibition of erythropoiesis during development (5). We subsequently observed by hematoxylin and eosin (H&E) staining that the livers of DNase II−/− embryos contained numerous infiltrating macrophages full of engulfed apoptotic cells responsible for producing high levels of cytokines (Fig. 2C). In contrast to control mice, the livers of Sting−/− DNase II−/− embryos exhibited a similar phenotype (Fig. 2C). Analysis of fetal livers by terminal deoxynucleotidyltransferase-mediated dUTP biotin nick-end labeling (TUNEL) confirmed that the Sting−/− DNase II−/− embryos and DNase II-deficient but not wild-type fetal livers contained numerous large inappropriately digested dying cells (Fig. 2C). In vitro analysis has indicated that macrophages from the embryos of wild-type or DNase II−/− mice engulf apoptotic cells adequately (4, 5, 7). However, although the DNA of the engulfed apoptotic cells is efficiently degraded in the lysosomes of wild-type macrophages, DNase II−/− macrophages accumulate engulfed nuclei and cannot digest DNA (1). This event leads to the stimulation of innate immune signaling pathways and production of autoimmune-related cytokines. Given this fact, we evaluated the ability of embryonic liver-derived macrophages that lacked both DNase II and STING to engulf apoptotic cells and digest DNA (Fig. S3). Similar to DNase II−/− macrophages, Sting−/− DNase II−/− macrophages were not able to digest the engulfed nuclei from Dex-treated apoptotic thymocytes compared with control macrophages taken from wild-type or Sting−/− mice (Fig. 2C, Feulgen staining). Thus, macrophages harvested from the livers of Sting−/− DNase II−/− embryonic mice similarly exhibit an inability to digest engulfed apoptotic cells, analogous to DNase II−/− macrophages.
Fig. 2.
STING-dependent gene regulation in E17 Sting−/− DNase II−/−embryos. (A) STING and DNase II expression by qPCR and Western blot from E17 fetal liver wild-type (eWT), Sting−/− DNase II+/+(eStingKO), Sting+/− DNase II−/− (eDNaseIIKO), and Sting−/− DNase II−/− (eDoubleKO) embryos. (B) Macroscopic view of E17 Sting+/− DNase II+/−(Left), eDNaseIIKO (Center), and eDoubleKO (Right) embryos. (C) H&E and TUNEL stains from mice livers same as A. Feulgen stain of BMDM after incubation with thymocytes (+) Dex. (Magnification: 200×.)
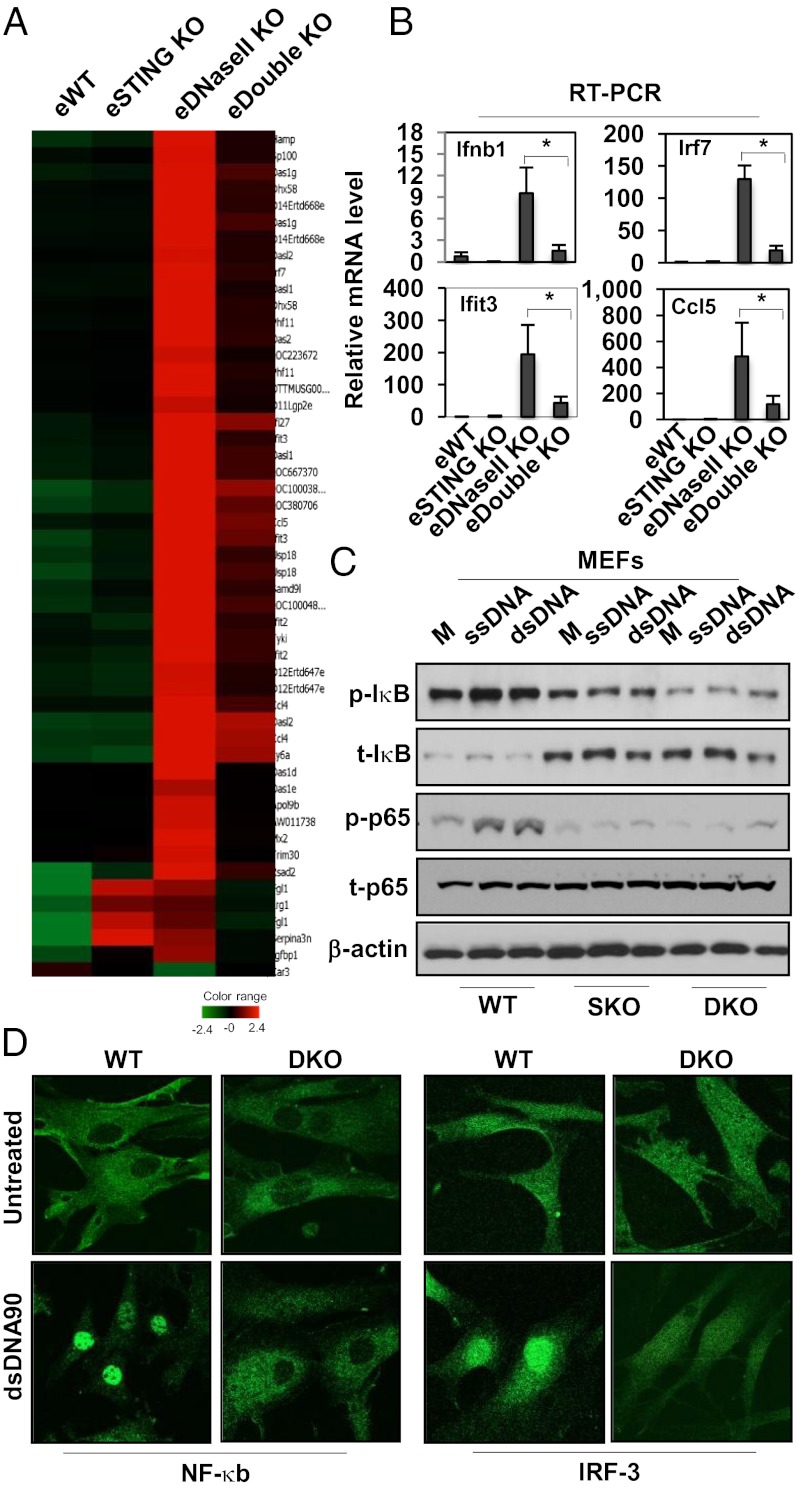
It has been demonstrated that the aDNA engulfed by the embryonic macrophages stimulates the overproduction of cytokines, including type I IFN that are responsible for lethal erythropoiesis (5, 7). We thus complemented our above study with analyzing mRNA expression levels, by microarray analysis, in the livers of the embryonic mice. This experiment indicated little inflammatory gene production in the livers of wild-type or Sting−/− embryos. However, we observed that the livers of DNase II−/− embryos contained abnormally high levels of cytokine-related mRNA, as reported (Fig. 3A) (5, 7). Significantly, the livers of Sting−/− DNase II−/− mice had dramatically reduced levels of innate immune gene expression activity compared with DNase II−/− mice (Fig. 3A). These results were confirmed by analyzing the mRNA expression levels of select innate immune genes in embryonic livers by RT-PCR. For example, the production of IFNβ was reduced severalfold in Sting−/− DNase II−/− mice compared with DNase II−/− mice (Fig. 3B). The production of key IFN-stimulated genes (ISGs) such as the 2′-5′ oligoadenylate synthetases (OAS), IFN-induced proteins with tetratricopeptide repeats (IFITs) IFN-inducible protein 27 (IFI27), and ubiquitin-like modifier (ISG15) were also dramatically reduced (Fig. S4A and Table S2). Proinflammatory cytokines such as TNFα and IL1β were also decreased in the embryonic livers of Sting−/− and Sting−/− DNase II−/− compared with DNase II−/− mice (Fig. S4B). Although the production of innate immune genes was dramatically suppressed in the absence of STING, the presence of some genes remained slightly elevated in Sting−/− DNase II−/− mice, albeit in low levels as determined by array analysis, which may be due to variation in mRNA expression between the animals analyzed, or perhaps due to the stimulation of alternate pathways (14). Many of these genes have been reported to be regulated by NF-κB and IFN regulatory factor (IRF) pathways (15). We thus evaluated the function of these transcription factors in Sting−/− DNase II−/− or control murine embryonic fibroblasts (MEFs), developed from E14. Principally, we observed a defect in NF-κB activity (p65 phosphorylation) in Sting−/− DNase II−/− MEFs when exposed to cytoplasmic DNA (Fig. 3C and Fig. S5A). This finding was confirmed by noting that NF-κB and IRF3 also failed to translocate in Sting−/− DNase II−/− as well as Sting−/− MEFs but not in WT and DNase II−/− MEFs after exposure to dsDNA (Fig. 3D and Fig. S5 B and C). Thus, STING is likely important for controlling self DNA-induced inflammatory cytokine production that is responsible for causing lethal embryonic erythropoiesis.
Fig. 3.
STING-dependent gene regulation in E17 Sting−/− DNase II−/− embryos. (A) Gene array analysis of fetal liver RNA isolated from E17 mice same as Fig. 2A. Data are from the mean of three independent embryos. (B) qPCR analysis of Ifnβ1, Irf7, Ifit3, and Ccl5 mRNA level from RNA same as (A). Error bars indicate SD. *P < 0.05, ***P < 0.0005 by Student’s t test. (C) Immunoblot analysis to determine the levels of p-Iκb and p-p65 in MEFs from Sting+/+DNase II+/+ (WT), Sting−/− DNase II+/+ (SKO), and Sting−/− DNase II−/− (DKO) treated with dsDNA90 and ssDNA90 for 3 h. M, mock. (D) Confocal analysis of MEFs isolated from WT and E17 DKO embryos transfected with dsDNA90 for 3 h and stained with anti–NF-κB and IRF-3 antibody. (Magnification: 1260×.)
DNase II−/−-Mediated Embryonic Lethality Is Prevented by Eliminating STING.
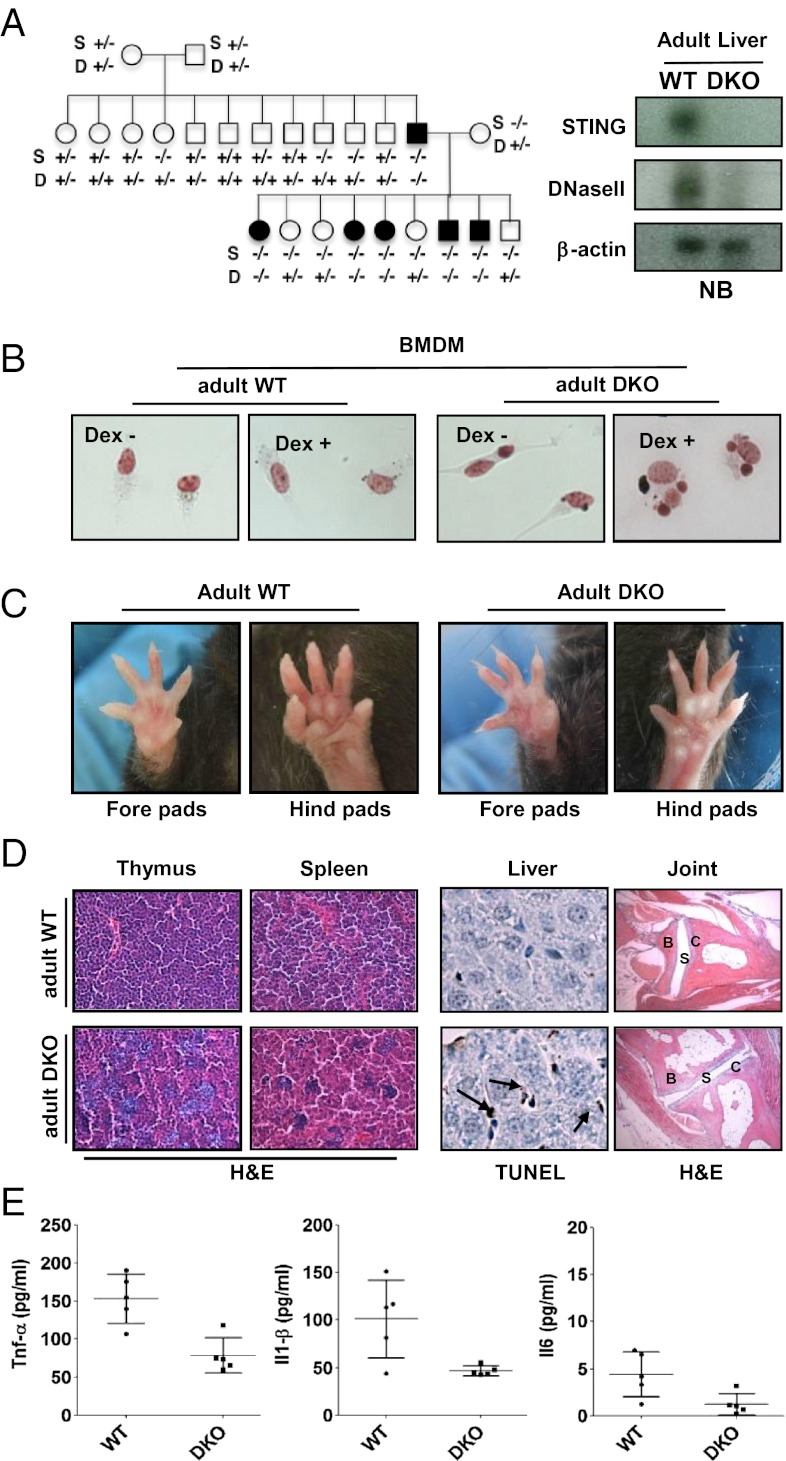
To extend our evaluation of the importance of STING in mediating self DNA-facilitated lethal erythropoiesis, we evaluated whether DNase II−/− mice could be born in the absence of STING. Significantly, we observed that DNase II−/− mice were born, with apparent Mendelian frequency, when crossed onto a Sting−/− background (Fig. 4A and Fig. S6A). PCR genotyping, Northern blot, RT-PCR, and immunoblot analysis confirmed DNase II and STING deficiency in the progeny mice (Fig. 4A and Fig. S6B). Sting−/− DNase II−/− double knockout mice (DKO) appeared to grow normally and exhibited similar size and weight compared with control mice although it was noted that Sting−/− mice were somewhat larger for reasons that presently remain unclear (Fig. S6C). Preliminary immunological evaluation also indicated that the Sting−/− DNase II−/− DKO animals shared a similar CD4+/CD8+ profile similar to Sting−/− and wild-type mice, although the DKOs were noted to develop splenomegaly as they aged (Fig. S6 D and E). Splenomegaly was also noted in surviving DNase II-deficient mice that lacked type I IFN signaling (DNase II−/− Ifnar1−/− mice) and has been reported to be due to enlargement of the red pulp (7). However, analysis of serum from Sting−/− DNase II−/− mice indicated no detectable abnormal cytokine production compared with control mice at 8 wk of age because of the general low immeasurable levels of cytokines produced. Through these studies, we similarly noted in vitro that Sting−/− DNase II−/− macrophages, similar to DNase II−/− macrophages, were not able to digest the engulfed nuclei from apoptotic thymocytes (Dex+) compared with control macrophages taken from wild-type or Sting−/− mice (Fig. 4B). The accumulation of undigested DNA in DNase II−/− Sting−/− macrophages was less pronounced when WT thymocytes were used as targets (Dex−). Thus, BMDM derived from Sting−/− DNase II−/− mice are also incapable of digesting DNA from apoptotic cells, although in contrast to DNase II−/− BMDM, do not produce major inflammatory cytokine responses.
Fig. 4.
Generation and characterization of Sting−/− DNase II−/− adult mice. (A) Mendelian analysis of the progeny DKO-deficient mice. Northern blotting with Sting and DNaseII cDNA as probe from liver of 8-wk-old Sting+/+DNase II+/+ (WT), and DKO mice. (B) Feulgen stain of peritoneal macrophages stained with Schiff reagent after incubation with thymocytes treated with or without Dex (10 μM, 5 h). (C) Macroscopic view of the normal fore and hind paws of DKO at the age of 12 mo. (D) H&E stain of joint sections from 6-mo-old WT and DKO mice. B, bone; C, cartilage; S; synovial. (Magnification: 200×.) (E) TNF-α, IL1-β, and IL-6 levels in serum from 8-mo-old DKO was measured by ELISA. Wild-type mice are shown as control for DKO. Data are shown with mean of five independent mice. Error bars indicate SD.
Sting−/− DNase II−/− Mice Do Not Exhibit Polyarthritis.
DNase II-mediated embryonic death can be avoided by crossing DNase II+/− mice with type I IFN defective Ifnar1−/− mice, indicating that the production of type I IFN is responsible for the observed lethal anemia. However, DNase II−/− Ifnar1−/− progeny suffer from severe polyarthritis ∼8 wk after birth (arthritis score of 2) because undigested DNA activates innate immune signaling pathways and triggers the production of inflammatory cytokines such as TNFα (5). However, we noted that Sting−/− DNase II−/− mice did not manifest any signs of polyarthritis after birth (Fig. 4C). Arthritis scores remained at approximately zero (no score) in the Sting−/− DNase II−/− up to 12 mo of age, in contrast to reported DNase II−/− Ifnar1−/− mice, which exhibited an arthritis score of up to 7 after a similar period (5, 7). Although H&E and TUNEL staining of spleen and thymus tissues of DNase II−/−Sting−/− mice illustrated the presence of infiltrating macrophages that also contained apoptotic DNA, histology of joints from 6-mo-old Sting−/− DNase II−/− mice exhibited normal bone (B), synovial joint (S), and cartilage (C) structure with no evidence of pannus infiltration in the joints (Fig. 4D) (5, 7). Levels of TNFα, IL1β, and IL6 from sera of Sting−/− DNase II−/− mice were reduced as predicted from our array analysis of BMDM that lacked STING (Fig. 4E, Table S1). Neither was there evidence of CD4, CD68, nor TRAP-positive cells infiltration within the joints of Sting−/− DNase II−/− mice (Fig. S7). Analysis of the serum of Sting−/− DNase II−/− mice also indicated no elevated levels of rheumatoid factor (RF), anti-dsDNA antibody, or MMP3 (Fig. S8). Thus, loss of STING largely eliminates proinflammatory cytokine production responsible for self DNA-mediated polyarthritis.
Discussion
The etiology of severe inflammation augmented autoimmune diseases remains unknown. However, the chronic stimulation of innate immune signaling pathways by aberrant DNA species likely plays an important role in inflammatory disorders (1). Conceivably, the overstimulation of such innate immune pathways could occur through inappropriately digested apoptotic DNA, necrotic cells, chronic infection, or by other forms of self DNA such as endogenous retroviral elements (16, 17). Here, we demonstrate that cellular STING facilitates the recognition of aberrant cytoplasmic DNA species derived from necrotic or inappropriately apoptosed cells to trigger the production of an array of innate immune and proinflammatory genes, including type I IFN in BMDM, and other cell types. Presumably, the DNA from the engulfed dead cells is leaked from the phagocytes lysosomal compartments to trigger STING function. Accordingly, loss of STING rescues lethal apoptotic DNA-mediated inflammatory disease. Moreover, DNase II−/−Sting−/− do not exhibit signs of arthritis, similar to DNase II−/− Ifnar1−/− mice (5, 7). Self-apoptotic DNA-triggered arthritis in such animals has been reported to be reminiscent of idiopathic arthritis or Still’s disease in humans (18). STING has also been shown to rescue Trex1-mediated inflammation-related disorders in Trex1−/− mice by mechanisms that remain unknown (19). The mode of intracellular DNA sensing and downstream signaling processes also remains to be fully clarified, although our data indicate that STING, an endoplasmic reticulum associated protein, may facilitate these processes by complexing with cytoplasmic DNA species, including apoptotic DNA. STING has also been shown to bind bacterial cyclic dinucleotides such as c-di-GMP or c-di-AMP (20). These events activate the IRF3/7 and NF-κB pathways, leading to gene induction (21). Our data indicates the significance of STING in self DNA-mediated inflammatory disease and potentially provides a target and pathway that could conceivably be therapeutically controlled to help avoid such disorders.
Materials and Methods
Generation of Double Knockout Mice.
STING knockout mice (Sting−/−) were generated in our laboratory. DNase II Hetero knockout mice (DNase II+/−) (RBRC01725) were purchased from RIKEN BRC BioResource Center. Sting−/− mice were crossed with DNase II+/− to generate the double knockout mice (Sting−/− DNase II−/−). Mice care and study were conducted under approval from the Institutional Animal Care and Use Committee of the University of Miami. Mouse genotypes from tail biopsies were determined by using real-time PCR with specific probes designed for each gene by commercial vendor (Transnetyx).
Primary Cells.
Bone marrow macrophages were isolated from hind-limb femurs of 8-wk-old Sting+/+ and Sting−/− mice. Briefly, the marrow cells were flushed from the bones with minimum essential medium (MEM) α (12561072; Invitrogen), 10% (vol/vol) heat-inactivated FCS (16000044; Invitrogen) with a 26 gauge needle, incubated at 37 °C for 4 h and changed with complete alpha MEM (Invitrogen) including 10 ng/mL mouse recombinant colony-stimulating factor (M-CSF, 416-ML) (R&D Systems). For dendritic cells, we used MEM including 20 ng/mL recombinant mouse GM-CSF (BioLegend; 576302). Additional details can be found in SI Materials and Methods.
Gene Array Analysis.
Total RNA was prepared from the fetal liver and bone marrow-derived macrophages by using RNeasy Mini kit (74104; Qiagen). RNA quality was analyzed by Bionalyzer RNA 6000 Nano (Agilent Technologies). Gene array analysis was examined by Illumina Sentrix BeadChip Array (Mouse WG6 version 2) (Affymetrix) at the Oncogenomics Core Facility, University of Miami. Gene expression profiles were processed and statistical analysis was performed at the Center of Computational Science, University of Miami.
ELISA.
Bone marrow-derived macrophages were seeded in 96 wells at 3 × 104 cells per well and transfected by using 1 μg/mL dsDNA90 and apoptotic DNA (1 μg/mL) by using Lipofectamine 2000 (11668019; Invitrogen) for 24 h. IFN-β levels were determined by using VeriKine Mouse IFN-Beta ELISA Kit (42400-1; PBL IFN Source). IL1-β, IL6, and TNFα levels were determined by using the Procarta Cytokine Assay kit (Affymetrix) and analyzed on the Luminex 100 System. Rheumatoid factor (RF), anti-dsDNA, and MMP-3 levels were determined by ELISA KIT (RF, 6200; Alpha Diagnostic; anti-dsDNA, 5110; Alpha Diagnostic; and MMP-3; R&D Systems).
Quantitative Real-Time PCR (qPCR).
Total RNA samples were reverse transcribed by using QuantiTect Reverse Transcription Kit (205310; Qiagen). Real-time PCR was performed by using SyBr green (Finnzyme, Thermo Scientific) for Sting (Sense: CATTGGGTACTTGCGGTT; Anti-sense: CTGAGCATGTTGTTATGTAGC) and DNaseII (Sense: GGAGACGGTGATCAAGAACCAA; Anti-sense: AATTTTGCCAGAACTGGACCT) (Sigma) genes, and Taqman Gene Expression Assay (Applied Biosystems) for innate immune genes and inflammatory cytokines (Ifnβ, Mm010439546; Irf7, Mm00516788; Tnf, Mm00443258; Il6, Mm00446190; Il1β, Mm01336189; Ifit3, Mm0170846; Ccl5, Mm01302427).
Statistical Analysis.
All statistical analysis was performed by Student’s t test. The data were considered to be significantly different when P < 0.05.
Supplementary Material
Acknowledgments
We thank Shigekazu Nagata for DNase II−/− and DNase II−/− Ifnar1−/− mice; Auristela Rivera for mouse maintenance; Daria Salyakina, Biju Issac, and Alan Goodman for array analysis; and Tianli Xia and Hiroyasu Konno for confocal microscopy. This work was supported by National Institutes of Health-National Institute of Allergy and Infectious Diseases Grant 5R01AI079336.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215006109/-/DCSupplemental.
References
- 1.Nagata S. Apoptosis and autoimmune diseases. Ann N Y Acad Sci. 2010;1209:10–16. doi: 10.1111/j.1749-6632.2010.05749.x. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, et al. National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Crow MK. Type I interferon in systemic lupus erythematosus. Curr Top Microbiol Immunol. 2007;316:359–386. doi: 10.1007/978-3-540-71329-6_17. [DOI] [PubMed] [Google Scholar]
- 4.Kawane K, et al. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292(5521):1546–1549. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6(1):49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6(5):280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 7.Kawane K, et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443(7114):998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 8.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202(10):1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455(7213):674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong B, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29(4):538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Jin L, et al. MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals. Mol Cell Biol. 2008;28(16):5014–5026. doi: 10.1128/MCB.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saitoh T, et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci USA. 2009;106(49):20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oka , et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 16.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131(5):873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134(4):587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawane K, Tanaka H, Kitahara Y, Shimaoka S, Nagata S. Cytokine-dependent but acquired immunity-independent arthritis caused by DNA escaped from degradation. Proc Natl Acad Sci USA. 2010;107(45):19432–19437. doi: 10.1073/pnas.1010603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gall A, et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36(1):120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdette DL, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478(7370):515–518. doi: 10.1038/nature10429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barber GN. Cytoplasmic DNA innate immune pathways. Immunol Rev. 2011;243(1):99–108. doi: 10.1111/j.1600-065X.2011.01051.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.